Abstract
Glutathione is an abundant and ubiquitous low-molecular-weight thiol that may play a role in many cellular processes, including protection against the deleterious effects of reactive oxygen species. We address here the role of glutathione in protection against hydrogen peroxide (H2O2) in Haemophilus influenzae and show that glutathione and catalase provide overlapping defense systems. H. influenzae is naturally glutathione deficient and imports glutathione from the growth medium. Mutant H. influenzae lacking catalase and cultured in glutathione-deficient minimal medium is completely devoid of H2O2 scavenging activity and, accordingly, substantial amounts of H2O2 accumulate in the growth medium. H. influenzae generates H2O2 at rates similar to those reported for Escherichia coli, but the toxicity of this harmful metabolite is averted by glutathione-based H2O2 removal, which appears to be the primary system for protection against H2O2 endogenously generated during aerobic respiration. When H2O2 concentrations exceed low micromolar levels, the hktE gene-encoded catalase becomes the predominant scavenger. The requirement for glutathione in protection against oxidative stress is analogous to that in higher and lower eukaryotes but is unlike the situation in other bacteria in which glutathione is dispensable for aerobic growth during both normal and oxidative stress conditions.
Cellular metabolism of molecular oxygen produces reactive and potentially toxic oxygen species such as superoxide radicals, hydrogen peroxide (H2O2), and hydroxyl radicals (5). Escherichia coli generates ca. 14 μM H2O2 per s when it grows exponentially in air in minimal glucose medium (36). Recently, Seaver and Imlay showed that E. coli mutants completely defective in H2O2 scavenging activity grow poorly in air and that growth often stopped entirely when these mutant cells were repeatedly subcultured (36). This implies that aerobically grown E. coli generates enough H2O2 to poison scavengerless cells. Indeed, H2O2 itself can potentially damage enzymes by oxidizing sulfhydryl and iron-sulfur moieties and, upon conversion to a hydroxyl radical, it produces mutagenic and lethal lesions (4, 21).
E. coli mainly expresses two systems to destroy H2O2. The first system is embodied by two types of catalase enzymes, a bifunctional catalase/peroxidase (HPI), encoded by katG (39), and a monofunctional catalase (HPII), encoded by katE (45), which are both heme-containing enzymes involved in the dismutation of H2O2 into O2 and H2O. Clear evidence shows that the two catalase genes are regulated differently in terms of growth phase and responsiveness to oxidative stress (reviewed in reference 22). HPI is transcriptionally induced during logarithmic growth in response to low micromolar concentrations of H2O2. This induction requires the positive transcriptional activator OxyR, which directly senses oxidative stress. On the other hand, HPII is not peroxide inducible and its gene, katE, is transcribed at the transition from the exponential growth phase to the stationary growth phase by RNA polymerase containing the alternative sigma subunit σs, the product of the rpoS (or katF) gene.
Next to these catalases, E. coli expresses a two-enzyme H2O2-scavenging system, the alkyl hydroperoxide reductase (AhpR) system, which was initially characterized as rapidly reducing diverse organic hydroperoxides such as cumene- and t-butylhydroperoxide (18). Later, Niimura et al. (26) showed that the specific activities of the Salmonella enterica serovar Typhimurium AhpR homolog for the reduction of cumene hydroperoxide and H2O2 are within the same order of magnitude. One component of the AhpR system, AhpF, is a flavoprotein that shuttles reducing equivalents from NAD(P)H (with a strong preference for NADH) to AhpC, the actual peroxidase and a member of the peroxiredoxin family of thiol peroxidases. The ahpC and ahpF genes are organized in a two-gene operon, and transcription is OxyR controlled (38).
In E. coli, AhpR and HPI have discrete roles in scavenging H2O2. AhpR is the primary scavenger of endogenous H2O2. HPI contributes little when H2O2 concentrations are low, but it becomes the more effective scavenger when H2O2 concentrations are high or, presumably, when the absence of a carbon source depletes the cell of the NADH necessary for AhpR activity (36).
Analogously, catalases are believed to be indispensable in eukaryotic cells to protect compartments subjected to high H2O2 concentrations, e.g., the peroxisomal matrix and membrane (6), whereas the H2O2 resulting from the cellular metabolism of molecular oxygen is primarily scavenged by thiol-dependent peroxidases (13, 29). In animals, these peroxidases are fueled by either the glutathione or the thioredoxin redox cycle. The tripeptide-reduced glutathione (GSH; γ-l-glutamyl-l-cysteinyl glycine) is an abundant and ubiquitous low-molecular-weight thiol with a presumed role in many cellular processes, ranging from oxidative stress defense to cysteine storage (reviewed in reference 31). During these processes, GSH is often converted into its symmetrical disulfide form, oxidized glutathione (GSSG), and regeneration occurs via the NADPH-dependent action of glutathione reductase. Glutathione deficiency in higher eukaryotes is at the basis of several diseases caused by the degradation of mitochondria (8, 19), an observation which allowed us to conclude that H2O2 generated through respiration is scavenged inside the mitochondria by GSH peroxidase (GPx). Furthermore, the finding that glutathione-deficient yeast strains are hypersensitive to H2O2 points to the importance of glutathione-based peroxide removal in eukaryotes (10, 17).
Haemophilus influenzae is a strictly human commensal organism. As unencapsulated species, the bacterium is found in the upper respiratory tracts of up to 80% of healthy adults and, as encapsulated species, in 3 to 5% of normal individuals (25). Encapsulated strains are capable of invasive infections, including meningitis, pneumonia, and epiglottitis.
An acatalasemic H. influenzae Rd mutant was constructed by transposon-mediated inactivation of one structural gene, hktE. Consequently, hktE accounts for total catalase activity in H. influenzae cells (3). The hktE gene product, HktE, is strongly homologous to E. coli KatE. However, the H. influenzae catalase is downregulated in the stationary growth phase and is upregulated by exposure to H2O2. As such, hktE regulation is similar to that of katG (3). Consistent with findings in other bacteria, disruption of catalase production in H. influenzae causes hypersensitivity to high H2O2 concentrations (2). On the other hand, deletion of the hktE gene produces only a modest reduction in the ability to cause lethal sepsis after parenteral challenge and causes no change in the ability to colonize after intranasal inoculation in the infant rat model of infection (2). Consequently, the H. influenzae hktE gene is not of major importance for the process of colonization and invasive infection.
Because H. influenzae catalase mutants do not show any growth disadvantage either in vitro in rich medium or in vivo after intranasal inoculation in infant rats, it is anticipated that at least one other H2O2-scavenging system supports peroxide removal in H. influenzae. AhpR is not involved because neither an ahpC nor an ahpF homolog is apparent from the H. influenzae Rd genome sequence (7).
Recently, we reported that H. influenzae is naturally deficient in glutathione biosynthesis (42) and showed that H. influenzae instead imports glutathione from the growth medium. In this way, extracellular glutathione (supplemented as GSSG to prevent chemical reactions with the oxidizing test chemicals) protects cultures against methylglyoxal, S-nitrosoglutathione, and t-butylhydroperoxide toxicity. Interestingly, an open reading frame (HI0572) was isolated that encodes glutathione-based t-butylhydroperoxide removal. On the other hand, the presence of GSSG in the growth medium of H. influenzae NCTC 8143 cultures does not influence the sensitivity to exogenous H2O2 stress (42). We show here, however, that glutathione is a crucial component in the metabolism of H2O2 endogenously generated during aerobic growth.
MATERIALS AND METHODS
Media.
Mueller-Hinton broth (MH broth) was prepared from a dehydrate (Fluka at Sigma-Aldrich, St. Louis, Mo.) and autoclaved. MH medium broth was MH broth supplemented with Haemophilus test medium supplement (Oxoid, Hampshire, United Kingdom), containing V-factor (NAD) and X-factor (hemin), according to the manufacturers' instructions.
H. influenzae specific minimal medium (MIc medium) was prepared essentially as described by Herriott et al. (15). l-Cystine (Sigma-Aldrich) was added to a final concentration of 50 μM, and Haemophilus test medium supplement was added according to the manufacturers' instructions. GSSG-supplemented MIc medium [MIc(GSSG)] was prepared by replacing l-cystine with GSSG (Sigma-Aldrich) at the same final concentration. Both l-cystine and GSSG were added from a sterile concentrated stock solution. For anaerobic growth, the recipe for the preparation of MIc minimal medium was adjusted as follows: (i) sodium lactate was replaced by disodium fumarate (final concentration, 5 mM), and (ii) the final glycerol concentration was raised from 0.3 to 0.5%. This medium is referred to as anMIc or anMIc(GSSG) medium throughout the text, depending on whether l-cystine or GSSG was added, respectively; 1.8% agar was added to prepare anMIc and anMIc(GSSG) agar plates.
Bacterial strains and growth conditions.
Wild-type strain H. influenzae Rd was purchased from the American Type Culture Collection (Manassas, Va.). The construction of the H. influenzae catalase-negative strain AB2593 (Rd hktE::mini-Tn10Cm) was reported previously (2). Strain AB2593 was kindly provided by William R. Bishai (Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine).
Cultures were routinely grown at 37°C under anaerobic conditions in anMIc medium. Anaerobic cultures were prepared in a Coy chamber (Coy Laboratory Products, Inc.) under an atmosphere of 85% N2-10% H2-5% CO2 in culture tubes that fit tightly into the cuvette holder of a Shimadzu 1240 Mini Single-Beam UV-VIS spectrophotometer (Shimadzu, Duisburg, Germany). The system enables cell density measurements without the need to transfer culture samples into regular cuvettes. The tubes were closed with a silicone stopper and were shaken outside the Coy chamber. As such, anaerobiosis was preserved, a finding confirmed in preliminary experiments by adding 0.002% resazurine to the growth medium (resazurine is a redox-sensitive dye added to media as a simple, qualitative indicator of the redox conditions of the media).
Tn10-linked mutations in hktE were confirmed by assaying catalase activity in whole cells as described earlier (12).
For aerobic shift experiments, precultures grown anaerobically overnight were diluted 1:100 in anMIc or anMIc(GSSG) medium to an optical density at 600 nm (OD600) of ∼0.005 and then grown anaerobically to an OD600 of ∼0.15. The silicone stopper was then removed in order to shift the cells into air. In the case of aerobic-shift experiments to monitor growth in the presence of nonenzyme H2O2 scavenging, pyruvate was added to the anMIc or anMIc(GSSG) cultures from a buffered sterile stock solution to a final concentration of 0.75%. In the case of aerobic-shift experiments to evaluate catalase activity in response to aerobiosis, cell extracts were prepared, and catalase activity was measured, as described previously (42), 20 min after the removal of the silicone stopper. These catalase activities were then compared to those derived from continuously anaerobically grown counterparts.
Heat-killed bacteria were prepared by incubating suspensions at 60°C for 1 h.
Disk diffusion.
Overnight anaerobically grown precultures were diluted 1:100 times in anMIc or anMIc(GSSG) medium to an OD600 of ∼0.005 and then grown anaerobically to an OD600 of 0.5 (late exponential phase). The following manipulations were performed inside the Coy chamber. Using a sterile cotton swab, cells were inoculated onto the entire surface of anMIc or anMIc(GSSG) plates. Round sterile filters (5.2-mm diameter) were placed in the center of the plates and spotted with either 5 μl of 3% H2O2 or 5 μl of 500 mM t-butyl hydroperoxide. The plates were placed in an anaerobic jar and incubated for 2 days at 37°C. The diameter of the zone of complete inhibition was recorded in millimeters. The experiment was performed in triplicate; mean values are reported.
H2O2 detection.
The Amplex red hydrogen peroxide/peroxidase assay kit (Molecular Probes, Eugene, Oreg.) was used to detect H2O2 according to the manufacturer's instructions. This assay can be performed either fluorometrically or spectrophotometrically. We chose the latter approach because the extinction coefficient of the Amplex red reagent oxidation product (resorufin) is high (54,000 cm−1 M−1), resulting in a detection limit of ca. 0.2 μM. The latter value is low enough to complete the experiments described here. Absorbance was measured at 563 nm by using a Shimadzu 1240 Mini Single-Beam UV-VIS spectrophotometer. The assay procedure was performed in a total volume of 400 μl. For each assay, no-H2O2 controls were monitored.
H2O2 scavenging by whole cells.
Overnight anaerobically grown precultures were diluted 1:100 times in anMIc or anMIc(GSSG) medium to an OD600 of ∼0.005 and grown anaerobically to an OD600 of 0.15. Cells were pelleted in a microcentrifuge, washed twice, and resuspended in room temperature phosphate-buffered saline (PBS) at an OD600 of 0.15. H2O2 was added to the appropriate final concentration (see the figure legends). At intervals, 200-μl samples were removed, and the reactions were terminated (i.e., the cells were removed) by filtering the reaction mixtures with sterile Millex-GV13 0.22-μm-pore-size filter units (Millipore Products Division, Bedford, Mass.). H2O2 was then assayed by the Amplex red method.
Measurement of H2O2 accumulation in cell cultures.
Cultures were grown as described for the aerobic-shift experiments. Immediately before and at intervals after the shift to air, the OD600s were measured, and 200-μl samples were removed. Cells were immediately removed from the samples by filtration with sterile Millex-GV13 0.22-μm-pore-size filter units. The cell-free culture media were then assayed for H2O2. H2O2 levels were also determined in sterile medium that was incubated at 37°C for an equivalent time period. These background levels, however, did not exceed the detection limit of ca. 0.2 μM. The rate of H2O2 production was normalized to the cytoplasmic volume of the suspended cells by using a standard ratio of 0.94-μl internal volume per 1 ml of H. influenzae at 1 OD600. The latter value is derived from the assumption that H. influenzae cells can be modeled as approximate cylinders with a length of 1.2 μm and a radius of 0.25 μm; as such, the volume per cell is 0.236 × 10−15 liters. The number of cells present in 1 ml of a 1-OD600 anMIc culture was determined by dilution plating to be 4.0 × 109.
RESULTS
H. influenzae Rd catalase null mutant strain AB2593 exhibits a severe aerobic growth defect in minimal medium.
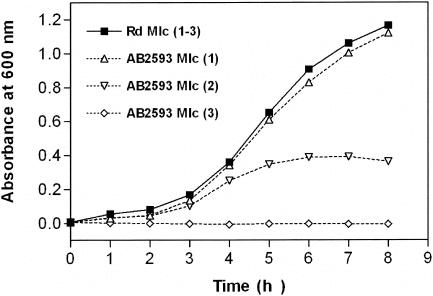
The H. influenzae strains examined here, the HktE-negative strain AB2593, and the wild-type isogenic parent strain Rd are identical to those studied by Bishai et al. (2), who used them to characterize catalase mutants of H. influenzae in terms of colonization and invasion abilities. This primary report illustrates that these strains share identical growth characteristics when grown aerobically in rich medium. We found that the growth curve of strain AB2593, grown in rich medium (MHs), is indeed similar to the growth curve obtained with wild-type cultures. No variation was observed when AB2593 cells were repeatedly subcultured in MHs medium. Also, no aberrations were detected for aerobic growth in minimal medium (MIc) inoculated with a stationary-phase MHs medium culture. However, these latter MIc cultures appeared to have lost one or more protective factors for surviving aerobic conditions. Indeed, second-generation AB2593 MIc subcultures showed severe growth defects when grown aerobically (Fig. 1), whereas growth in the absence of oxygen proceeded at wild-type levels (data not shown). Growth even stopped entirely in third-generation AB2593 MIc subcultures (Fig. 1).
FIG. 1.
Aerobic growth deficiency of acatalasaemic H. influenzae in minimal medium. Overnight precultures of wild-type H. influenzae Rd and mutant AB2593 (hktE) grown in rich medium (MHs) were diluted 1:100-fold in MIc medium [Rd (1), ▪; AB2593 (1), ▵], and growth was monitored at 600 nm. After 8 h of growth, these cultures were subcultured (1:100) in MIc broth, and growth was recorded [Rd (2), ▪; AB2593 (2), ▿). Again, 8 h later, growth was monitored of the third-generation subcultures in MIc broth [Rd (3), ▪; AB2593 (3), ⋄].
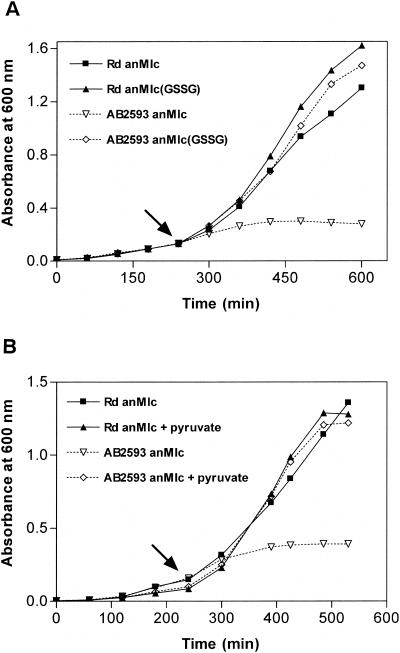
A clear representation of the aerobic growth defect of catalase-negative AB2593 cells could be obtained by shifting cultures, after repeated anaerobic subculturing in anMIc medium, from anaerobic to aerobic conditions (Fig. 2). Whereas Rd cultures immediately responded to the shift by the expected growth acceleration due to the initiation of aerobic respiration, AB2593 cultures responded by no more than a slight increase of the doubling time as a step toward complete growth arrest and death.
FIG. 2.
Both exogenous pyruvate and GSSG conceal the aerobic growth defect in minimal medium of H. influenzae AB2593. The assay was done by aerobic shift growth measurements. Overnight precultures of wild-type H. influenzae Rd and mutant AB2593 (hktE) anaerobically grown in anMIc broth were diluted 1:100-fold in anMIc medium (Rd, ▪; AB2593, ▿) and anMIc(GSSG) broth (Rd, ▴; AB2593, ⋄) (A) or in anMIc medium (Rd, ▪; AB2593, ▿) and in anMIc medium supplemented with 0.75% pyruvate (Rd, ▴; AB2593, ⋄) (B). Anaerobic growth was monitored. At an OD600 of ∼0.15, the cultures were shifted to air (indicated by an arrow), and aerobic growth was recorded.
As mentioned before, catalases are ubiquitous among aerobic organisms, where they serve to detoxify the H2O2 that arises from exposure to oxygen. So, we first studied whether it is this H2O2 that poisons AB2593 grown aerobically in minimal medium. Therefore, we repeated the aerobic-shift experiments in the presence of sodium pyruvate, a nonenzyme scavenger of peroxides (41). We observed that catalase-negative H. influenzae cells grown in pyruvate-supplemented anMIc medium doubled at wild-type rates after the aerobic shift (Fig. 2). A question arises as to why endogenously generated H2O2 kills catalase-negative H. influenzae cells in MIc medium but fails to do so in rich MHs medium. Note that we here refer to endogenously generated H2O2. We indeed tested anMIc medium and anMIc(GSSG) medium for the nonenzymatic production of H2O2 and found that concentrations were below the detection limit (ca. 0.2 μM). For comparison, Luria-Bertani medium chemically generates ca. 1.2 μM H2O2 per min (14).
Exogenous glutathione conceals the aerobic growth defect in minimal medium of H. influenzae AB2593.
The fact that even fairly dense cultures of AB2593 died as a result of an aerobic shift (Fig. 2) rules out the possibility that a dilution effect causes growth inhibition in minimal medium. More likely, the aerobic growth defect is the result of the different composition of the growth media. One particular component that is not present in (an)MIc medium compared to rich medium is glutathione. When supplied to the growth medium in oxidized form, this thiol tripeptide was recently shown to strengthen the oxidative defense of naturally glutathione-deficient H. influenzae (42). We therefore repeated the aerobic-shift experiments in the presence of GSSG (Fig. 2) and observed that AB2593 cultures grown in glutathione-supplemented minimal medium are completely relieved from defective growth under aerobic conditions.
Effects of intracellular glutathione-based peroxide reduction on the metabolism of H2O2 produced during aerobic respiration.
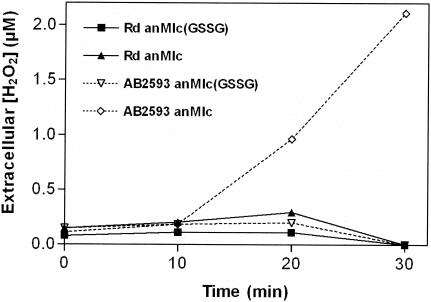
In order to elucidate whether glutathione-deficient AB2593 cells indeed exhibit problems in detoxifying the H2O2 that is produced during aerobic respiration, we repeated our aerobic-shift experiment and monitored the H2O2 concentrations in the medium (Fig. 3). For all conditions tested, we measured higher H2O2 concentrations immediately after the aerobic shift. This most likely represents the adaptation time required to build up adequate antioxidant activity. Table 1 illustrates that the shift to oxidative metabolism caused a substantial 6.6-fold increase in catalase activity in glutathione-deficient H. influenzae Rd cells, indeed suggesting that, in response to the shift to air, H2O2 is generated at a rate greater than what could be metabolized to a steady-state level (i.e., <0.2 μM) by the antioxidant machinery present in anaerobically growing cells. This hypothesis, however, appears not to be valid for glutathione-supplemented cells. Note that the experiment illustrated in Table 1 only focuses on catalase activity, which, as described below, appears to be less efficient compared to glutathione-based peroxide removal. Thus, in response to the aerobic shift, induction of the latter system may be sufficient to alleviate the toxicity of respiratory-generated H2O2 to levels too low to induce the synthesis of catalase.
FIG. 3.
H2O2 production by glutathione- and catalase-deficient H. influenzae. Wild-type H. influenzae Rd and mutant AB2593 (hktE) were grown anaerobically in anMIc(GSSG) medium (Rd, ▪; AB2593, ▿) or anMIc medium (Rd, ▴; AB2593, ⋄) to an OD600 of 0.15. The cultures were then shifted to air, and the H2O2 concentration of the medium was measured at various time points after the shift.
TABLE 1.
Effect of shift to oxidative metabolism on catalase activity monitored in H. influenzae Rd extracts derived from cultures grown in the presence or absence of GSSGa
| Growth medium (growth condition) | Mean catalase (HktE)b activity (μmol of H2O2 decomposed/min/mg of protein) ± SEM |
|---|---|
| anMIc(GSSG) (anaerobiosis) | 34.0 ± 2.3 |
| anMIc(GSSG) (aerobiosis) | 33.6 ± 4.5 |
| anMIc (anaerobiosis) | 34.4 ± 1.2 |
| anMIc (aerobiosis) | 228 ± 22 |
See Materials and Methods for experimental details.
Results are expressed as the mean values from three independent experiments.
Twenty minutes after the shift to air, the growth of glutathione-supplemented AB2593 cultures continued, metabolizing H2O2 to concentrations below the detection limit. On the other hand, glutathione-deficient catalase-negative cells clearly experienced problems in controlling H2O2 production, as noticed by the steady H2O2 accumulation in the growth medium.
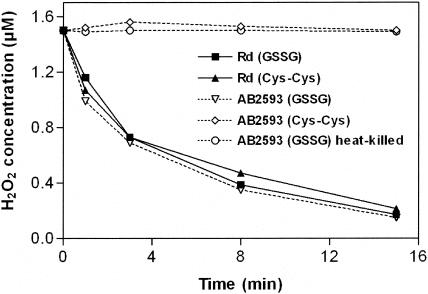
Once we had determined that both glutathione-based H2O2 removal and catalase activity are important during aerobic growth, we wondered whether these two systems represent the entire H2O2-scavenging activity of H. influenzae. Therefore, we set up a time-response experiment exemplifying the in vivo H2O2-scavenging activities of whole metabolically active cells (Fig. 4). We observed that, whereas glutathione-supplemented AB2593 scavenged 1.5 μM H2O2 as well as did its wild-type parent, the glutathione-deficient hktE mutant exhibited virtually no H2O2-scavenging activity. Also, heat-killed glutathione-supplemented AB2593 cells failed to remove H2O2 from solution. Thus, H. influenzae relies on two systems for the destruction of H2O2 generated through respiration: a glutathione-based system that requires metabolically active cells on the one hand and catalase on the other.
FIG. 4.
Glutathione-supplemented acatalasaemic H. influenzae and wild-type H. influenzae Rd remove low micromolar concentrations of extracellular H2O2 at similar rates. Cultures of Rd and AB2593 were grown anaerobically in anMIc(GSSG) medium (Rd, ▪; AB2593, ▿) and in anMIc medium (Rd, ▴; AB2593, ⋄), and resuspended in PBS at an OD600 of 0.075. Heat-killed bacteria (○) were prepared by incubating the PBS suspension at 60°C for 1 h. H2O2 was added to a final concentration of 1.5 μM. The H2O2 concentration was measured at various time points after addition of H2O2 as described in Materials and Methods.
HktE and glutathione-based peroxidatic activities have discrete roles in scavenging H2O2.
Since we had now shown that glutathione-based scavenging activity alone is sufficient to scavenge H2O2 derived from the own metabolism, we sought to determine to what extent this type of peroxidatic activity is beneficial for H. influenzae at high, supranormal levels of H2O2. Therefore, we set up disk diffusion assays, which test the ability of strains to degrade high concentrations of H2O2 to a level that permits growth (Table 2). The glutathione-deficient wild type appeared to be somewhat more sensitive than its glutathione-supplemented counterpart. On the other hand, the glutathione-supplemented hktE mutant turned out to be hypersensitive, indicating that HktE represents the more effective scavenging system at supranormal H2O2 concentrations in H. influenzae.
TABLE 2.
Effect of glutathione deficiency on sensitivity to exogenous supranormal H2O2 concentrations in wild-type and acatalasaemic H. influenzaea
| Strain (medium) | Zone of inhibition (mm) |
|---|---|
| Rd (MIc) | 26 |
| Rd [MIc(GSSG)] | 21 |
| AB2593 (MIc) | 46 |
| AB2593 [MIc(GSSG)] | 33 |
Each assay was done by disk diffusion of 5 μl of 3% H2O2. See Materials and Methods for experimental details.
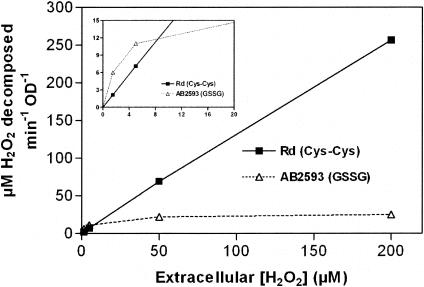
To verify this result in terms of kinetic behavior of the HktE and glutathione-based peroxidatic systems, we set up a concentration-response experiment exemplifying the rate of H2O2 decomposition achieved by a steady number of metabolically active whole cells (Fig. 5). The peroxidatic activity inside glutathione-supplemented AB2593 cells became saturated when extracellular H2O2 concentrations exceeded 25 μM. In contrast, HktE-expressing cells (glutathione-deficient wild type) were not saturated by the highest H2O2 concentrations tested, a finding consistent with the millimolar Km values reported for other catalases (1, 20, 33). Furthermore, the H2O2 decomposition rates of whole glutathione-deficient wild-type cells at high extracellular H2O2 concentrations were much greater than those for whole cells that only relied on the glutathione-based peroxidatic system. On the other hand, at extracellular H2O2 concentrations of up to 5 μM, glutathione-based scavenging was significantly more efficient than that propelled by catalase.
FIG. 5.
Dependence of scavenging rate on extracellular H2O2 concentration. Rates of H2O2 decomposition were measured in dilute suspensions (0.075 OD600) of H. influenzae Rd grown in anMIc medium (▪) and of AB2593 (hktE) grown in anMIc(GSSG) medium (▵). Rates were normalized to a value of 1.0 OD600.
Calculation of endogenous H2O2 production during aerobic growth.
As shown in Fig. 3, substantial H2O2 accumulated in the medium of glutathione-deficient AB2593 cultures. These cultures were shown to be completely deficient in H2O2 scavenging at low micromolar concentrations, so that virtually all of the H2O2 that enters or is formed within these cells diffuses out without being scavenged (36, 37). For this reason, the measurement of excreted H2O2 is a valid proxy for measurement of endogenous H2O2 formation. Between 20 and 30 min after the shift to air, glutathione-deficient AB2593 cultures at an OD600 of 0.15 accumulated 1.05 μM H2O2 (mean of three independent experiments). Using the relation that 1 ml of bacteria at an OD600 of 1.0 contains 0.94 μl of cytoplasmic volume (see Materials and Methods), we calculated that aerobically grown log-phase H. influenzae forms H2O2 at a rate of ∼12.4 μM per s.
DISCUSSION
In 1983, Meister and Anderson (23) proposed that glutathione protects mammalian cells from oxidative damage. Two decades and several studies later, it has become definite that glutathione is indispensable for eukaryotic cells to deal with reactive oxygen species generated by aerobic respiration. For the most part, this results from the fact that GSH functions as a cofactor for peroxidatic H2O2 removal (16, 35). In contrast, Greenberg and Demple (11) reported that a glutathione-deficient mutant of E. coli K-12 has normal resistance to H2O2 and cumene hydroperoxide. Thus, even though intracellular GSH can efficiently scavenge free radicals nonenzymatically and can reach millimolar levels, it is not essential for protecting E. coli from oxidative damage. Moreover, no growth defects were reported for mutant acatalasaemic E. coli genetically inactivated for glutathione biosynthesis (32, 44).
We demonstrated here that the acatalasaemic H. influenzae mutant strain AB2593 requires glutathione in its growth medium in order to survive endogenous H2O2 stress associated with aerobic growth. In a previous study (42), we showed that H. influenzae, although it possesses glutathione metabolizing enzymes, is naturally glutathione deficient and that glutathione is acquired by importing the thiol tripeptide (either as GSH or as reducible symmetrical and asymmetrical [mixed] disulfide forms of GSH) from the growth medium. Therefore, since H. influenzae AB2593 is deficient in catalase, the restoration of wild-type growth rates in glutathione-supplemented (an)MIc medium could be the result of pure glutathione-based nonenzymatic H2O2 scavenging inside the cell (28). However, the fact that glutathione-supplemented AB2593 scavenges H2O2 at wild-type rates (Fig. 4) and the fact that the glutathione-based H2O2 removal attains a maximum initial velocity with increasing H2O2 concentrations (Fig. 5) indicate that intracellular GSH donates electrons to a powerful peroxidase.
Glutathione-dependent peroxidatic H2O2 removal requires a continuous flow of GSH. Glutathione reductase is the central enzyme in glutathione redox cycling, and this reductase exclusively exploits NADPH as a reductant. Heat-killed glutathione-supplemented AB2593 is not able to generate reducing power. This probably explains our observation that these cells were totally ineffective in H2O2 scavenging (Fig. 4). Consequently, the rate of glutathione-based H2O2 scavenging most likely depends on the rate at which metabolism generates NADPH. This may well explain why exogenous H2O2 stress results in a twofold increase in glucose-6-phosphate dehydrogenase (G6PD) activity (42), whereas G6PD levels remain unaltered in response to peroxides in E. coli. Indeed, G6PD is the first and rate-limiting enzyme of the pentose phosphate pathway, and for both lower and higher eukaryotes G6PD expression is regulated in order to maintain NADPH levels for the glutathione-based enzymatic pathway of peroxide removal (40). In E. coli, NADH is the preferred reductant for AhpR-dependent peroxidatic activity (26) and, it therefore appears that no G6PD induction is required in response to H2O2 stress.
Recently, we reported that the H. influenzae genome encloses a gene (ORF HI0572) encoding a novel GSH-dependent t-butylhydroperoxide reductase (Prx/Grx) (42). Naturally glutathione-peroxidase-deficient E. coli expresses a vast amount of GSH-dependent t-butylhydroperoxide reductase activity when transformed with a multicopy plasmid carrying the HI0572 locus. The Prx/Grx protein shares 63% sequence identity with the garA gene product of Chromatium gracile (30). Because the C. gracile peroxidase reduces both t-butylhydroperoxide and H2O2 at high rates (43), we anticipated that Prx/Grx might be the major peroxidase involved in H2O2 metabolism of H. influenzae. To examine this premise, we are currently generating both an H. influenzae HI0572 mutant and an H. influenzae HI0572 hktE double mutant. In the meantime, we recently obtained supportive biochemical evidence through the characterization of the H. influenzae Rd Prx/Grx peroxidase. We observed that H2O2 binds very efficiently to the GSH-dependent peroxidase, as can be demonstrated by deducing the Km value from an empirical fit to the data given by the concentration-response curve shown in Fig. 5; note that the abscissa displays extracellular H2O2 concentrations and, therefore, the deduced Km value cannot be interpreted as real Michaelis constant. The extracellular concentration at half-maximal rate was ca. 5.8 μM, which is very similar to the Michaelis constant (2.29 μM) obtained via in vitro kinetics of the Prx/Grx peroxidase (30).
As observed for E. coli (36), our results strongly suggest that aerobically grown log-phase H. influenzae scavenges the majority of endogenous H2O2 through one catalase and one peroxidase. Indeed, as reported for catalase and AhpR-deleted E. coli (36), glutathione-deficient H. influenzae lacking catalase is almost completely devoid of H2O2-scavenging activity (Fig. 4). Notably, in contrast to the fact that H2O2 scavengerless E. coli grows, albeit poorly, in rich and minimal media in air (36), we observed that glutathione-deficient AB2593 is completely unable to grow aerobically. So, the continual accumulation of H2O2 inside scavengerless aerobically growing cells appears to be more damaging to H. influenzae than to E. coli. In growing cells, the steady-state concentration of H2O2 depends on the rates of its formation and of its dissipation. Given that H2O2 removal is essentially absent in both strains, the growth difference could be the result of differences in H2O2 formation rates. We observed rates (∼12.4 μM per s) that are 1 order of magnitude higher than those reported by Gonzales-Flecha and Demple (ca. 1 to 2 μM per s) for E. coli (9). However, Seaver and Imlay (36) reported rates of H2O2 production in exponentially growing E. coli (∼14 μM) similar to those reported here for H. influenzae. This inconsistency is the result of differences in experimental procedure to measure H2O2 formation rates (36). Since our procedure is very similar to the one used by Seaver and Imlay (i.e., actual H2O2 formation rates are measured by using H2O2 scavengerless log-phase cultures grown at 37°C), we suggest that H. influenzae and E. coli cells generate equal amounts of H2O2 per s. Thus, the growth difference between H2O2 scavengerless H. influenzae and E. coli cannot be explained in terms of differences in H2O2 formation rates. Since GSH is a potent nonenzyme scavenger of reactive oxygen species and since it also functions as a cofactor for enzymes involved in oxidative stress defense, such as glutaredoxins and glutathione S-transferases, deprivation of intracellular GSH not only blocks GSH-dependent H2O2 removal but also prevents all other processes that require GSH in the H. influenzae cytoplasm. As such, glutathione-deficient AB2593 is subjected to additional, e.g., thiol-disulfide, stress (32) compared to H2O2 scavengerless E. coli, which explains why glutathione-deficient AB2593 is not viable under aerobic conditions.
For E. coli, it was proposed that AhpR and HPI have discrete roles in scavenging H2O2. AhpR scavenges low levels of H2O2, and HPI scavenges high levels of H2O2 (36). We obtained analogous results showing that glutathione-based peroxidatic activity is more effective at scavenging very low concentrations of H2O2, whereas HktE is the more effective enzyme at higher concentrations. Omitting GSSG from the media of H. influenzae NCTC 8143 cultures results in a twofold increase in catalase activity (42). Moreover, the catalase activity of glutathione-deficient H. influenzae NCTC 8143 is similar compared to the activity of glutathione-supplemented counterparts stressed with 50 μM H2O2 for 1 h. This implies that glutathione deficiency and micromolar H2O2 stress affect hktE gene expression to a similar extent (42). Moreover, Table 1 illustrates that aerobiosis caused a drastic 6.6-fold increase in catalase activity in glutathione-deficient H. influenzae Rd cells. We and others observed no such regulation of catalase for E. coli deficient in glutathione biosynthesis compared to the isogenic parent (28; data not shown). Thus, in H. influenzae, debilitation of the glutathione-based peroxidatic system causes catalase induction, which may be a compensatory response to provide enough, though less efficient, H2O2-scavenging activity. This compensatory response may well explain our observation illustrated in Fig. 4 that either catalase or glutathione-based peroxidase activities alone give the same rate of H2O2 scavenging by whole cells as when both are present. Compensatory interactions between catalase and peroxidase (AhpR) synthesis have been observed previously in a wide range of bacteria. In E. coli (36), Xanthomonas campestris (24), Bacteroides fragilis (34), and Pseudomonas aeruginosa (27), mutations in the peroxidatic system, AhpR, cause catalase induction.
Acknowledgments
We are grateful to William R. Bishai for providing strain AB2593.
J.J.V.B. is indebted to the Fund for Scientific Research-Flanders (grant 3G003601). F.P. is a beneficiary of a doctorate research grant provided by the Institute IWT, Flanders, Belgium.
REFERENCES
- 1.Abe, K., N. Makino, and F. K. Anan. 1979. pH dependency of kinetic parameters and reaction mechanism of beef liver catalase. J. Biochem. 85:473-479. [DOI] [PubMed] [Google Scholar]
- 2.Bishai, W. R., N. S. Howard, J. A. Winkelstein, and H. O. Smith. 1994. Characterization and virulence analysis of catalase mutants of Haemophilus influenzae. Infect. Immun. 62:4855-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishai, W. R., H. O. Smith, and G. J. Barcak. 1994. A peroxide/ascorbate-inducible catalase from Haemophilus influenzae is homologous to the Escherichia coli katE gene product. J. Bacteriol. 176:2914-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brawn, K., and I. Fridovich. 1981. DNA strand scission by enzymically generated oxygen radicals. Arch. Biochem. Biophys. 206:414-419. [DOI] [PubMed] [Google Scholar]
- 5.Chance, B., H. Sies, and A. Boveris. 1979. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59:527-605. [DOI] [PubMed] [Google Scholar]
- 6.del Rio, L. A., L. M. Sandalio, J. M. Palma, P. Bueno, and F. J. Corpas. 1992. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic. Biol. Med. 13:557-580. [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Ruiz, C., A. Colell, A. Morales, N. Kaplowitz, and J. C. Fernandez-Checa. 1995. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-κB: studies with isolated mitochondria and rat hepatocytes. Mol. Pharmacol. 48:825-834. [PubMed] [Google Scholar]
- 9.Gonzalez-Flecha, B., and B. Demple. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270:13681-13687. [DOI] [PubMed] [Google Scholar]
- 10.Grant, C. M., G. Perrone, and I. W. Dawes. 1998. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 253:893-898. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg, J. T., and B. Demple. 1986. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J. Bacteriol. 168:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald, R. A. 1985. Therapeutic benefits of oxygen radical scavenger treatments remain unproven. J. Free Radic. Biol. Med. 1:173-177. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell, B. 1991. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 91:14S-22S. [DOI] [PubMed]
- 14.Hassett, D. J., E. Alsabbagh, K. Parvatiyar, M. L. Howell, R. W. Wilmott, and U. A. Ochsner. 2000. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 182:4557-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herriott, R. M., E. M. Meyer, and M. Vogt. 1970. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J. Bacteriol. 101:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue, Y., T. Matsuda, K. Sugiyama, S. Izawa, and A. Kimura. 1999. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 274:27002-27009. [DOI] [PubMed] [Google Scholar]
- 17.Izawa, S., Y. Inoue, and A. Kimura. 1995. Oxidative stress response in yeast: effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 368:73-76. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson, F. S., R. W. Morgan, M. F. Christman, and B. N. Ames. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage: purification and properties. J. Biol. Chem. 264:1488-1496. [PubMed] [Google Scholar]
- 19.Jain, A., J. Martensson, E. Stole, P. A. Auld, and A. Meister. 1991. Glutathione deficiency leads to mitochondrial damage in brain. Proc. Natl. Acad. Sci. USA 88:1913-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita, H., H. Atomi, M. Ueda, and A. Tanaka. 1994. Characterization of the catalase of the n-alkane-utilizing yeast Candida tropicalis functionally expressed in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 40:682-686. [DOI] [PubMed] [Google Scholar]
- 21.Lesko, S. A., R. J. Lorentzen, and P. O. Ts'o. 1980. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry 19:3023-3028. [DOI] [PubMed] [Google Scholar]
- 22.Loewen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 23.Meister, A., and M. E. Anderson. 1983. Glutathione. Annu. Rev. Biochem. 52:711-760. [DOI] [PubMed] [Google Scholar]
- 24.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 182:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moxon, E. R. 1986. The carrier state: Haemophilus influenzae. J. Antimicrob. Chemother. 18(Suppl. A):17-24. [DOI] [PubMed] [Google Scholar]
- 26.Niimura, Y., L. B. Poole, and V. Massey. 1995. Amphibacillus xylanus NADH oxidase and Salmonella typhimurium alkyl-hydroperoxide reductase flavoprotein components show extremely high scavenging activity for both alkyl hydroperoxide and hydrogen peroxide in the presence of S. typhimurium alkyl-hydroperoxide reductase 22-kDa protein component. J. Biol. Chem. 270:25645-25650. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner, U. A., M. L. Vasil, E. Alsabbagh, K. Parvatiyar, and D. J. Hassett. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J. Bacteriol. 182:4533-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oktyabrsky, O. N., G. V. Smirnovam, and N. G. Muzyka. 2001. Role of glutathione in regulation of hydroperoxidase I in growing Escherichia coli. Free Radic. Biol. Med. 31:250-255. [DOI] [PubMed] [Google Scholar]
- 29.Park, S. G., M. K. Cha, W. Jeong, and I. H. Kim. 2000. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 275:5723-5732. [DOI] [PubMed] [Google Scholar]
- 30.Pauwels, F., B. Vergauwen, F. Vanrobaeys, B. Devreese, and J. J. Van Beeumen. 2003. Purification and characterization of a chimeric enzyme from Haemophilus influenzae Rd that exhibits glutathione-dependent peroxidase activity. J. Biol. Chem. 275:16658-16666. [DOI] [PubMed] [Google Scholar]
- 31.Penninckx, M. 2000. A short review on the role of glutathione in the response of yeasts to nutritional, environmental, and oxidative stresses. Enzyme Microb. Technol. 26:737-742. [DOI] [PubMed] [Google Scholar]
- 32.Potamitou, A., A. Holmgren, and A. Vlamis-Gardikas. 2002. Protein levels of Escherichia coli thioredoxins and glutaredoxins and their relation to null mutants, growth phase, and function. J. Biol. Chem. 277:18561-18567. [DOI] [PubMed] [Google Scholar]
- 33.Regelsberger, G., C. Obinger, R. Zoder, F. Altmann, and G. A. Peschek. 1999. Purification and characterization of a hydroperoxidase from the cyanobacterium Synechocystis PCC 6803: identification of its gene by peptide mass mapping using matrix assisted laser desorption ionization time-of-flight mass spectrometry. FEMS Microbiol. Lett. 170:1-12. [DOI] [PubMed] [Google Scholar]
- 34.Rocha, E. R., and C. J. Smith. 1999. Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate anaerobe Bacteroides fragilis. J. Bacteriol. 181:5701-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki, K., S. Bannai, and N. Makino. 1998. Kinetics of hydrogen peroxide elimination by human umbilical vein endothelial cells in culture. Biochim. Biophys. Acta 1380:275-288. [DOI] [PubMed] [Google Scholar]
- 36.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartaglia, L. A., G. Storz, and B. N. Ames. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 210:709-719. [DOI] [PubMed] [Google Scholar]
- 39.Triggs-Raine, B. L., B. W. Doble, M. R. Mulvey, P. A. Sorby, and P. C. Loewen. 1988. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J. Bacteriol. 170:4415-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ursini, M. V., A. Parrella, G. Rosa, S. Salzano, and G. Martini. 1997. Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem. J. 323:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varma, S. D., and S. M. Morris. 1988. Peroxide damage to the eye lens in vitro prevention by pyruvate. Free Radic. Res. Commun. 4:283-290. [DOI] [PubMed] [Google Scholar]
- 42.Vergauwen, B., F. Pauwels, M. Vaneechoutte, and J. J. Van Beeumen. 2003. Exogenous glutathione completes the defense against oxidative stress in Haemophilus influenzae. J. Bacteriol. 185:1572-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergauwen, B., F. Pauwels, F. Jacquemotte, T. E. Meyer, M. A. Cusanovich, R. G. Bartsch, and J. J. Van Beeumen. 2001. Characterization of glutathione amide reductase from Chromatium gracile: identification of a novel thiol peroxidase (Prx/Grx) fueled by glutathione amide redox cycling. J. Biol. Chem. 276:20890-20897. [DOI] [PubMed] [Google Scholar]
- 44.Vlamis-Gardikas, A., A. Potamitou, R. Zarivach, A. Hochman, and A. Holmgren. 2002. Characterization of Escherichia coli null mutants for glutaredoxin 2. J. Biol. Chem. 277:10861-10868. [DOI] [PubMed] [Google Scholar]
- 45.von Ossowski, I., M. R. Mulvey, P. A. Leco, A. Borys, and P. C. Loewen. 1991. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J. Bacteriol. 173:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]