Abstract
We identified a previously unknown integrin, α9β1, on OCLs and their precursors. Antibody to α9 inhibited OCL formation in human marrow cultures, and OCLs from α9 knockout mice had a defect in actin ring reorganization and an impaired bone resorption capacity.
Introduction
Integrins play important roles in osteoclast (OCL) formation and function. Mature OCLs mainly express αvβ3 integrin, a heterodimer adhesion receptor that has been implicated in osteoclastic bone resorption. We identified ADAM8, a disintegrin and metalloproteinase, as a novel stimulator of OCL differentiation and showed that the disintegrin domain of ADAM8 mediated its effects on OCL formation. Because the disintegrin domain of ADAM8 does not bind Arg-Gly-Asp (RGD) sequences, we determined which integrin bound ADAM8 and characterized its role in OCL formation and activity.
Materials and Methods
Chinese hamster ovary cells (CHO) expressing different integrin subunits were tested for their capacity to bind the disintegrin domain of ADAM8. Mouse or human bone marrow cells and purified OCL precursors were tested for α9β1 integrin expression by Western blot, immunocytochemistry, and real-time RT-PCR. A monoclonal antibody to human α9 was used to block α9β1 on OCL precursors stimulated by 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] or RANKL. Vertebrae of 7-day-old α9−/− mice and wildtype (WT) littermates were compared using bone histomorphometry and 3D μCT analysis.
Results
α9 integrin was expressed by mouse and human bone marrow–derived OCLs and their precursors. Importantly, the anti-α9 antibody inhibited human OCL formation stimulated by 1α,25(OH)2D3 or RANKL dose-dependently. Furthermore, analysis of OCLs formed in marrow cultures from α9−/− mice showed that the OCLs formed were more contracted and formed significantly less bone resorption pits on dentin slices. Histologic analysis of α9−/− vertebrae showed thickened trabecular regions and retained cartilage within vertebral bodies of α9−/− mice. 3D μCT analysis of α9−/− vertebrae also showed a significant increase in trabecular bone volume/total tissue volume and a tendency for decreased trabecular separation compared with WT mice.
Conclusions
These results support a previously unknown role for α9β1 integrin in OCL formation and function.
Keywords: α9β1 integrin, osteoclast, ADAM8, α9 knockout mice
INTRODUCTION
Osteoclasts (OCLs) are multinucleated cells formed by the fusion of precursors in the monocyte/macrophage lineage.(1,2) OCL formation and function involve a series of developmental and regulatory steps including homing to bone by hematopoietic progenitor cells, migration of OCL precursors to the area of bone to be remodeled, cell proliferation, attachment of OCL progenitors to the bone, and fusion of mononuclear precursors to form multinucleated OCLs that are activated and resorb bone. Many of these steps involve cell–cell and cell–matrix adhesion. Several factors affect osteoclastogenesis at different stages of development, including 1α,25-dihydroxy-vitamin D3 [1α,25(OH)2D3], RANKL, macrophage-colony-stimulating factor (M-CSF), interleukin-1 (IL-1), TGF-β, TNF-α, IL-6, and PTH.(1) In addition, several autocrine/paracrine factors are produced by OCLs that regulate OCL formation and activity.(3–5)
ADAM8, a member of the ADAM gene family, is an autocrine factor expressed by OCLs that is involved in the later stages of OCL formation.(6) The ADAM family is comprised of proteins that have transmembrane and cytoplasmic domains containing metalloproteinase (MMP)-like, disintegrin-like, cysteine-rich, and epidermal growth factor (EGF)-like domains. ADAM family members have been implicated in cell fusion and cell-to-matrix adhesion(5,7) and can act as signaling molecules. These functions are vital for normal cellular processes such as cell morphogenesis, wound healing, tumor cell invasion, and metastases.(8,9) ADAM8 is expressed primarily in cells of the monocyte/macrophage lineage as a type 1 membrane–anchored protein.(10) We previously reported that the disintegrin domain of ADAM8 mediated its stimulatory effects on OCL formation.(6) However, the integrin mediating the effects of ADAM8 on OCL formation had not been previously identified. αvβ3 is abundantly expressed by OCLs, and specifically binds to the Arg-Gly-Asp (RGD) domain. αvβ3 plays an important role in the resorptive process.(11) OCLs also express other integrins at lower levels, such as α2β1, αvβ1, αvβ5, and α5β1. (12) ADAM8 lacks an RGD motif and has the RX6DLPEF sequence, which is an α9β1 recognition motif. This study shows that α9β1 integrin is expressed on OCLs and their precursors and that α9β1 integrin is the receptor for ADAM8 that mediates its effects on osteoclastogenesis. Furthermore, we show that OCLs lacking α9 have an abnormal cytoskeleton and a decreased bone resorption capacity.
MATERIALS AND METHODS
Reagents and antibodies
RANKL (Amgen, Seattle, WA, USA) and 1α,25(OH)2D3 (Teijin, Tokyo, Japan) were generously provided for these experiments. Recombinant human M-CSF was purchased from R&D Systems (Minneapolis, MN, USA). Restriction enzymes, Taq DNA polymerase, FCS, and tissue culture media were purchased from Invitrogen (Grand Island, NY, USA). Mouse anti-human α9β1 monoclonal antibody was purchased from US Biological (Swampscott, MA, USA). The polyclonal antibody against murine α9 was generously provided by Dr Dean Sheppard (University of California at San Francisco), and all other chemicals were obtained from Sigma (St Louis, MO, USA).
Animals
Four- to 6-week-old C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). α9 heterozygote mice were generously provided by Dr Dean Shep-pard(13) and bred under conditions approved by the IACUC at Virginia Commonwealth University. Seven-day-old α9−/− mice were used for cell culture, μCT, and histologic studies.
Adhesion assays
Adhesion assays were performed as reported by Eto et al.(14) Briefly, 96-well Immulon-2 microtiter plates (Dynatech Laboratories, Chantilly, VA, USA) were coated with 100 μl of PBS containing 20 μg/ml of glutathione S-transferase (GST)-ADAM8 disintegrin fusion protein or control GST protein and incubated overnight at 4°C. The 96-well plates were blocked with 1% BSA (Calbiochem, San Diego, CA, USA) for 1 h at room temperature. After washing with PBS, 105 CHO cells stably expressing αV, α9 and/or β1, β3, integrin (generously provided by Dr Takada, Y Scripps Research Institute, La Jolla, CA, USA) were added to the wells in 100 μl of DMEM supplemented with 1% BSA and incubated at 37°C for 1 h. Unbound cells were removed by washing with PBS and the remaining cells counted with an inverted microscope. GST protein was used as a negative control. In selected experiments, the GST-ADAM8–coated plates were also pretreated with a blocking antibody to α9 integrin (clone Y9A2; Chemicon, Temecula, CA, USA) before the CHO cells were added.
OCL formation assays
Mouse bone marrow cultures were performed as previously described.(4) Briefly, nonadherent mouse bone marrow cells from 4–6-week-old α9+/− mice, 7-day-old α9+/−, or α9−/− C57BL/6 mice were cultured for 6–9 days in either eight-well chamber slides (Nunc), 48- or 96-well plates in α-MEM containing 10% FCS with 1α,25(OH)2D3 (10−9 M), or 10 ng/ml M-CSF, and 50 ng/ml hRANKL. At different times during the culture period, the cells were fixed and stained for TRACP or total RNA was extracted from the cells using RNA-BEE (Tel Test, Friendswood, TX, USA). In selected experiments, bone marrow cells were cultured on sperm whale dentin slices. After 1 week of culture, the dentin slices were stained with hematoxylin, and the number of OCLs was counted. The cells on the dentin slices were removed by rubbing the slices, and the number of resorption pits was scored microscopically.
Human bone marrow cultures were performed as previously described.(2,15) The studies were approved by the Institutional Review Board of the University of Pittsburgh. After obtaining informed consent, 2 ml of bone marrow was aspirated from the posterior superior iliac crest of healthy normal volunteers into a 20-ml syringe containing 1 ml of α-MEM and 1000 U/ml of heparin. The mononuclear cell fraction was obtained by Ficoll-Hypaque density gradient centrifugation, and the mononuclear cells (5 × 106 cells/ml) were collected and incubated in α-MEM containing 20% FCS overnight at 37°C in 100-mm tissue culture plates to deplete adherent cells. Nonadherent bone marrow cells were cultured in 96-well plates in α-MEM containing 20% horse serum in the presence of RANKL (50 ng/ml) and M-CSF (10 ng/ml) or 10−8 M 1α,25(OH)2D3. The cultures were fed every 3 days by replacing one half the media, and after 21 days of culture, the cells were fixed with 1% formaldehyde. OCL-like cells were identified by cross-reactivity with the monoclonal antibody 23c6 using a Vectastatin-ABC-AP kit (Vector Laboratories, Burlingame, CA, USA). The 23c6-positive OCLs were scored using an inverted microscope.
In selected experiments, nonadherent mononuclear cells were cultured in methylcellulose culture as described previously(16) to form CFU-GM colonies. In brief, CFU-GM colonies were formed by culturing 105 nonadherent marrow mononuclear cells/ml in 0.3% methylcellulose in α-MEM with 30% FCS in 35-mm tissue culture plates in presence of 100 pg/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) for 7–10 days. The CFU-GM colonies (>40 cells) were morphologically identified by light microscopy and individually collected. CFU-GM–derived cells are highly purified early OCL precursors.(16)
RT-PCR and real-time RT-PCR analysis
Total RNA was extracted from bone marrow cells cultured for 1–7 days using RNA-BEE (Tel Test), and the cDNA was synthesized from 1 μg of total RNA using the SuperScript reverse transcriptase system (Invitrogen, Carlsbad, CA, USA) in a total volume of 20 μl. The resulting cDNA products were subjected to PCR analysis using primers specific for α9 integrin (5′-CCTAACGTTGCACTGCAACC-3′ sense, 5′-AGCAGAAAAATGAGGATCCCC-3′ antisense; accession no. NM_133721); αv (5′-AATCTCCAGTGGCCTTACAA-3′ sense, 5′-AGGAAGCAGATGACTTCAGG-3′ antisense; accession no. NM_008402); ADAM8 (5′-ATGCTACTGTCCTGAACCAC-3′ sense, 5′-ACTGAGACGACACACCTCTC-3′; accession no. NM_007403); GAPDH (5′-ACCACAGTCCATGCCATCAC-3′ sense, 5′-TCC-ACCACCCTGTTGCTGTA-3′ antisense; accession no. NM_001001303). The PCR amplification was performed by incubating the sample at 94°C for 30 s, 58°C for 30 s, and 68°C for 1 minute for 20–35 cycles. The PCR products were electrophoresed on a 1.5–2% agarose gel containing ethidium bromide. For real-time RT-PCR analysis, α9 primers were 5′-TCAACATCACAGCACCTGAG-3′ (forward) and 5′-AGCCGTCAGATTGTAGTTCAG-3′ (reverse); for ADAM8, 5′-ATGCTACTGTCCTGAACCAC-3′ (forward) and 5′-TGCTACACCTGCTGAATATCC-3′ (reverse); and for GAPDH, 5′-GCCTTCCGTGTTCCTACC-3′ (forward) and 5′-GCCTGCTTCACCACCTTC-3′ (reverse). The correlation coefficients for each amplification were 0.99 or higher, and the amplicons were visualized on 4% agarose gel as well. A Bio-Rad iCycler and iQ SYBR green supermix were used for the real-time RT-RCR analysis. The running conditions were incubation at 94°C for 2 minutes and 40 cycles of incubation of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s, with final incubation at 72°C for 7 minutes.
Western blot analysis
Cells were lysed in a buffer containing 1% Triton X-100 in 50 mM Tris-HCl (pH 7.4) containing 150 mM NaCl, 2 mM Na3VO4, 100 mM NaF, and protease inhibitors and subjected to Western blot analysis. The protein concentration of the samples was measured using the BioRad Brad-ford reagent according to the manufacturer’s protocol. Protein samples (20 μg) were subjected to electrophoresis, using 8–12% polyacrylamide gels. The proteins were transferred onto nitrocellulose membranes for immunoblot analysis. After blocking with 5% nonfat dry milk or 2% BSA in 150 mM NaCl, 50 mM Tris (pH 7.2), 0.05% Tween 20 (TBST) buffer, the membrane was incubated for 1 h with an anti-α9β1 or β-actin antibodies diluted 1:1000 in 5% non-fat dry milk-TBST. The blots were incubated for 1 h with horseradish peroxidase (HRP)–conjugated goat anti-mouse or anti-rabbit IgG diluted 1:2500 in 5% nonfat dry milk-TBST or 2% BSA and visualized using the ECL system (Amersham, Arlington Heights, IL, USA).
Immunocytochemistry
Cells were fixed with 3.7% formaldehyde for 20 minutes. After being rinsed, cells were blocked with H2O2 for 30 minutes and blocked with 2% horse serum for 30 minutes. Cells were incubated overnight with primary antibody at 4°C and for 1 h at room temperature with an HRP-labeled secondary antibody; goat anti-mouse or anti-rabbit IgG (Santa Cruz). For phalloidin-Texas red (Invitrogen) staining, the cells were fixed with 3.7% formaldehyde and per-meabilized with 0.1% Triton-X 100 in PBS before staining. Control staining was performed by replacing the primary antibody with normal mouse IgG. Cell morphology was observed with an Olympus epi-fluorescence microscope and recorded with a Magnafire digital camera (Optronics), or a Leica TCS-SL confocal microscope system (Leica Microsystems, Weltzlar, Germany) with a ×40 oil (NA 1.25) lens.
μCT Analysis
Fifth lumbar vertebrae of 7-day-old α9−/− and wildtype littermate mice (five mice of each genotype) were fixed in 3.7% formaldehyde and used for 3D μCT analysis. First, 2D CT horizontal slices of the lumbar were obtained with a Scanco vivaCT40 (Scanco Medical, Bassersdorf, Switzerland). The thickness of slices was 10 μm. 3D images were reconstructed using the software provided by Scanco. The trabecular bone was measured in the tissue area. Parameters analyzed from 3D images included the ratio of trabecular bone volume and total bone volume (TV/BV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp). The threshold for the 3D analysis was set at 175.
Histology
The undecalcified fifth lumbar vertebrae of 7-day-old α9−/− and WT littermate mice (n = 5) were sectioned into 4 μm on a cryostat (CryoJane Tape-Transfer System). The sections were fixed in citrate/acetone solution or 3.7% formaldehyde for TRACP staining or H&E staining, respectively. TRACP activity was detected by incubation with a mixture of 0.1 mg/ml naphthol AS-MX phosphate (Sigma), 0.5% N,N-dimethylformamide, and 0.6 mg/ml fast red AL salt (Sigma) in 0.1 M acetate buffer solution (pH 5.0) at 37°C for 30 minutes. H&E staining was performed using standard procedures. The slides were viewed with a Nikon TE2000 inverted microscope equipped with a Spot 12 bit 1600 × 1200 pixel CCD camera. Histomorphometric analysis was performed using the Fovea Pro Analysis System (Reindeer Graphics, Asheville, NC, USA) according to the American Society of Bone and Mineral Research His-tomorphometry Committee.(17)
Data analysis
Data were expressed as mean ± SE. Statistical differences were determined using ANOVA for repeated measures; p values <0.05 were considered to be significant.
RESULTS
Binding of CHO cells expressing integrin αvβ3 and α9β1 to a GST-ADAM8 fusion protein
We previously reported that the disintegrin domain of ADAM8 mediated its stimulatory effects on OCL formation.(3) To identify which integrin subunit interacted with ADAM8, we tested the adherence of CHO cells homogenously expressing human integrin αVβ3 or α9β1 to plates coated with the disintegrin domain of GST-ADAM8 or control GST protein. CHO cells expressing the integrin α9β1 subunit significantly bound the ADAM8 disintegrin domain, whereas CHO cells expressing integrin αvβ3 did not significantly bind to ADAM8 (Fig. 1A). All transformed CHO cells minimally bound the control GST protein (data not shown). To confirm the specificity of the interaction of ADAM8, plates coated with GST-ADAM8 fusion protein were pretreated with an anti-α9 antibody and the CHO cells transformed with αvβ3 or α9β1 cDNA were allowed to attach to the plates. The addition of α9 antibody completely inhibited the binding of α9β1 to GST-ADAM8 fusion protein (Fig. 1B), but did not alter background binding to αvβ3.
FIG. 1.
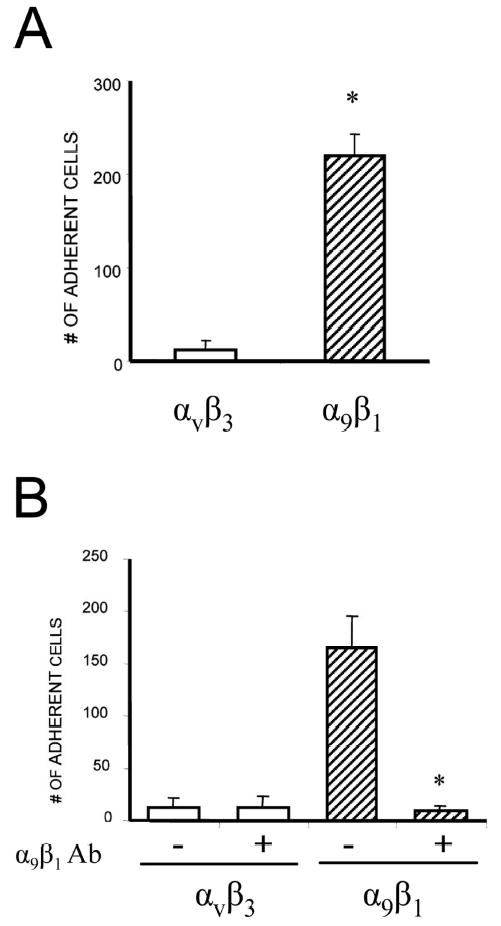
Adhesive capacity of CHO cells expressing heterodimeric integrin subunits. Adhesion of CHO cells stably expressing α9β1 or αvβ3 integrin to the disintegrin domain of ADAM8. (A) Adhesion was measured by counting the number of adherent cells with an inverted microscope. Adhesion capacity of CHO cells expressing α9β1 integrin to the disintegrin domain of ADAM8 was significantly enhanced compared with integrin, αvβ3. (B) Effects of anti-α9β1 antibody on CHO cell adhesion. In addition to being coated with the GST-ADAM8 fusion protein, the culture plates were pretreated with a blocking antibody against α9β1 (10 μg/ml). The adhesion of stably expressing α9β1 CHO cells to the culture plates was significantly decreased. Similar results were found in three independent experiments. Results from one typical experiment are shown as mean ± SE (*p < 0.05).
α 9 integrin expression on mouse and human OCL precursors
α9 integrin is expressed in airway epithelial cells, airway and gut smooth muscle, keratinocytes, hepatocytes, and neutrophils, but expression of α9 on OCLs or their precursors has not been reported. To examine if α9 integrin is expressed during OCL differentiation, real-time quantitative RT-PCR analysis for α9 and ADAM8 mRNA was performed on mouse bone marrow cultures treated with RANKL/M-CSF for 1–7 days of culture. Expression of α9 mRNA progressively increased during OCL differentiation (Fig. 2A), as did the expression of ADAM8. In addition, the expression levels of αv were similar between α9−/− and WT mice (Fig. 2B).
FIG. 2.
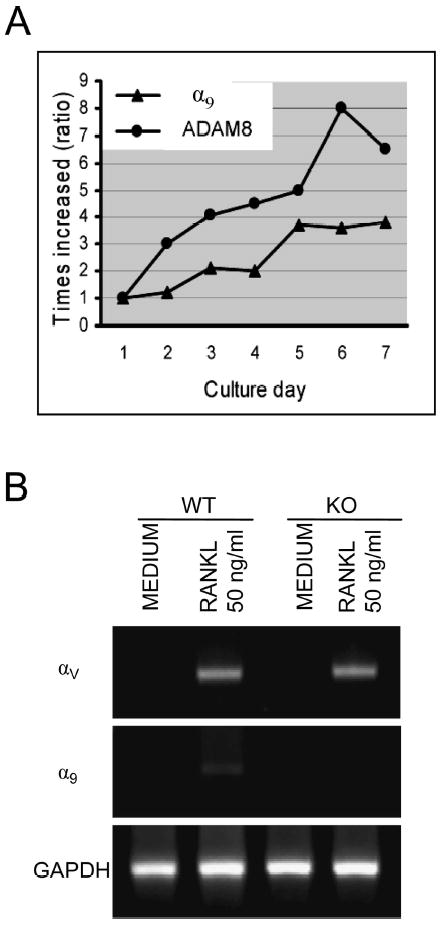
Expression of α9 during mouse bone marrow OCL differentiation. (A) Mouse bone marrow nonadherent cells were treated with RANKL (50 ng/ml) and M-CSF (10 ng/ml) for varying time intervals. The cells were harvested at different time-points as indicated and analyzed for α9 and ADAM8 mRNA expression by real-time RT-PCR using specific primers. (B) Non-adherent bone marrow cells from α9−/− and WT mice were incubated with factors as indicated for 3 days. The αv integrin expression levels were analyzed by RT-PCR. Similar results were found in three independent experiments. Results from one typical experiment are shown.
Purified human OCL precursors were tested for α9 integrin expression. Western blot analysis of lysates from highly purified human OCL precursors showed a 140-kDa α9 integrin band (Fig. 3A). No band was detected in the control lane. OCL precursors were positively stained (Fig. 3B) by a monoclonal anti-human α9 antibody. The dark stained area surrounding the cells denoted a positive reaction. Control staining performed without primary antibody showed no staining (data not shown). When highly purified CFU-GM derived and human OCL precursors were treated with RANKL (50 ng/ml) and M-CSF (10 ng/ml) for 7 days, both α9 and ADAM8 mRNA was expressed (Figs. 3C and 3D) as shown by RT-PCR analysis.
FIG. 3.
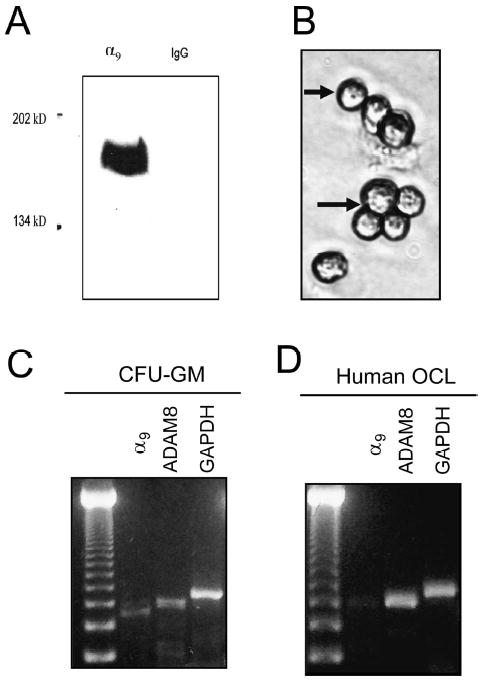
Expression of α9 integrin on human osteoclasts and precursors. (A) Western blot analysis of human CFU-GM derived cell lysates with monoclonal anti-human α9 antibody. A 140-kDa α9 integrin band was detected in lysates from highly purified OCL precursors. No band was detected in the control lane. (B) Immunocytochemistry for α9 expression on osteoclast precursors (CFU-GM–derived cells) treated with 1α,25(OH)2D3(10−8 M) for 5 days. RT-PCR analysis for α9 and ADAM8 expression in (C) CFU-GM–derived cells and (D) OCLs formed from human CFU-GM–derived OCL precursors induced with 50 ng/ml RANKL and 10 ng/ml M-CSF in liquid cultures.
Anti-α9 antibody inhibits OCL formation
We previously reported that ADAM8 stimulated OCL formation and bone resorption and that an antisense construct to ADAM8 inhibited OCL formation induced by RANKL or 1α,25(OH)2D3. (6) To determine if α9 integrin was involved in OCL formation, we tested the effects of a blocking α9 antibody on OCL formation in human marrow cultures treated with 1α,25(OH)2D3 (10−8 M). When human bone marrow cultures were treated with an antibody to α9 for 3 weeks, OCL formation was reduced significantly (Fig. 4A) at an antibody concentration of 1.0 μg/ml compared with the control mouse IgG (1.0 μg/ml). Addition of 10μg/ml of anti-a9 antibody did not further inhibit OCL formation (data not shown). Because OCL formation from bone marrow nonadherent cells occurs in the presence of other cells in the culture, we also used purified OCL precursors to test if the α9 antibody inhibited OCL formation by OCL precursors. Highly purified human CFU-GM–derived cells were treated with RANKL (50 ng/ml) and M-CSF (10 ng/ml) in the presence or absence of anti-α9 for 3 weeks. Treatment with α9 antibody significantly decreased OCL formation (Fig. 4B).
FIG. 4.
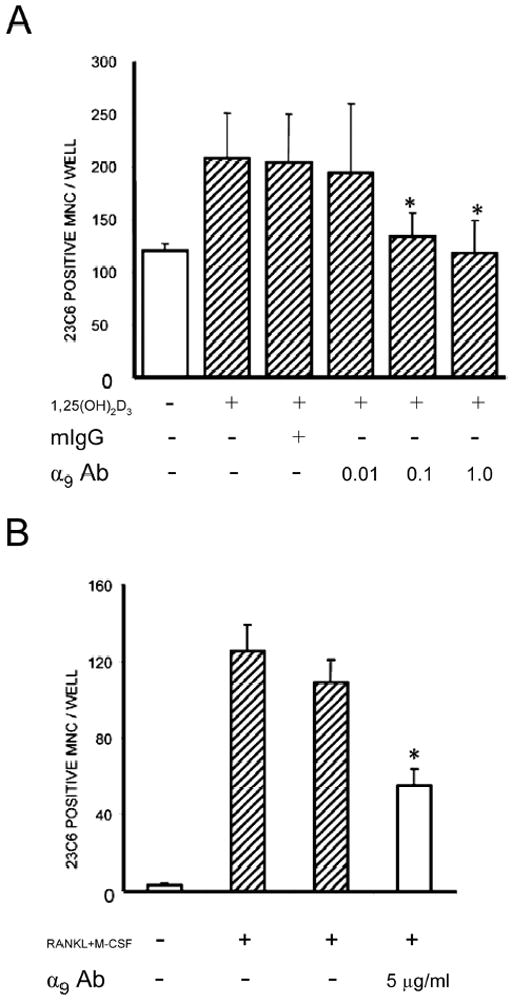
Anti-α9 integrin inhibits OCL formation by human CFU-GM–derived cells. (A) Human bone marrow cells stimulated with 1α,25(OH)2D3 (10−8 M) were treated with varying concentrations of anti-α9 antibody (μg/ml as indicated in the figure) and cultured for 3 weeks. (B) CFU-GM–derived cells were treated with RANKL (50 ng/ml) and M-CSF (10 ng/ml) in the presence or absence of anti-α9 for 3 weeks. At the end of the culture period, cells were fixed and stained with the 23C6 monoclonal antibody that identifies the osteoclast vitronectin receptor. 23c6+ multinucleated cells containing three or more nuclei per cell were scored as OCLs. Mouse IgG was used as a control. Results represent the mean ± SE for the number of OCL formed (*p < 0.05) for a typical experiment. Similar results were found in three independent experiments.
OCL formation and the bone histomorphometry of α9−/− mice
To assess the role of α9β1 in OCL differentiation, we analyzed OCL formation in marrow cultures in α9−/− mice, because α9 only forms a heterodimer with the β1 subunit, and β1 knockout mice are embryonic lethal. Mice homozygous for a null mutation in α9 die of chylothorax at 7–10 days after birth.(13) Therefore, nonadherent bone marrow cells from 7-day-old α9−/− or WT mice were cultured with RANKL (50 ng/ml) and M-CSF (10 ng/ml) for 7 days to induce OCL formation. The cultures were fixed and stained for TRACP. TRACP+ OCLs from α9−/− mice were smaller compared with WT (Fig. 5A). Approximately 10% of the OCLs present in the α9−/− cultures appeared normal in size. When bone marrow cells from α9−/− or WT mice were cultured on dentin slices for 7 days, WT OCLs formed normal numbers of resorption pits and with serpentine-like moving trails. In contrast, α9−/− OCLs formed dramatically fewer resorption pits (Fig. 5B). Furthermore, the total area resorbed on dentine slices was dramatically decreased when cultured with α9−/− OCLs (Fig. 5C). In addition, OCLs from α9−/− mice had decreased bone resorption efficiency per cell, as indicated in Fig. 5D. These data suggest that OCLs from α9−/− had an impaired bone resorption capacity and that this is not simply caused by decreased numbers of OCLs formed.
FIG. 5.
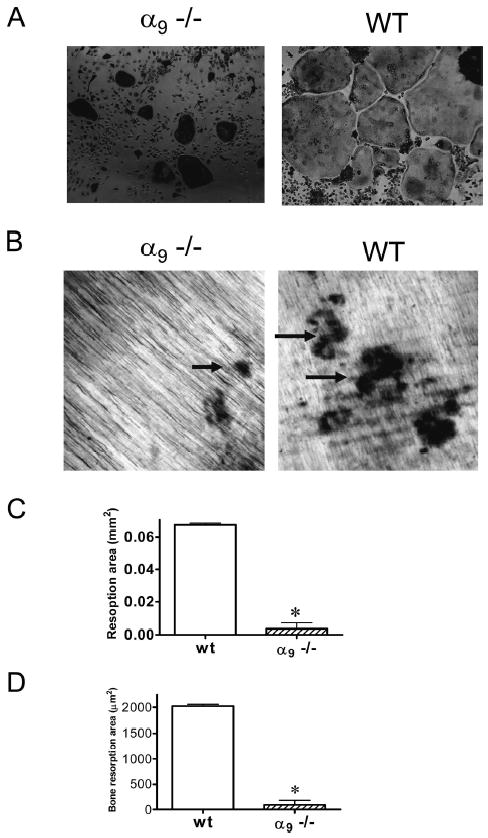
OCL formation and bone resorption in bone marrow cultures from α9−/− mice. Bone marrow cells from 7-day-old α 9−/− or WT mice were cultured with RANKL (50 ng/ml) and M-CSF (10 ng/ml) for 7 days. The cultures were fixed and stained for TRACP. (A) OCLs formed from α9−/− were smaller and more contracted than those in WT cultures. After 7 days, the cells were removed from dentin. The resorption pits formed on dentin were visualized by light microscopy after staining with hematoxylin. (B) α9−/− OCLs formed dramatically fewer resorption pits. In contrast, WT OCLs formed numerous and serpentine-like resorption pits. In addition, OCL resorption capacity was determined by measuring (C) total bone resorption areas and (D) the average resorption area per OCL. Magnification: ×10. Similar results were found in three independent experiments. Results from one typical experiment are shown as mean ± SE (*p < 0.05).
Actin ring formation is required for OCL bone resorption. Because α9−/− OCLs had an impaired bone resorption capacity, we examined actin ring formation in these cells with phalloidin staining. As shown in Fig. 6A, although α9−/− OCLs could form an actin-ring/podosome belt, they did not form well-defined lamellipodia in contrast to WT OCLs. In addition, some podosomes were loosely scattered around the dense podosome belt in α9−/− OCLs, as seen by the enlarged podosome belt at the right of the actin ring. To further evaluate the OCL cytoskeleton, we measured the osteoclast height (the distance between the basolateral membrane facing the bone marrow space and the ruffled border). Osteoclast height has been used as an index of osteoclast polarization.(18) α9−/− OCLs had a significantly decreased height compared with WT OCLs, suggesting α9−/− OCLs had impaired polarization (Fig. 6B).
FIG. 6.
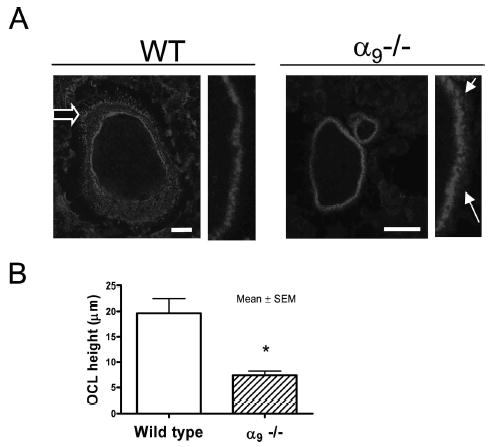
Cytoskeleton dysfunction and actin-ring deformation in α9−/− OCLs. (A) OCLs formed from α9−/− and WT marrow cultures were stained with Texas-red-phalloidin (magnification, ×10; scale: 25 μm). (A) Left: actin-ring formation in WT and α 9−/− OCL; fine structure of the podosome belt is shown at higher power. Open arrow shows the lamellipodium. Solid arrows show the loosely distributed podosomes at the edge of actin-ring. (B) Osteoclast height in WT and α9−/− OCLs. Similar results were found in three independent experiments. Results from one typical experiment are shown.
Histological analysis showed that vertebrae from 7-day-old α9−/− mice had moderately thickened trabecular regions, retained cartilage within vertebral bodies, an odd convex shape, and more abundant trabecular bone in the vertebrae compared with WT littermates (Fig. 7A). 3D reconstruction of the fifth lumbar vertebrae (Fig. 7B) showed denser bone and intervertebral discs in α9−/− mice compared with WT. There was no difference in OCL numbers or surface between WT and α9−/− vertebrae (data not shown). Furthermore, 3D μCT analysis of the fifth lumbar vertebrae revealed a modest but significant increase in trabecular bone volume/total tissue volume (WT = 16 ± 1.6%; α9−/− = 20 ± 4.1%; p =0.04; Fig. 7C), and a tendency of decreased trabecular separation (p =0.06) in α9−/− mice compared with WT mice.
FIG. 7.
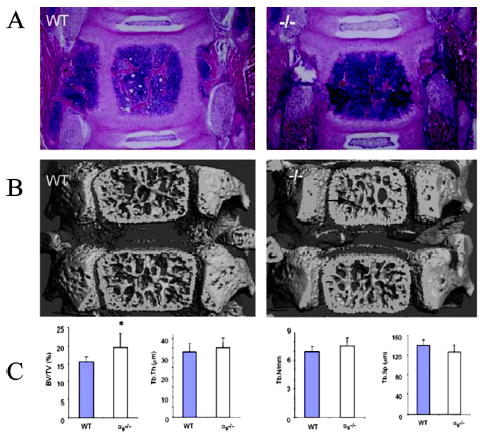
Histologic and μCT analysis of α9−/− mice vertebrae. (A) Coronal section of a fifth vertebrae stained with H&E staining outlined thickened trabeculae in α9−/− mice compared with WT (magnification, ×4). (B) μCT reconstructed fifth lumbar vertebrae images of 7-day-old WT mice. (C) Bone morphometric analyses of the fifth vertebrae from μCT indices of the fifth lumbar vertebrae bone showed a significant increase of the ratio of trabecular bone volume to total bone volume (BV/TV) in α9−/− mice compared with WT. BV/TV, bone volume/total volume; Tb.Th, trabecular bone thickness; Tb.N, trabecular bone number; Tb.Sp, trabecular bone separation. Mean ± SE for five mice/group are shown. *p < 0.05 by Student t-test.
DISCUSSION
Integrins are matrix receptors that transduce the signals bidirectionally inside-out and outside-in the cell.(19,20) They play a key role in OCL activity by mediating OCL adhesion and regulating cytoskeleton organization.(21,22) OCLs express very high levels of the αvβ3 integrin,(23) which regulates osteoclastic bone resorption.(11,24) OCLs also express lower levels of other type integrins, including α2β1, αvβ1, αvα5, and α5β1.(12,25) In this study, we showed that α9β1 integrin is expressed on OCL precursors and mature OCLs and that it interacts with ADAM8. This conclusion is based on our studies that showed that ADAM8 binds α9β1 and not αvβ3. ADAM8 lacks an RGD motif; therefore, it is not surprising that it does not bind αvβ3. ADAM family members that lack an RGD sequence can bind or α9β1 α4β1 because these integrins have high structural homology. However, we and others have been unable to detect α4β1 integrin on OCL precursors either by PCR or by Western blot analysis.(26)
Time-course studies suggested that ADAM8/α9β1 effects on OCL formation start at the early stage of OCL precursor differentiation. Murine and human OCL precursors expressed α9β1 integrin at a very early stage, and α9β1 integrin and ADAM8 expression increased with subsequent differentiation. The mRNA expression level of both and α9 ADAM8 was increased significantly over time. These data suggest that the effects of ADAM8 on OCL formation are mediated through interactions with α9β1.(3,27) These results are consistent with our previous time-course studies in which ADAM8 was added at various times during the culture period and was found to act at the later stages of OCL precursor differentiation.(6) Further support for a role for α9β1 in OCL formation are our experiments using an anti-body to α9, which showed that it significantly inhibited OCL formation induced by 1α,25(OH)2D3 or RANKL and M-CSF.
α9β1 did not seem to play a critical role in OCL formation because OCL numbers in vitro were only decreased by 30%, but rather its primary effects were on OCL function. OCLs formed in marrow cultures from α9−/− mice were small and contracted. Although α9−/− OCLs can form actin rings, they did not form lamellipodia, indicating impaired OCL mobility. In support of this conclusion, WT OCL formed a serpentine-like bone-resorption trail, whereas α9−/− OCLs formed only dot-like resorption pits. Furthermore, α9−/− OCLs had impaired polarization as indicated by the decreased osteoclast height. These defects resulted in a decreased bone resorption capacity for the α9−/− OCLs. This is despite the fact that α9−/− OCLs express normal amounts of αvβ3. In preliminary experiments, we found that activation of by ADAM8 induced phosphorylation of α9 paxillin in OCL precursors. These data are consistent with previous reports of the α9 signaling pathway in other cell types.(28) Multiple signaling pathways have been implicated in the induction of OCL formation. Because of cross-talk between these signaling pathways, it is likely that more than one signaling pathway is involved in α9 regulation of osteoclastogenesis.
Histologic analysis of bones from α9−/− mice further suggests that α9−/− OCLs are dysfunctional in vivo. Bones from 7-day-old α9−/− mice showed decreased resorption of cartilage and thickened trabeculae and had a significant increase in the BV/TV ratio on μCT analysis. However, trabecular number was not significantly reduced. This is not surprising because we could only examined mice at 7 days of age. The lack of a definitive bone phenotype at this very early age is similar to results published for mice lacking the β3 integrin, which do not develop osteosclerosis until ~6 months of age.(11) Yamamoto et al.(26) have also suggested a role for α9 in bone resorption and examined the molecular mechanisms of osteopontin (OPN) action in rheumatoid arthritis (RA), using collagen-induced arthritis (CIA) as an in vivo model. Levels of thrombin-cleaved OPN (OPN-T) are increased in synovial fluid and serum from patients with RA. OPN-T expresses a cryptic epitope, SLAYGLR, which is chemotactic for monocytes expressing α9 and α4 integrins. Monocytes from animals with CIA had increased expression of integrin mRNA. When these animals were α9 treated with an antibody to SLAYGLR, M5, there was less proliferation of the synovium and a marked decrease in bone erosion. These data are consistent with our findings that plays an important role in OCL function and α9β1 suggest that may contribute to the increased OCL α9β1 activity in inflammatory bone loss.
Acknowledgments
This study was support by NIH Grants RO1-AR41336, AG12951, and AR47700.
Footnotes
Dr Roodman received corporate appointments from Amgen, Merck & Co., Millennium, Novartis, and Scios, and he has received funding from Celgene. All other authors state that they have no conflicts of interest.
References
- 1.Roodman GD. Cell biology of the osteoclast. Exp Hematol. 1999;27:1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara N, Chenu C, Miller M, Civin C, Roodman GD. Identification of committed mononuclear precursors for osteoclast-like cells formed in long-term human marrow cultures. Endocrinology. 1990;126:2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- 3.Choi SJ, Devlin RD, Reddy SV, Chung H, Boyce BF, Roodman GD. Identification of human asparaginyl endopeptidase (legumain) as an autocrine inhibitor of osteoclast formation and bone resorption. J Biol Chem. 1999;274:27747–27753. doi: 10.1074/jbc.274.39.27747. [DOI] [PubMed] [Google Scholar]
- 4.Oba Y, Chung HY, Roodman GD, Choi SJ. Eosinophil chemotactic Factor-L (ECF-L): A novel osteoclast stimulating factor. J Bone Miner Res. 2003;18:1332–1341. doi: 10.1359/jbmr.2003.18.7.1332. [DOI] [PubMed] [Google Scholar]
- 5.Huovila APJ, Almeia EA, White JM. ADAMs and cell fusion. Curr Opin Cell Biol. 1996;8:692–699. doi: 10.1016/s0955-0674(96)80111-6. [DOI] [PubMed] [Google Scholar]
- 6.Choi SJ, Han JH, Roodman GD. ADAM8: A novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–822. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 7.Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, novel family of membrane proteins containing a disintegrin and metalloprotease domain: Multipotential functions in cell-cell and cell-matrix interaction. J Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu E, Croucher PI, McKie N. Expression of members of the novel membrane linked metalloproteinase family ADAM in cells derived from a range of hematological malignancies. Biochem Biophys Res Commun. 1997;235:437–442. doi: 10.1006/bbrc.1997.6714. [DOI] [PubMed] [Google Scholar]
- 9.Bohm BB, Aigner T, Gehrsitz A, Blobel CP, Kalden JR, Burkhardt H. Upregulation of MDC15 (metargidin) messenger RNA in human osteoarthritic cartilage. Arthritis Rheum. 1999;42:1946–1950. doi: 10.1002/1529-0131(199909)42:9<1946::AID-ANR21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD 156 (human ADAM8): Expression, primary amino acid sequence, and gene location. Genomics. 1997;41:56–62. doi: 10.1006/geno.1997.4607. [DOI] [PubMed] [Google Scholar]
- 11.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 13.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- 15.Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–614. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menaa C, Kurihara N, Roodman GD. CFU-GM-derived cells form osteoclasts at a very high efficiency. Biochem Biophys Res Commun. 2000;267:943–946. doi: 10.1006/bbrc.1999.2042. [DOI] [PubMed] [Google Scholar]
- 17.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 18.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicx VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: Versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 21.Faccio R, Grano M, Colucci S, Zallone AZ, Quaranta V, Pelletier AJ. Activation of alphav beta3 integrin on human osteoclast-like cells stimulates adhesion and migration in response to osteopontin. Biochem Biophys Res Commun. 1998;249:522–525. doi: 10.1006/bbrc.1998.9180. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, Novack DV, Faccio R, Ory DS, Aya K, Boyer MI, McHugh KP, Ross FP, Teitelbaum SL. A Glanzmann’s mutation in beta 3 integrin specifically impairs osteoclast function. J Clin Invest. 2001;107:1137–1144. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 24.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19:97–105. doi: 10.1016/s0945-053x(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, Okazaki I, Seki N, Fujii T, Uede T. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112:181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biskobing DM, Fan X, Rubin J. Characterization of MCSF-induced proliferation and subsequent osteoclast formation in murine marrow culture. J Bone Miner Res. 1995;10:1025–1032. doi: 10.1002/jbmr.5650100706. [DOI] [PubMed] [Google Scholar]
- 28.Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, Sheppard D. The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]


