Abstract
An experimental tracking optical coherence tomography (OCT) system has been clinically tested. The prototype instrument uses a secondary sensing beam and steering mirrors to compensate for eye motion with a closed-loop bandwidth of 1 kHz and tracking accuracy, to within less than the OCT beam diameter. The retinal tracker improved image registration accuracy to <1 transverse pixel (<60 μm). Composite OCT images averaged over multiple scans and visits show a sharp fine structure limited only by transverse pixel size. As the resolution of clinical OCT systems improves, the capability to reproducibly map complex structures in the living eye at high resolution will lead to improved understanding of disease processes and improved sensitivity and specificity of diagnostic procedures.
Optical coherence tomography (OCT) has emerged over the past decade as an outstanding tool for imaging biological structures. Ultrahigh-resolution, Doppler, spectroscopic, polarization-sensitive, parallel (spectral domain), and three-dimensional (3-D) OCT imaging modes, among others, have been demonstrated by numerous researchers.1–5 These tools have found important applications in quantitative retinal imaging and detection and characterization of retinal pathology. Clinical OCT instruments have been shown to produce highly accurate maps of the retinal nerve fiber layer.6 We have modified a commercial clinical OCT system by integrating a prototype of an active, hardware-based retinal tracker for OCT image stabilization. The new experimental system, called tracking optical coherence tomography (TOCT), uses a secondary sensing beam and steering mirrors to compensate for eye motion7 with a closed-loop bandwidth of up to 1 kHz.
Involuntary eye movements during image acquisition, and particularly between scans, currently limit the multi-image acquisition potential of OCT and thus its feasibility for several advanced clinical applications. To build an undistorted image voxel-by-voxel or co-add multiple images correctly, the absolute coordinates of each voxel relative to a fixed retinal reference point must be known. Even in patients that can fixate well (hold a precise gaze angle), eye-motion amplitude may be several hundred micrometers at the retina, much larger than the resolution of second-and third-generation OCT instruments [Carl Zeiss Meditec, Inc. (CZMI)]. High-speed spectral-domain optical coherence tomography (SDOCT) systems can capture images with A-scan rates of tens of kilohertz.5 However, even for these systems, retinal tracking may still be useful to lock and return to the same retinal position from visit to visit, to average sets of images accurately, and to generate 3-D retinal maps that will still require many seconds to acquire. As imaged volume and resolution increase, a means of retinal tracking is increasingly important. Retinal position tracking permits extended scan periods and frame averaging of multiple scans to reduce OCT speckle with minimal effect on transverse or longitudinal resolution. Stabilized two-dimensional (2-D) scans can be concatenated into motion artifact-free 3-D image cubes. Because the retinal tracker uses physical landmarks, precisely aligned scans can be compared months or years apart. Retinal morphology can thus be accurately recorded and monitored over time to detect subtle changes. Such capabilities may provide greater diagnostic sensitivity and specificity for glaucomatous changes in the eye.
The prototype retinal tracking instrument illustrated in Fig. 1, employed in the clinical tests described below, projects a low-power tracking beam (<25 μW at 860 nm) onto small retinal features of modest light or dark contrast relative to neighboring areas. Such features may include blood vessel junctions, the lamina cribrosa (a bright region within the optical nerve head), retinal lesions, drusen, scars, or irregular pigmentation. The tracking beam is circularly dithered at 8 kHz and an amplitude of 150 μm (0.5°) on the retina with a pair of resonant scanners, detected with a confocal reflectometer, and processed with a dual-phase lock-in amplifier to produce directional error signals. A split aperture is used in the confocal reflectometer to minimize interference from corneal reflections. The tracking beam is directed onto the retina by a galvanometer-driven tracking mirror pair. Real-time digital signal processing extracts position and orientation information from optical error signals to make precise closed-loop beam-steering corrections much faster than the highest possible image-framing rate. Because the OCT scans created with the galvanometer pair are also reflected from the tracking mirrors, the OCT beam automatically follows any movement of the retina with the same speed and precision as the tracking beam. There was no discernible effect of tracking-induced frequency shifts on OCT images. The rms tracking error of <0.05° (~15 μm) is typically smaller than the OCT beam diameter (~20 μm). After this tracking technology was integrated into a clinical OCT II (CZMI) system, precise eye-motion correction was demonstrated within and between 2-D OCT scans in both normal subjects and glaucoma patients.
Fig. 1.
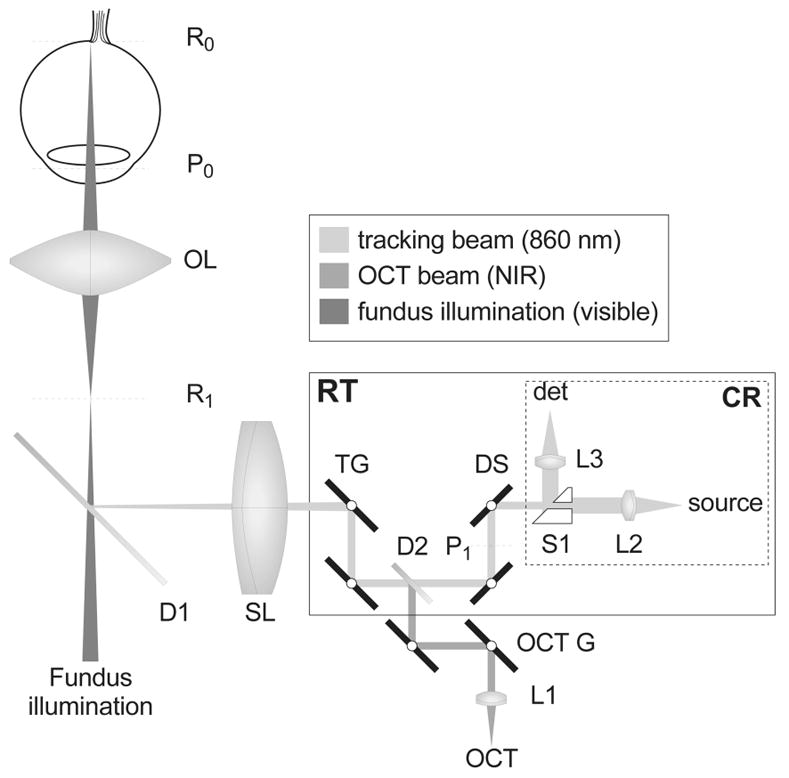
Optical schematic of TOCT. The 3-D layout is flattened to two dimensions for clarity. R0 and P0 indicate the retinal and pupil planes of the eye, respetively; R1 and P1 are optically conjugate to those planes. OL and SL are the ophthalmoscopic and scan lenses; RT, retinal tracker; CR, confocal reflectometer; D1, D2, dichroic beam splitters; L1–L3, fiber collimating lenses; TG, tracking galvanometer-driven mirrors; OCT G, OCT beam galvanometer-driven mirrors; DS, dither resonant scanners; S1, split aperture; NIR, near-infrared.
The clinical tests consisted of a series of OCT scans performed on three separate visits of more than 20 subjects with normal or glaucomatous eyes. The scans were collected both with and without retinal tracking. The tracking feature selected for all scans was the lamina cribrosa, which is generally a stable, robust tracking feature. The sequence of OCT scans used in this protocol for each visit includes peripapillary scans (nominal angular diameter of 12° and a transverse resolution of 110 μm) and sequences of radial scan through the disk and the macula (six scans successively rotated by 60° with a nominal scan length of 20° and transverse resolution of 60 μm). The peripapillary scan is generally used to measure retinal nerve fiber layer thickness around the optic disk to screen or monitor subjects with glaucoma. The radial scan is used to generate a map of a large region of the retina for measurement of retinal nerve fiber layer or retinal thickness. OCT scans were processed and analyzed with custom software designed to register and co-add multiple images within and between visits and to extract quantitative information on the location of retinal features with sharp edges (e.g., optic disk cup). In the co-added OCT scans, the qualitative criteria for improvement as a result of eye-motion stabilization were the ability to resolve blood vessel lumen, the number and width of blood vessel shadow edges, and the clarity of clearly identifiable features such as distinct retinal and subretinal layers. Quantification of tracking performance compared with fixation by measurement of transverse image registration was limited in this study by the OCT transverse pixel resolution. The typical amplitude of eye motion for a good fixator, including involuntary microsaccades and drift, is ~0.5° (~150 μm), or approximately 1.4 and 2.5 pixels for peripapillary and radial OCT II scans, respectively. The accuracy of a similar tracking system in other higher resolution imaging implementations8 was found to be <0.05° (~15 μm). In the clinical trials a qualitative improvement in OCT II peripapillary scans was found in 97% of all subject visits. Analysis of the mean variation in disk edge position in OCT II radial disk scans found an improvement in every subject.
Figure 2 shows average OCT peripapillary images co-added from three successive visits to the clinic (~60 total scans) with and without tracking. Note the absence of speckle in the averaged OCT images. The measured signal-to-noise ratio for both Figs. 2b and 2c is 5.6 times greater than that of a single OCT image from the same set, implying significant decorrelation of speckle between images. Horizontal retinal layers are generally less affected because the shallow gradients of these layers, even in subjects with glaucoma, render the sharpness of such boundaries (once they are vertically aligned) less sensitive to transverse motion parallel to the layers. Nevertheless, the sharpness of the retinal layers is clearly improved, as presumably is the precision of the delineation of these boundaries with automated analysis. It is in the appearance of nonlayered fine structures that the advantage of tracking is immediately apparent in the co-added cross-sectional images. Blood vessel shadows (caused by the relatively high light extinction by blood at these wavelengths) show good contrast and are precisely aligned. Details of the vessel cross section, including lumen, emerge with tracking. Fine structure within the choroid is evident in the tracking average. The limitation in image detail is determined by the coarse pixelation of the OCT II system rather than by motional broadening.
Fig. 2.
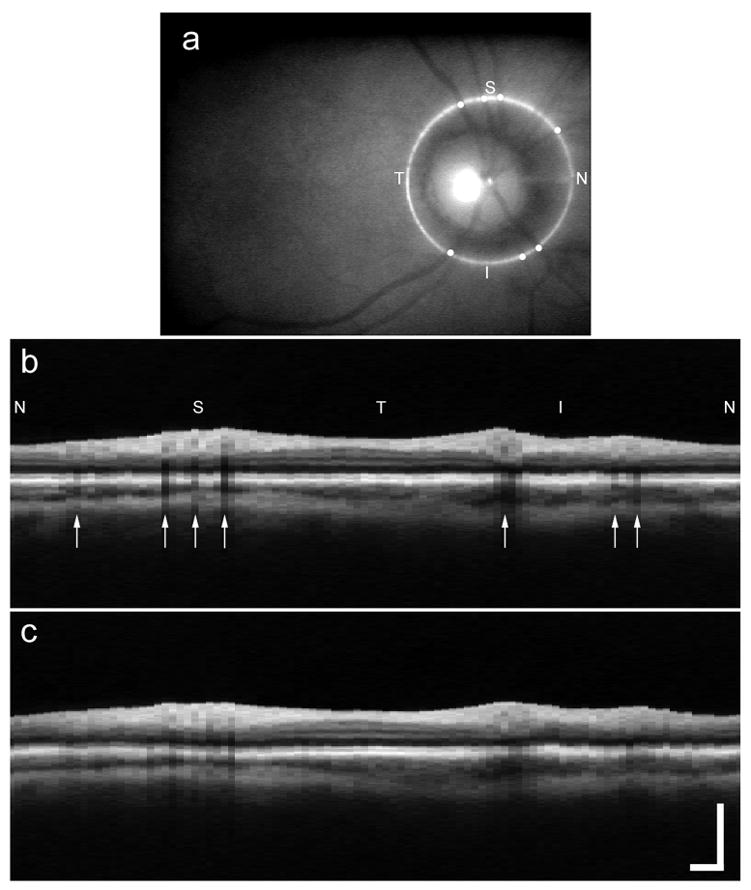
Composite peripapillary OCT images co-added from three visits of 20 scans each: a, fundus image (single frame); b, with tracking; c, without tracking. S, superior; T, temporal; I, inferior; N, nasal. The scale bar is 1.0 mm. The small white circles in a and the arrows in b indicate the location of blood vessels.
Figure 3 shows a set of three single OCT radial disk scans for one orientation (of six) acquired on three successive visits with (left column) and without tracking (right column). The average OCT image is shown in the final frame of each column (Figs. 3j and 3t). With tracking, the position and morphology of the disk and cup appear to be quite stable across many frames. Without tracking, not only is the in-plane transverse alignment and distortion problematic but also out-of-plane motion (normal to the image plane) increases the probability that a different section of the disk is scanned. These motions are uncorrectable by postprocessing and further distort the image when we are executing the vertical alignment software by coupling transverse alignment error into vertical distortion. Without tracking, the average, although smoothed, is without diagnostic significance. The mean variance in disk cup edge position for the individual visits for this subject, a good fixator, was 0.35 pixels with tracking and 1.22 pixels without tracking. One of the significant improvements expected from TOCT is the improved ability to measure the same retinal location every time a patient is examined to monitor longitudinal progression of a disease or defect. To test the hypothesis that tracking improved the ability to return to a fixed retinal position, we examined the variance in disk cup edge position for multiple visits and found it to increase to 0.45 pixels with tracking and 3.76 pixels without tracking.
Fig. 3.
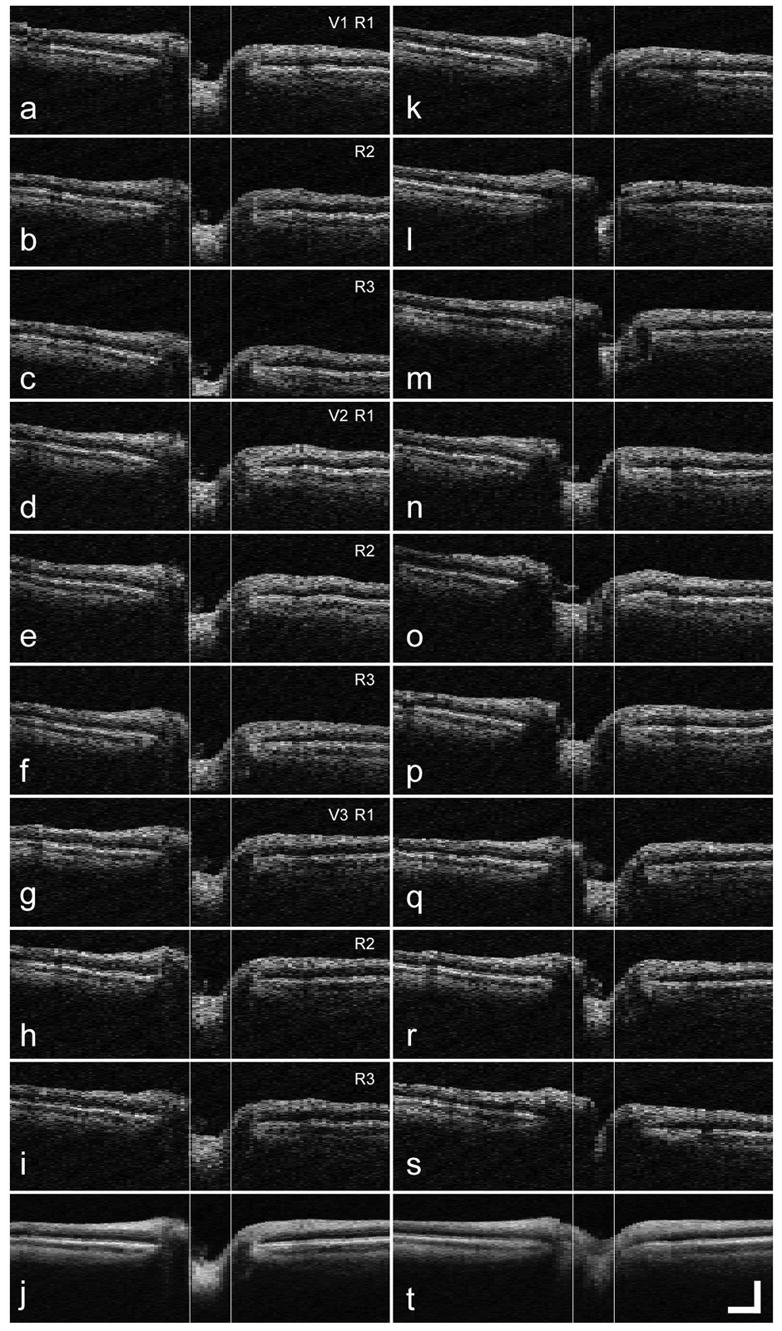
Radial disk OCT scans from one subject on three successive visits: a–i, scans taken with tracking; j, composite image created from scans a–i; k–s, scans taken without tracking. t, composite image created from scans k–s. The vertical lines indicate the FWHM edge of the disk cup. V, visit number; R, run number. The scale bar is 0.5 mm.
In the third-generation clinical OCT system (Stratus_OCT)system, there is significant improvement in both transverse and longitudinal OCT image resolution. With the additional capabilities provided by TOCT, retinal boundaries can become even better defined by averaging because high image spatial frequencies are preserved. Direct, high-resolution 2-D and 3-D imaging of the disk and other retinal structures and pathologies with steeper vertical features will benefit even more dramatically from retinal tracking.
Acknowledgments
This work was funded by the National Institutes of Health (National Eye Institute) under grants EY13036 and EY13178. Both Physical Sciences, Inc., and CZMI provided partial support for the clinical tests. R. D. Ferguson’s e-mail address is ferguson@psicorp.com.
References
- 1.Aguirre AD, Hsiung P, Ko TH, Hartl I, Fujimoto JG. Opt Lett. 2003;28:2064. doi: 10.1364/ol.28.002064. [DOI] [PubMed] [Google Scholar]
- 2.Westphal V, Yazdanfar S, Rollins AM, Izatt JA. Opt Lett. 2002;27:34. doi: 10.1364/ol.27.000034. [DOI] [PubMed] [Google Scholar]
- 3.Wojtkowski M, Kowalczyk A, Keitgeb R, Fercher AF. Opt Lett. 2002;27:1415. doi: 10.1364/ol.27.001415. [DOI] [PubMed] [Google Scholar]
- 4.Cense B, Chen TC, Park GH, Pierce MC, de Boer JF. Opt Lett. 2002;27:1610. doi: 10.1364/ol.27.001610. [DOI] [PubMed] [Google Scholar]
- 5.Nassif N, Cense B, Park BH, Yun SH, Chen TC, Bouma BE, Tearney GJ, de Boer JF. Opt Lett. 2004;29:480. doi: 10.1364/ol.29.000480. [DOI] [PubMed] [Google Scholar]
- 6.Guedes V, Schuman JS, Hertzmark E, Correnti A, Mancini R, Wollstein G, Lederer D, Voskanian S, Velazquez L, Pakter HM, Pedut Kloizman T, Fujimoto JG, Mattox C. Ophthalmology. 2003;110:177. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson RD. Servo tracking system utilizing phase-sensitive detection of reflectance. 5,767,941. US patent. 1998 June 16;
- 8.Hammer DX, Ferguson RD, Magill JC, White MA, Elsner AE, Webb RH. Appl Opt. 2003;42:4621. doi: 10.1364/ao.42.004621. [DOI] [PubMed] [Google Scholar]


