Abstract
Motility on surfaces is an important mechanism for bacterial colonization of new environments. In this report, we describe detection of rapid surface motility in the wild-type Bacillus subtilis Marburg strain, but not in several B. subtilis 168 derivatives. Motility involved formation of rapidly spreading dendritic structures, followed by profuse surface colonies if sufficient potassium ion was present. Potassium ion stimulated surfactin secretion, and the role of surfactin in surface motility was confirmed by deletion of a surfactin synthase gene. Significantly, this motility was independent of flagella. These results demonstrate that wild-type B. subtilis strains can use both swimming and sliding-type mechanisms to move across surfaces.
There is increasing interest in how bacteria establish symbiotic or pathogenic biofilms on plant or animal cell surfaces. Several recent reviews (5, 15, 16, 24, 27) describe an important role for bacterial motility in such interactions, including motility as a component of the initial development of microbial biofilms. Many types of bacterial motility are known, as reviewed by Henrichsen (8), allowing increased access to nutrients, movement to favorable colonization sites, or avoidance of antimicrobial substances produced by the host.
We are interested in the possible presence and role of Bacillus subtilis biofilms on plant roots (R. Fall, R. F. Kinsinger, and K. A. Wheeler, submitted for publication). B. subtilis is normally considered as a soil bacterium that can be transferred to associated plants and plant materials as well as animals and aquatic habitats (20), but there are also few reports of its direct association with roots (17, 26). While testing the presence of B. subtilis and related strains on plant roots, we observed two types of rapid surface motility on semisolid media, including (i) dendritic growth, reminiscent of the branched colony morphology exhibited by some Bacillus, Paenibacillus, Serratia, and Salmonella isolates on agar surfaces, as reviewed by Kozlovsky et al. (9); and (ii) profuse surface colony formation if the medium was supplemented with a source of K+ ion. Furthermore, the rapid surface motility of our Bacillus isolates might be explained by a lubricating model, in which secretion of biosurfactants is a component of this type of surface growth (9). These considerations led us to examine the role of surfactin, an extracellular lipopeptide with exceptional surfactant properties (19), in the surface motility of wild-type B. subtilis.
Media for rapid surface motility.
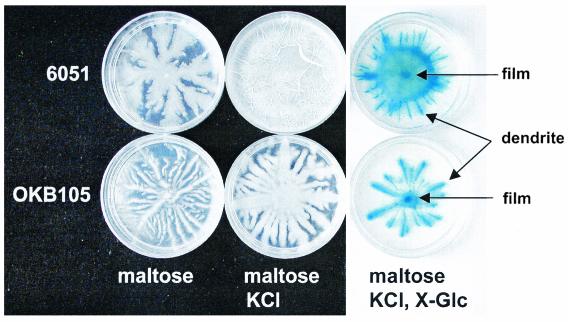
In this work, the wild-type B. subtilis Marburg strain was used (strain 6051, Table 1), and a distinctive feature of its growth on semisolid media is dendritic surface motility. To routinely demonstrate this, a casein digest-based agarose medium was used, as typically prepared with 0.3% (wt/vol) agarose (low electroendosmosis grade; Fisher Scientific) in 60-mm-diameter petri dishes; this medium is described in detail elsewhere (Fall et al., submitted). In various experiments described here, different carbon sources were used with very similar results, including 0.3% (wt/vol) glucose or maltose or 1% (wt/vol) mannitol: termed CG, CMal, and CM media, respectively. The same media were supplemented to contain a source of K+ ion, such as KCl or K2HPO4, and then used to demonstrate extensive surface colony formation. Figure 1 (upper left plates) shows results on CMal versus CMal-KCl agarose media. In the absence of added potassium ion, and with plates inoculated in the center with a sterile toothpick, surface growth appeared as branched dendrites, and the surface was not completely covered (16 h, 40°C). Gram staining of cells in these dendrites showed tightly clustered cells. In contrast, addition of a potassium source, typically 2 to 14 mM KCl or 7 mM K2HPO4, to the casein digest medium resulted in coverage of the agarose surface within 8 h at 40°C (or overnight at 37°C) with a pellicle-like colony (Fig. 1, upper center) similar to pellicles seen on the surfaces of liquid cultures (3). These surface colonies often had a wrinkled appearance, and if grown on 100-mm-diameter petri dishes usually collapsed onto the lid of the dish with visible bubbles indicative of the presence of a surface-active agent. Very similar results were obtained with all three of the carbon sources mentioned above.
TABLE 1.
B. subtilis strains used and some of their properties
| B. subtilis strain | Relevant phenotype or genotype | Source or referencea | Phenotype score
|
|
|---|---|---|---|---|
| Surfactin production (hemolysis) | Dendritic on CM agarose/surface film on CMK agarose; no added surfactinb | |||
| 6051 | Wild type (Marburg) | ATCC | + | +/+ |
| M1 | Surfactin negative, ΔsrfA-A | This work | − | −/− |
| 1A1 (168) | trpC2 | BGSC | − | −/− |
| JH642 | Surfactin negative pheA1 trpC2 | P. Zuber (14) | − | −/− |
| OKB105 | Surfactin-positive transformant of JH642, pheA1 sfp | P. Zuber (14) | + | +/+ |
| OKB120 | Surfactin negative, pheA1 srf::Tn917 sfp | P. Zuber (14) | − | −/− |
| 1A139 | Flagellumless, flaA4 hag-1 lys trpC2 | BGSC | − | −/− |
ATCC, American Type Culture Collection (http://www.atcc.org/); BGSC, Bacillus Genetic Stock Center (http://bacillus.biosci.ohio-state.edu/).
Phenotypes were scored as shown in Fig. 1 after 16 h, of growth at 37°C and, as described in the text.
FIG. 1.
Effect of KCl on the surface growth of B. subtilis 6051and OKB105 and visualization of the stages of surface growth with X-Glc. Shown are CMal agarose plates (pH 7.5) with no added KCl (left), with 2 mM KCl added (center), or with 2 mM KCl and 80 μg ml−1 X-Glc added to visualize β-glucosidase activity (right). The arrows indicate (i) some of the many blue-stained dendrites of cells growing out from the central point of inoculation and (ii) a slower-growing surface film that fills in between the individual dendrites. It should be noted that in the presence of X-Glc in the CMal-KCl agarose medium, both dendritic growth and surface film growth were slower than in its absence (center plates). All plates were inoculated in the center and grown overnight at 37°C, and the results were replicated in three independent experiments.
Specificity of K+-dependent surface motility and its relation to surfactin.
To determine the specificity of dendritic surface growth for monovalent cations, the chloride salts of LiCl, NaCl, KCl, CsCl, or NH4Cl were tested; CG agarose medium was used for these experiments. Very similar growth, although with subtle differences in the dendritic patterns, was seen if the medium was supplemented with common monovalent cations, 2 to 20 mM LiCl, NaCl, or NH4Cl, and no growth was seen in the presence of 20 mM CsCl (data not shown). In contrast, addition of as little as 0.25 mM KCl converted dendritic growth to nearly full coverage of the plate with a surface colony, with full coverage at KCl concentrations of 0.5 to 1.0 mM. These results were each scored visually, but the extent of surface biomass was not quantified. The finding of film growth stimulation by such low levels of K+ ion may simply represent a threshold for the putative high-affinity K+ transporter in B. subtilis (22) or could signify induction of potassium-dependent genes that control colony growth.
If plates lacking added K+ ions like those shown in Fig. 1 (upper left) were observed at intermediate times (6 to 8 h), the appearance of a fluid preceding each dendritic “finger” of growth could be seen. Placement of 10-μl drops of water near such fluid regions, but not on other bare portions of the plates, led to a rapid spreading of the water drop—indicative of the presence of a surfactant. It is known that wild-type B. subtilis strains secrete surfactin (13). In addition, as mentioned above, growth on casein digest agarose medium with K+ ions added suggested the presence of a biosurfactant.
To determine if strain 6051 produces extracellular surfactin and if addition of K+ ion affects growth and/or surfactin formation, 6051 was grown in liquid CM medium with or without addition of 7 mM K2HPO4. With or without the addition of the K2HPO4, in duplicate experiments, there were similar rates of logarithmic growth at 37°C and similar final optical densities at 600 nm after 6 h (±10%) (data not shown). However, the production of extracellular surfactin was stimulated by the presence of 7 mM K2HPO4, as determined by the drop collapse method (2). The cell-free culture media of early-stationary-phase cultures contained 5.7 to 8.6 μg of surfactin ml−1 (CM broth) and 38.4 to 48.6 μg of surfactin ml−1 (CM plus 7 mM K2HPO4 broth) (data from three separate experiments). Smaller stimulation of surfactin formation was obtained by addition of 14 mM KCl with or without control of the pH of the medium (pH 6.6 or 7.3 to mimic the absence or presence of 7 mM K2HPO4, respectively). Thus, the stimulation of surface motility and colony formation by potassium salts like that seen in Fig. 1 and 2 could to be due in large part to different levels of extracellular surfactin, controlled by K+ levels in the medium.
FIG. 2.
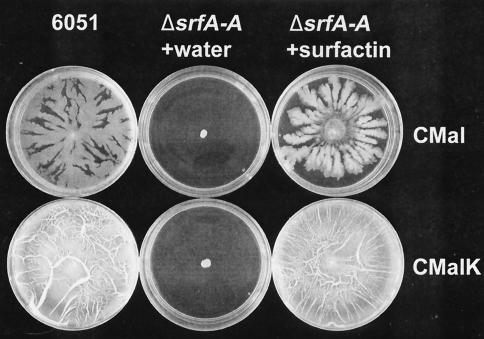
Insertional inactivation of the srfA-A gene in B. subtilis M1 blocks dendritic growth on a low-potassium medium and K+-dependent surface film formation on a high-potassium medium, and addition of authentic B. subtilis surfactin restores rapid surface growth. (Top row) Plates of CMal agarose (low potassium) were inoculated in duplicate with 6051 (B. subtilis Marburg), the ΔsrfA-A mutant, or the ΔsrfA-A mutant plus surfactin. For the latter two inoculations, the center of each plate was spotted with 10 μl of water or a surfactin solution (10 mg ml−1 in 20 mM NaOH) and dried 45 min before inoculation. The bottom row is the same as the top row, but plates containing 7 mM K2HPO4 (CMalK agarose) were used. The center of the plates was inoculated with sterile toothpicks from cultures grown on CMal agarose plates, and then the plates were incubated for 8 h at 40°C. All of the results shown are from multiple, independent experiments; similar results were seen if surfactin was dissolved in 20 mM Tris base.
Isolation and characterization of a ΔsrfA-A mutant.
To verify the role of surfactin in the two phases of surface motility, we used insertional disruption of an essential gene, sfrA-A, of the surfactin operon (25). We used the pJM103 integration vector (18) to disrupt the surfactin synthase srfA-A gene in the B. subtilis Marburg genetic background. The protocols used by Shirk et al. (23) were followed. Primer sequences were obtained by using the published srfA-A sequences of Schneider et al. (21). The forward primer 3′-srfA-BamHI (5′-CGCGGATCCGTGAGCTTGATGTTGAAAGG-3′) and the reverse primer 3′-srfA-EcoRI (5′-GCGGAATTCGAGTACGAATGTAAGCCC-3′) were located within the 10,764-bp wild-type gene, generating a 1,040-bp DNA fragment. All primers were designed with BamHI or EcoRI tails for directional cloning. Primers were purchased from Invitrogen (Frederick, Md.). The integration vector, pJM103, with a chloramphenicol resistance element, was obtained from M. Perego (18). Chemically competent DH5α cells, purchased from Invitrogen, were transformed according to the manufacturer's protocol. B. subtilis chromosomal DNA was isolated from the Marburg strain as described by Cutting and Vander Horn (4). B. subtilis 6051 cells were prepared and transformed as described by Anagnostopoulus and Spizizen (1), using chloramphenicol (5 μg/ml; Sigma) added to tryptose blood agar base (TBAB) plates to select and maintain the mutant strain. The srfA-A mutant was designated strain M1, and was maintained on TBAB agar containing 5 μg of chloramphenicol ml−1.
Mutant M1 no longer produced surfactin, as assessed by hemolysis of erythrocytes, as described by Nakano et al. (14), and by high-performance liquid chromatography analysis of lipopeptides in growth medium (H. Pal Bais, J. Vivanco, and R. Fall, unpublished observations). As shown in Fig. 2 (center plates), the resulting ΔsrfA-A mutant (M1) exhibited no significant dendritic structures on low-potassium medium or complete surface colony coverage on high-potassium medium compared to its wild-type parent (Fig. 2, left plates). To confirm that the growth phenotype effects of the ΔsrfA-A mutation were specific to loss of surfactin production, the M1 mutant was grown on plates supplemented with authentic B. subtilis surfactin (Sigma-Aldrich). As shown in Fig. 2 (right plates), addition of surfactin to the surface of the plates restored both the low-potassium dendritic growth and the high-potassium surface colony coverage.
The optimal concentration of surfactin needed to support surface motility was determined according to dendritic growth on semisolid CM agarose medium and also on CM medium solidified with agar (0.6% [wt/vol]; Difco). On the latter medium, surface spreading was more compact, and it was easier to measure the diameter of the surface colony. On either agarose or agar surfaces, the effects of surfactin could be seen with as little as 0.2 μg of surfactin applied per plate: the approximate effective concentration to give a 50% response (EC50) in different experiments ranged from 0.3 to 0.6 μg of surfactin per plate (data not shown). If the applied surfactin had diffused throughout the medium in the plate (10 ml), this would give an EC50 of 0.03 to 0.06 μg ml−1. This is much below the reported critical micellar concentration of 7.8 μg ml−1 for surfactin (10). However, when CM agarose plates were prepared with surfactin added at the time the plates were poured, the EC50 was approximately five times higher (∼2.5 to 4 μg per plate). It is noteworthy that typical values for surfactin secretion in liquid broth experiments with various B. subtilis strains range widely—from zero to >3,000 μg ml−1 depending on the growth conditions (see reference 25)—and that during the surfactin production phase, these concentrations are usually much higher than the levels necessary for effective surface translocation seen here.
Surface motility defects in B. subtilis 168 strains.
The commonly used genetic strain JH642, derived from widely used B. subtilis strain 168, does not produce surfactin, as a result of a defect in the sfp gene (13), and when casein digest agarose medium was used with various carbon sources, we could not detect the type of surface motility seen here with the 6051 strain (data not shown). We found that addition of surfactin was sufficient to restore dendritic motility and K+-dependent surface colony formation to both strains JH642 and 168 (data not shown). In addition, the surface motilities of OKB105 (sfp+) and its surfactin-negative derivative, OKB120, which has a Tn917 insertion in the srfA-B gene (13), were also tested. OKB105, as shown in Fig. 1 (lower left and lower center), showed both dendritic motility and K+-stimulated surface colony formation without addition of surfactin. Surfactin-negative OKB120 only showed these surface growth phenotypes if surfactin was added to the plates (data not shown), confirming the results we obtained with JH642, 168, and the surfactin-negative ΔsrfA-A mutant (Fig. 2). However, the formation of K+-dependent surface colonies was less extensive in the 168-derived strains (in the presence of surfactin) than with the Marburg strain or the M1 ΔsrfA-A mutant, which may reflect additional mutations in the 168 parent, which was derived by X-ray mutagenesis of the Marburg strain or a close relative (1).
Stages of surface motility.
It was possible to visualize the intermediate stages of surface motility and colony formation by hydrolysis of a colorimetric substrate as described by Mendelson and Salhi (11). CMal agarose was prepared and supplemented with X-Glc (5-bromo-4-chloro-3-indolyl-β-glucoside; 80 μg ml−1; Sigma-Aldrich). X-Glc forms a blue product upon hydrolysis of the glycosidic bond, which helps visualize the dendritic stages of surface motility. As shown in Fig. 1 (upper right), for B. subtilis 6051, addition of X-Glc to these plates slowed the surface spreading and led to blue staining of the central rib of each dendrite and a central colony that had much less of the blue stain. Even after plates were covered with the surface colony that grew between the dendritic structures, the blue-stained features of the underlying dendrites remained (when plates were observed from the bottom; data not shown). These findings correspond to visual inspection of plates without X-Glc during the time course of surface motility. Early rapid dendritic growth, which could reach 5 mm h−1, was seen on several carbon sources, such as glucose, mannitol, sorbitol, maltose, and cellobiose, and was always followed by slower film formation that eventually covered the plate, as long as a source of K+ ion (≥1 mM) was added.
For comparison, a B. subtilis strain derived from the 168 genetic background was tested on CMal plates with X-Glc. As shown in Fig. 1 (lower right), the surfactin-producing strain OKB105 described above also showed the blue staining of surface dendrites in the presence of X-Glc. No such dendrite formation or staining was seen in surfactin-negative strains OKB 120, 168, and JH642 (data not shown). These results demonstrate that the dendritic stage of surfactin-dependent surface motility in B. subtilis strains can be readily visualized with X-Glc.
Rapid surface motility can be independent of flagella.
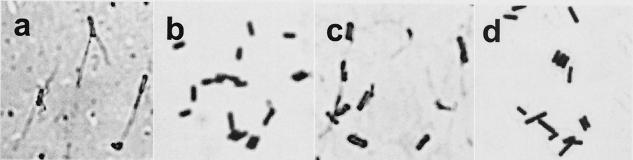
Bacillus species are well known to use flagella for surface motility (6), and in the case of B. anthracis, it is reported that these bacilli can use sliding motility (8). To determine if the rapid motility seen here is due to flagella, we used Ryu stain as used by Heimbrook et al. (7) to attempt to detect flagella in dendritic and surface colonies of the Marburg strain and the ΔsrfA-A mutant. Motile cells from the leading edge of these surface structures were stained, and there was no consistent detection of flagella in these bacteria on CM or CMK agarose (Fig. 3). As a control, these strains were grown in a semisolid swimming medium (Bsa) used to measure swimming motility in B. subtilis (12). These cells contained readily visualized polar flagella (Fig. 3). For comparison in each case (Fig. 3), Gram-stained cells show the typical gram-positive rods.
FIG. 3.
Flagellar staining of motile B. subtilis 6051cells from the leading edges of growth on motility agar (Bsa) (a) versus CM-K2HPO4 agarose (c). Gram stains are also shown for motile cells from Bsa (b) and CM-K2HPO4 agarose (d). Polar flagella are readily visible on motile cells from Bsa motility agar, but not on those from CM-K2HPO4 agarose. In numerous images like that shown in panel c, faint background structures were due to components of the stain and not detached flagella.
As a further test of the independence of surface motility from expression of flagella, we obtained B. subtilis 1A139, with mutations in the flaA4 and hag-1 genes that control flagellar expression and assembly (12). The flagellum-negative phenotype (determined by flagellar staining) and tryptophan and lysine requirements of 1A139 (Table 1) were confirmed, and when the strain was grown on CG or CG-KCl agarose, the results were essentially identical to those seen with the ΔsrfA-A, as in Fig. 2. That is, dendritic motility and surface colony formation were only seen when the plates were supplemented with surfactin or surfactin-KCl, respectively. We conclude that dendritic motility and surface colony formation in B. subtilis are independent of flagella.
Concluding comments.
A striking feature of the surface motility of wild-type B. subtilis shown here is that in the presence of K+ ion, the cells rapidly spread to cover the surface of the casein digest agarose media used. This spreading occurs in two phases, including initial dendritic growth of tightly clustered cells, followed by complete surface film formation that often continues over the edge of the petri dish onto the lid. Both of these stages of surface motility are independent of flagella and thus are not examples of typical swarming motility, which is usually associated with the movement of rafts of multiflagellated cells (6). Instead, this surface spreading is likely to be more akin to sliding motility, which was defined by Henrichsen (8) as a kind of translocation produced by expansive forces in combination with reduction of friction between the cell and the surface. Our finding that the biosurfactant surfactin is required for the surface motility seen here is consistent with this view. Secretion of surfactin at the edges of growing surface dendrites would result in lowering the surface tension, allowing rapid cell spreading. It remains to be determined how addition of K+ ion stimulates surfactin production, which in liquid-phase growth of B. subtilis is under the control of a complex quorum-sensing system (25).
Acknowledgments
This work was supported by grant DE-FG03-97ER20274 from the U.S. Department of Energy, Office of Basic Energy Sciences.
We thank Daniel Kearns and Peter Zuber for advice and for providing strains.
REFERENCES
- 1.Anagnostopolous, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudor, A. A., and R. M. Miller-Maier. 1998. Application of a modified drop-collapse technique for surfactant quantitation and screening of biosurfactant-producing microorganisms. J. Microbiol. Methods 32:273-280. [Google Scholar]
- 3.Branda, S. S., J. E. González-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 5.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser, G. M., and C. Hughes. 1999. Swarming motility. Curr. Opin. Microbiol. 2:630-635. [DOI] [PubMed] [Google Scholar]
- 7.Heimbrook, M. E., W. L. L. Wang, and G. Campbell. 1989. Staining bacterial flagella easily. J. Clin. Microbiol. 27:2612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlovsky, Y., I. Cohen, I. Golding, and E. Ben-Jacob. 1999. Lubricating bacteria model for branching growth of bacterial colonies. Phys. Rev. E 59:7025-7035. [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama, T., and Y. Nakagawa. 1996. Bacterial wetting agents working in colonization of bacteria on surface environments. Colloids Surfaces B Interfaces 7:207-214. [Google Scholar]
- 11.Mendelson, N. H., and B. Salhi. 1996. Patterns of reporter gene expression in the phase diagram of Bacillus subtilis colony forms. J. Bacteriol. 178:1980-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirel, D. B., V. M. Lustre, and M. J. Chamberlin. 1992. An operon of Bacillus subtilis motility genes transcribed by the σD form of RNA polymerase. J. Bacteriol. 174:4197-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano, M. M., N. Corbell, J. Besson, and P. Zuber. 1992. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232:313-321. [DOI] [PubMed] [Google Scholar]
- 14.Nakano, M. M., M. A. Marahiel, and P. Zuber. 1988. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 16.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 17.Pandy, A., and L. M. S. Palni. 1997. Bacillus species: the dominant bacteria of the rhizosphere of established tea bushes. Microbiol. Res. 152:359-365. [DOI] [PubMed] [Google Scholar]
- 18.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. Biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 19.Peypoux, F., J. M. Bonmatin, and J. Wallach. 1999. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 51:553-563. [DOI] [PubMed] [Google Scholar]
- 20.Priest, F. G. 1993. Systematics and ecology of Bacillus, p. 3-16. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. Biochemistry, physiology, and molecular biology. American Society for Microbiology, Washington, D.C.
- 21.Schneider, A., T. Stachelhaus, and M. A. Marahiel. 1998. Targeted alteration of the substrate specificity of peptide synthetases by rational module swapping. Mol. Gen. Genet. 257:308-318. [DOI] [PubMed] [Google Scholar]
- 22.Sebestian, J., Z. Petrmichlová, S. Sebestianová, J. Náprstek, and J. Svobodová. 2001. Osmoregulation in Bacillus subtilis under potassium limitation: a new inducible K+-stimulated, VO43−-inhibited ATPase. Can. J. Microbiol. 47:1116-1125. [PubMed] [Google Scholar]
- 23.Shirk, M. C., W. P. Wagner, and R. Fall. 2002. Isoprene formation in Bacillus subtilis: a barometer of central carbon assimilation in a bioreactor? Biotechnol. Prog. 18:1109-1115. [DOI] [PubMed] [Google Scholar]
- 24.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerson. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan, E. R. 1998. Molecular genetics of biosurfactant production. Curr. Opin. Biotechnol. 9:263-269. [DOI] [PubMed] [Google Scholar]
- 26.Vullo, D. L., C. E. Cotto, and F. Sineriz. 1991. Characteristics of an inulinase produced by Bacillus subtilis 430A, a strain isolated from the rhizosphere of Veronica herbacea (Vell-Rusby). Appl. Environ. Microbiol. 57:2392-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]