Abstract
We report the production, purification, and characterization of a type IA DNA topoisomerase, previously designated topoisomerase I, from the hyperthermophilic archaeon Sulfolobus solfataricus. The protein was capable of relaxing negatively supercoiled DNA at 75°C in the presence of Mg2+. Mutation of the putative active site Tyr318 to Phe318 led to the inactivation of the protein. The S. solfataricus enzyme cleaved oligonucleotides in a sequence-specific fashion. The cleavage occurred only in the presence of a divalent cation, preferably Mg2+. The cofactor requirement of the enzyme was partially satisfied by Cu2+, Co2+, Mn2+, Ca2+, or Ni2+. It appears that the enzyme is active with a broader spectrum of metal cofactors in DNA cleavage than in DNA relaxation (Mg2+ and Ca2+). The enzyme-catalyzed oligonucleotide cleavage required at least 7 bases upstream and 2 bases downstream of the cleavage site. Analysis of cleavage by the S. solfataricus enzyme on a set of oligonucleotides revealed a consensus cleavage sequence of the enzyme: 5′-G(A/T)CA(T)AG(T)G(A)X↓XX-3′. This sequence bears more resemblance to the preferred cleavage sites of topoisomerases III than to those of topoisomerases I. Based on these data and sequence analysis, we designate the enzyme S. solfataricus topoisomerase III.
Topoisomerases are ubiquitous enzymes that participate in nearly all cellular activities involving DNA transactions (5, 31). Based on their catalytic mechanisms, topoisomerases are classified into two classes: type I enzymes, which generate a transient break on one DNA strand to allow the passage of the intact strand with a linking change of one in a single reaction cycle, and type II enzymes, which introduce a transient double-stranded break to allow the passage of another duplex DNA segment with a change in linking number by steps of two (5, 30). Each class of topoisomerases is further divided into two structurally and mechanistically distinct subfamilies on the basis of the polarity of enzyme attachment to the broken strands (i.e., IA, IB, IIA, and IIB). A cellular organism possesses multiple forms of topoisomerases. For example, Escherichia coli has two type IA enzymes (topoisomerases I and III) and two type IIA enzymes (gyrase and topoisomerase IV) (30). Humans carry two enzymes of each of the subfamilies IA (topoisomerases IIIα and IIIβ), IB (topoisomerase I and mitochondrial topoisomerase I), and IIA (topoisomerases IIα and IIβ) (31). Sulfolobus, a group of hyperthermophilic archaea, possesses two type IA enzymes (putative topoisomerase I, identified by gene annotation, and reverse gyrase) and a type IIB enzyme (topoisomerase VI) (2, 8, 10). It appears that all organisms have at least one type IA topoisomerase (31).
E. coli DNA topoisomerase I is the most extensively studied representative of type IA enzymes. The E. coli enzyme is composed structurally of an N-terminal transesterification domain and a C-terminal DNA binding domain (1, 17). The N-terminal domain has the active site tyrosine for DNA cleavage and is capable of forming a covalent adduct with single-stranded DNA. But this domain cannot relax negatively supercoiled DNA. The C-terminal domain consists of five copies of a zinc ribbon motif and is suspected to interact with the passing strand of DNA in the relaxation of negatively supercoiled DNA (1, 12). Each of the first three zinc ribbon motifs from the N terminus in this domain contains four cysteine residues at required sites and is likely to bind zinc (28). The fourth and fifth motifs have one and no cysteine residue, respectively. Sequence analysis of type IA topoisomerase-encoding genes has revealed a significant variation with respect to the copy number of zinc ribbon motifs (0 to 5) as well as the number of tetracysteine motifs in the enzymes (12). These data suggest that zinc ribbon motifs may not be required for DNA relaxation by at least some type IA topoisomerases. This suggestion is consistent with reports that zinc binding is not necessary for the relaxation activity of topoisomerases from Mycobacterium smegmatis and Thermotoga maritima (3, 29).
Although type IA topoisomerases share considerable sequence and structural similarity, they display significant diversity in substrate cleavage specificity. For example, E. coli and Micrococcus luteus topoisomerases I show a limited sequence preference (5′-CXXX↓-3′) (27), whereas E. coli topoisomerase III and M. smegmatis topoisomerase I are more specific in cleavage (5′-CT↓T-3′ and 5′-CG/TCT↓T-3′, respectively) (23, 33). Analysis of the cleavage sequences of topoisomerases is of importance to the understanding of the molecular basis of the interaction between the enzymes and their DNA substrates.
Both bacterial and eucaryal type IA topoisomerases have been extensively characterized, but no archaeal enzymes except for reverse gyrase have been biochemically investigated so far. In this study, we cloned and expressed in E. coli a putative type IA topoisomerase-encoding gene from the hyperthermophilic archaeon Sulfolobus solfataricus. We demonstrate that the recombinant protein is capable of relaxing negatively supercoiled DNA at 75°C and, therefore, is a thermophilic topoisomerase. A number of divalent cations (e.g., Mg2+, Ca2+, Cu2+, Co2+, Mn2+, and Ni2+) were capable of supporting the cleavage activity of the enzyme. No cleavage was detectable in the absence of a metal cofactor. Furthermore, the S. solfataricus enzyme was not sensitive to inhibition by spermidine. Intriguingly, while most type IA topoisomerases showed weak sequence preference in DNA cleavage, the S. solfataricus topoisomerase cleaved oligonucleotides with relatively high specificity at the sequence 5′-G(A/T)CA(T)AG(T)G(A)X↓XX. This sequence bears more resemblance to the preferred cleavage sites of topoisomerases III than to those of topoisomerases I. Based on these data and phylogenetic analysis, we propose that the S. solfataricus enzyme (previously postulated to be a topoisomerase I) and its archaeal homologues be classified as archaeal topoisomerases III.
MATERIALS AND METHODS
Gene cloning and expression.
The putative type IA topoisomerase gene was amplified from the genomic DNA of S. solfataricus by PCR using Pfu DNA polymerase (Stratagene, La Jolla, Calif.) with the following pair of primers: 5′-GTGGGGGATCCCATATGAATTTATGTAATGTAAAC/ and 5′-GTGGGAAGCTTTTATTCACTGCTTAGCATATAAG (NdeI and HindIII sites are underlined). The PCR product was cleaved with NdeI and HindIII and was inserted into the NdeI/HindIII sites of the expression vector pET-30a(+) (Novagen, Madison, Wis.). The resulting plasmid was transformed into E. coli BL21(DE3) after a passage through E. coli DH5α. The sequence of the insert was verified by DNA sequencing.
For protein production, the transformant was grown at 37°C to an optical density at 600 nm of ∼0.6 in Luria-Bertani medium containing 30 μg of kanamycin/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. Incubation was continued for 6 h.
Protein purification.
Induced cells of the Sso Topo III overproducer were harvested and resuspended in lysis buffer containing 20 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, and 1 mM dithiothreitol (DTT). The cells were disrupted by three cycles of freezing in liquid nitrogen and thawing at 4°C. After removal of cell debris by centrifugation (150,000 × g, 30 min, 4°C), the supernatant was heat treated at 75°C for 15 min. The sample was clarified by centrifugation (13,000 × g, 10 min, 22°C) and was dialyzed against buffer A (50 mM Tris-HCl, pH 7.8, 0.1 mM EDTA, 0.1 mM DTT, and 10% glycerol). The dialyzed material was loaded onto a Mono Q column (1 ml; Amersham Pharmacia Biotech, Uppsala, Sweden) equilibrated with buffer A. The column was washed with buffer A (5 ml), and bound proteins were eluted with a KCl gradient (0 to 1 M) in buffer A (10 ml). Peak topoisomerase fractions, eluted at 0.8 to 1.0 M, were pooled, dialyzed against buffer A, and stored at −70°C. All purification steps were carried out at 4°C. Protein concentrations were determined by the Lowry method with bovine serum albumin (BSA) as the standard.
Phylogenetic analysis.
A multiple alignment of protein sequences, retrieved from public sequence databases, was obtained by using ClustalW (26). The phylogenetic tree was constructed by the neighbor-joining method.
Site-directed mutagenesis.
Site-directed mutagenesis within the Sso Topo III gene, changing Tyr318 (ACC) into Phe318 (TCC), was performed by using the Altered Sites II in vitro mutagenesis system (Promega, Milwaukee, Wis.) with a mutagenic oligonucleotide primer (5′-GTCTAATAAGTTACCCAAGAACTAA) according to the manufacturer's instructions. The mutation was confirmed by DNA sequencing.
DNA relaxation assays.
A standard reaction mixture (20 μl) contained Sso Topo III (40 fmol, unless otherwise specified) and negatively supercoiled pUC18 DNA (300 ng) in 50 mM Tris-HCl, pH 8.8, 1 mM DTT, 0.1 mM EDTA, 10 mM MgCl2, 90 mM NaCl, 30 μg of BSA/ml, and 12% (vol/vol) ethylene glycol. The mixture was overlaid with a thin layer of mineral oil and was incubated for 30 min at 75°C. The reaction was terminated by the addition of 4 μl of a gel loading solution (2.5% sodium dodecyl sulfate [SDS], 50 mM EDTA, 25% glycerol, and 0.2% bromophenol blue). Samples were electrophoresed through 1.5% agarose in 0.5× Tris-phosphate-EDTA buffer. The gel was stained with ethidium bromide and was photographed under UV light.
Nick closure by Pfu DNA ligase.
The single-nick plasmid pUC18 was prepared as described previously (7). The nicked plasmid (1 μg) was ligated for 30 min at 75°C with Pfu DNA ligase (4 Weiss units; Stratagene) in the same reaction buffer as that employed in the DNA relaxation assays.
Oligonucleotide cleavage assays.
Oligonucleotides employed in the assays were labeled at the 5′ end with T4 polynucleotide kinase and [γ-32P]ATP and were purified by using a quick spin column. A standard cleavage reaction was performed by incubating Sso Topo III (4 pmol) with a radiolabeled oligonucleotide (0.1 pmol) for 30 min at 75°C in 50 mM Tris-HCl, pH 8.8, 1 mM DTT, 0.1 mM EDTA, 2 mM MgCl2, 90 mM NaCl, 30 μg of BSA/ml, and 12% (vol/vol) ethylene glycol in a total volume of 20 μl. The reaction was terminated by the addition of SDS to a final concentration of 0.5%. The sample was mixed with a gel loading solution (20 μl) containing 97.5% formamide, 10 mM EDTA, and 0.5% (wt/vol) bromophenol blue. The mixture was heated for 3 min at 95°C and was loaded onto a 19 or 25% polyacrylamide gel (19:1) containing 7 M urea in 1× Tris-borate-EDTA. After electrophoresis, the gel was dried and exposed to X-ray film or was analyzed with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
Phylogenetic analysis of a putative type IA topoisomerase from S. solfataricus.
A sequence search in the genome of S. solfataricus revealed the presence of an open reading frame (GenBank accession no. G90241) encoding a putative type IA topoisomerase, which was termed topoisomerase I based on gene annotation. The putative protein contains 668 amino acid residues and has a calculated molecular mass of 76.6 kDa. Among known type IA topoisomerases, the S. solfataricus protein is most closely related to its homologues from other archaeal species, being 37 and 28% identical at the amino acid sequence level to homologues from Aeropyrum pernix (GenBank accession no. Q9YB01) and Methanococcus jannaschii (Q59046), respectively. These archaeal type IA topoisomerases form a clad phylogenetically separated from their eucaryal and bacterial homologues (Fig. 1). Interestingly, the archaeal enzymes show closer relationships to topoisomerases III than to topoisomerases I. For example, the putative S. solfataricus protein shares 19 and 13% sequence identity with E. coli DNA topoisomerase III (GenBank accession no. P14294) and topoisomerase I (P06612), respectively. Based on this and the oligonucleotide cleavage specificity shown below, we designate the enzyme S. solfataricus topoisomerase (Sso Topo III). As reported earlier, reverse gyrases of both archaeal and bacterial origins are closely related to bacterial topoisomerases I (4). The conserved sequence motifs and sites (e.g., the active site tyrosine, the acidic triad, etc.) common to type IA topoisomerases are present in Sso Topo III. However, it is notable that Sso Topo III has a single zinc ribbon motif in which only three cysteine residues exist. Therefore, Sso Topo III does not appear to possess a zinc binding site. This is consistent with the notion that zinc binding may not be necessary for the activity of a type IA topoisomerase (3, 29).
FIG. 1.
Phylogenetic relationship of type IA DNA topoisomerases from Archaea, Bacteria, and Eucarya. Multiple-sequence alignments were obtained by using ClustalW. The tree was constructed by using the neighbor-joining method. Bootstrap values were obtained with 1,000 replicates. The source organism and accession number for each protein are as follows: Sso, S. solfataricus (Topo III: G90241; Top R: F90247); Pfu, Pyrococcus furiosus (Topo III: O73954; Top R: NP_578224); Ape, A. pernix (Topo III: Q9YB01); Mth, Methanobacterium thermautotrophicum (Topo III: O27661); Mja, M. jannaschii (Topo III: Q59046); Tma, T. maritima (Topo I: P46799; Top R: C72409); Fis, Fervidobacterium islandicum (Topo I: O34204); Eco, E. coli (Topo I: P06612; Topo III: P14294); Hin, Haemophilus influenzae (Topo I: P43012; Topo III: P43704); Pmu, Pasteurella multocida (Topo I: Q9CN30; Topo III: Q9CP53); Vch, Vibrio cholerae (Topo I: Q9KRB2; Topo III: Q9KQF5); Cel, Caenorhabditis elegans (Topo III: O61660); Sce, S. cerevisiae (Topo III: P13099); and Hsa, Homo sapiens (Topo IIIα: Q13472). Sso Topo III, Pfu Topo III, Ape Topo III, Mth Topo III, and Mja Topo III were previously known as Sso Topo I, Pfu Topo I, Ape Topo I, Mth Topo I, and Mja Topo I, respectively. The topoisomerase domain of reverse gyrase, indicated by amino acid residues in parentheses, was used in the analysis.
Expression and purification of recombinant Sso Topo III.
The gene encoding Sso Topo III was amplified by PCR from the genomic DNA of S. solfataricus and cloned into the expression pET-30a(+). Upon induction with IPTG, E. coli cells harboring the recombinant plasmid overproduced a polypeptide with an estimated molecular mass of 75.3 kDa, a size similar to that expected for the putative S. solfataricus topoisomerase III (Fig. 2A). The protein was purified to apparent homogeneity by using a simple procedure involving heat treatment, which eliminated most of the E. coli proteins, and column chromatography on Mono Q Sepharose. The protein was identified as the product of the cloned gene by N-terminal amino acid sequencing. Approximately 2 mg of Sso Topo III was purified from 1 liter of culture.
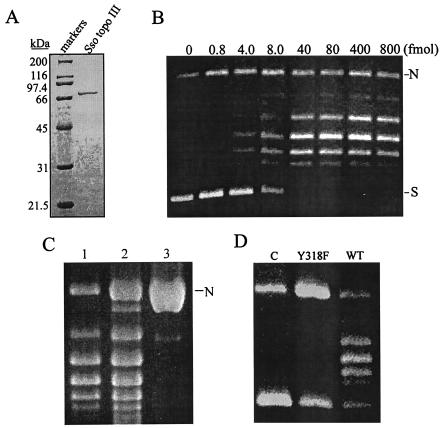
FIG. 2.
Purification and topoisomerase activities of Sso Topo III. (A) SDS-polyacrylamide gel electrophoresis of purified Sso Topo III. A sample (1.5 μg) of purified Sso Topo III was electrophoresed through an SDS-polyacrylamide gel (10% acrylamide). The gel was silver stained. Molecular masses of protein standards are indicated. (B) DNA relaxation by Sso Topo III. Serial dilutions of Sso topo III were incubated at 75°C for 30 min with pUC18 (300 ng) in 50 mM Tris-HCl, pH 8.8, 1 mM DTT, 0.1 mM EDTA, 10 mM MgCl2, 90 mM NaCl, 30 μg of BSA/ml, and 12% (vol/vol) ethylene glycol. Reaction mixtures were electrophoresed through 1.5% agarose. N, nicked plasmid; S, supercoiled plasmid. (C) Determination of the end point of DNA relaxation catalyzed by Sso Topo III. Sso Topo III (2 pmol) was incubated with pUC18 (300 ng) at 75°C for 30 min in the standard DNA relaxation assay (lane 1). Single-nick pUC18 (1 μg, lane 3) was incubated with Pfu DNA ligase (4 Weiss units) under the same conditions as those used for DNA relaxation to yield a completely relaxed plasmid (lane 2). Samples were electrophoresed in agarose. (D) Mutational analysis of the putative active site of Sso Topo III. A sample of wild-type or mutant Y318F enzyme was incubated with pUC18 (300 ng) at 75°C for 30 min in the standard DNA relaxation assay. Products were analyzed by agarose gel electrophoresis.
Catalytic properties of Sso Topo III.
The putative Sso Topo III was tested for its ability to relax negatively supercoiled DNA. The purified enzyme was serially diluted and incubated with native pUC18 DNA under the standard assay conditions. As shown in Fig. 2B, 40 fmol of the enzyme was capable of relaxing the input plasmid (300 ng) to the end point of the reaction within 30 min of incubation at 75°C. These data indicate that the recombinant protein is indeed a topoisomerase. To measure the extent of DNA relaxation catalyzed by the enzyme, we prepared single-nick plasmid pUC18 and ligated it under the same conditions as those used for the relaxation assays. The ligated pUC18 DNA was then used as a reference for the relaxed plasmid under the relaxation assay conditions. By comparing the degree of supercoiling in plasmid DNA relaxed by a saturating amount of Sso Topo III to that of the ligated plasmid, we found that both DNA samples had the same average number of supercoils (Fig. 2C). As expected, they were both negatively supercoiled under the electrophoresis conditions employed in this study (data not shown). These results indicate that Sso Topo III is capable of complete relaxation of DNA. Like other type IA topoisomerases, Sso Topo III was unable to relax positively supercoiled DNA (data not shown). Mutation that converted a tyrosine to a phenylalanine residue at the 318th position in Sso Topo III abolished the relaxation activity of the protein, a result consistent with the prediction based on sequence analysis that Tyr318 is the active site of the enzyme (Fig. 2D).
Effects of temperature, metal ions, and salts on DNA relaxation by Sso Topo III.
In the standard relaxation assay, Sso Topo III showed detectable activity at 65°C, was most active at 75°C, and became inactive at ≥ 85°C (Fig. 3A). In order to examine the thermal stability of Sso Topo III, we preincubated the enzyme at a test temperature for various lengths of time and then determined the activity of the treated enzyme under the standard assay conditions. We found that the enzyme was stable for up to 4 h at 80°C or 3 h at 85°C. The enzyme was inactivated in less than 1 h when incubated at 90°C. These data indicate that Sso Topo III is well adapted to the growth temperature of S. solfataricus.
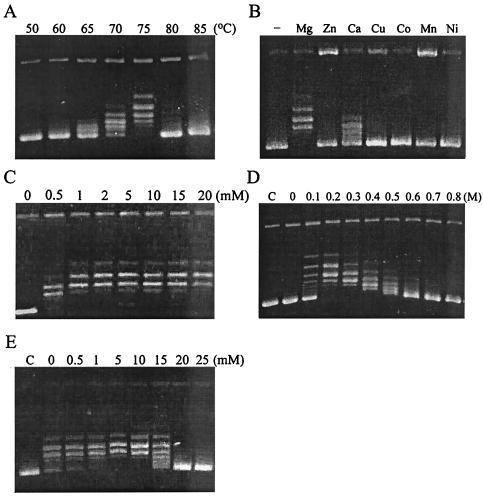
FIG. 3.
Characterization of the DNA relaxation activity of Sso Topo III. (A) Effect of temperature on DNA relaxation by Sso Topo III. Relaxation reactions were performed at indicated temperatures for 30 min. Reaction products were resolved by electrophoresis in agarose. (B) Effect of divalent cations on DNA relaxation by Sso Topo III. Reactions were performed at 75°C for 30 min in the presence of a tested divalent cation (10 mM). (C) Effect of Mg2+ concentration on DNA relaxation by Sso Topo III. Reactions were performed at 75°C for 30 min with various concentrations of MgCl2. (D) Effect of NaCl on DNA relaxation by Sso Topo III. Reactions were performed at 75°C for 30 min with various concentrations of NaCl. C, a control in which negatively supercoiled pUC18 DNA alone was electrophoresed. (E) Effect of spermidine on DNA relaxation by Sso Topo III. Reactions were carried out under the standard assay conditions at various concentrations of spermidine. C, control.
A divalent cation is indispensable for the relaxation activity of a type IA topoisomerase. We examined the effect of various metal ions (Mg2+, Zn2+, Ca2+, Cu2+, Co2+, Mn2+, and Ni2+) on DNA relaxation catalyzed by Sso Topo III. The enzyme was inactive in the absence of a metal cofactor. Mg2+ and, to a lesser extent, Ca2+ were able to support the DNA relaxation activity of the enzyme (Fig. 3B). A minimum of 1 mM Mg2+ was sufficient for the enzyme to be optimally active in DNA relaxation (Fig. 3C).
Sso Topo III displayed an unusual salt requirement in DNA relaxation. The enzyme was active over a wide range of NaCl concentrations from 100 to 600 mM but showed no detectable activity in the absence of added salt (Fig. 3D). At low salt concentrations (e.g., 100 mM NaCl), the enzyme catalyzed DNA relaxation in an apparently processive mode so that, while a fraction of the substrate molecules were relaxed to the end point of the reaction, the rest of the substrate molecules remained highly negatively supercoiled. However, as the salt concentration increased, the enzyme appeared to have switched to a distributive mode in which all of the input DNA molecules were relaxed to a similar but progressively smaller extent. The similar switch in the mode of action in response to the change in salt concentration was described previously for M. smegmatis topoisomerase I (3). It is possible that high concentrations of salt reduce the affinity of a topoisomerase for DNA, facilitating the dissociation of the enzyme from the substrate before the completion of the relaxation reaction.
The ability of a type IA topoisomerase to unpair double-stranded DNA prior to strand cleavage depends on the single-stranded nature of negatively supercoiled DNA. Spermidine at physiological concentrations (e.g., 1.5 mM) was found to inhibit E. coli topoisomerases I and III in 60 to 180 mM KCl (24, 25). We assayed DNA relaxation by Sso Topo III in the presence of an increasing concentration of spermidine. Surprisingly, the enzyme was not inhibited by spermidine at concentrations up to at least 10 mM (Fig. 3E).
Cleavage of oligonucleotides by Sso Topo III.
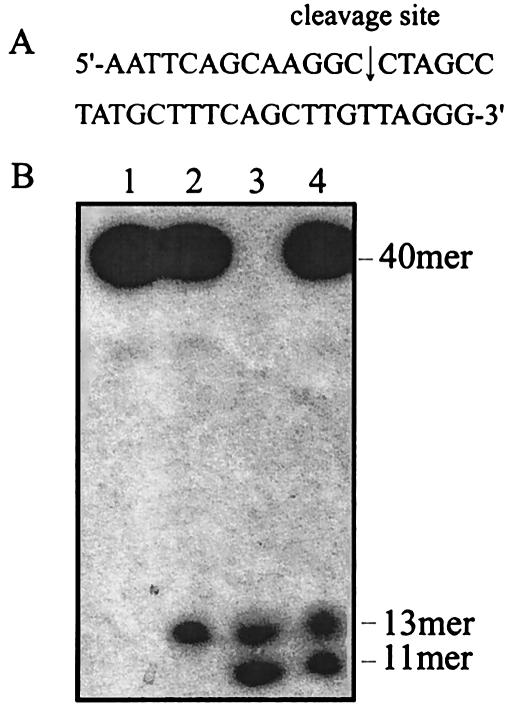
A type IA topoisomerase transiently cleaves one of the DNA strands to permit the passage of another strand through the nick during the relaxation of negatively supercoiled DNA. Type IA enzymes characterized so far show remarkable variation in their preferred cleavage sequences. To identify the preferred cleavage sequence of Sso Topo III, we performed cleavage assays on a number of 5′-radiolabeled oligonucleotides. We first tested a 22-nt oligonucleotide (5′-GAATGAGCCGCAACTTCGGGAT), which was readily cleaved by E. coli topoisomerase I and T. maritima topoisomerase I (29). The oligonucleotide, however, was not detectably cleaved by Sso Topo III. We then decided to screen a total of 40 randomly selected oligonucleotides (25 to 40 nt in size) available in our laboratory for the presence of cleavage sites for Sso Topo III. Only a 40-nt sequence (5′-AATTCAGCAAGGCCTAGCCTATGCTTTCAGCTTGTTAGGG) was significantly cleaved (Fig. 4A), and three other sequences were weakly cut. Therefore, it appears that Sso Topo III is sequence specific in oligonucleotide cleavage. The 40-mer was later found to contain a nearly perfect sequence for the Sso Topo III-catalyzed cleavage. To determine the site of cleavage by the enzyme on the 40-mer, we subjected the cleavage products along with markers of known sizes to electrophoresis on the same gel. As shown in Fig. 4B, the labeled cleavage product comigrated with a 13-nt marker. Since the 40-mer was 32P labeled at the 5′ end, cleavage occurred between C-13 and C-14.
FIG. 4.
Identification of the site of oligonucleotide cleavage by Sso Topo III. (A) The sequence of the 40-nt cleavage substrate. The cleavage site is indicated by the arrow. (B) Cleavage of the 40-mer by Sso Topo III. Sso Topo III (2 pmol) was incubated with the 5′ end radiolabeled 40-mer (0.1 pmol) at 75°C for 30 min in 50 mM Tris-HCl, pH 8.8, 1 mM DTT, 0.1 mM EDTA, 10 mM MgCl2, 90 mM NaCl, 30 μg of BSA/ml, and 12% (vol/vol) ethylene glycol. Reaction mixtures were analyzed by urea-polyacrylamide gel electrophoresis. Lane 1, 40-mer; lane 2, cleavage reaction products; lane 3, oligonucleotide size markers (13-mer [AATTCAGCAAGGC] and 11-mer [AATTCAGCAAG]); and lane 4, a mixture of the cleavage products and the size markers.
Effects of temperature, metal ions, and salts on oligonucleotide cleavage by Sso Topo III.
We then investigated the cleavage properties of Sso Topo III by using the 40-mer as a substrate. Cleavage of the 40-mer by the enzyme depended strongly on temperature (Fig. 5A). Little cleavage was detected at 0°C. The cleavage activity was very low but was measurable at 37°C and peaked at 65 to 75°C. By contrast, oligonucleotide cleavage by T. maritima topoisomerase I was unchanged over a range of temperatures from 24 to 75°C (29). The basis for this discrepancy remains to be understood.
FIG. 5.
Characterization of the oligonucleotide cleavage activity of Sso Topo III. (A) Effect of temperature on DNA cleavage by Sso Topo III. Cleavage reactions were performed at indicated temperatures for 30 min. Reaction products were resolved by urea-polyacrylamide gel electrophoresis. C, a control in which the oligonucleotide substrate was incubated for 30 min at 75°C. (B) Effect of divalent cations on DNA cleavage by Sso Topo III. Reactions were performed at 75°C for 30 min in the presence of a tested divalent cation (2 mM). C, control. (C) Effect of Mg2+ concentration on DNA cleavage by Sso Topo III. Reactions were performed at 75°C for 30 min at various concentrations of MgCl2. C, control. (D) Effect of salt concentration on DNA cleavage by Sso Topo III. Reactions were performed at 75°C for 30 min at various concentrations of NaCl.
Sso Topo III-catalyzed oligonucleotide cleavage depended on the presence of a divalent cation (Fig. 5B). No cleavage was detected in the absence of a metal cofactor. Among the tested divalent cations, Mg2+ appeared to be the best metal cofactor for the enzyme. The enzyme was optimally active with 2 to 5 mM Mg2+ (Fig. 5C). These data appear to contradict the finding that Mg2+ stimulated but was not required for DNA cleavage by E. coli topoisomerase I (9, 36). However, we cannot rule out the possibility that the Sso Topo III-catalyzed cleavage in the absence of Mg2+ is below the detection limit of our assay and that Mg2+-mediated stimulation of cleavage is far greater with Sso Topo III than with E. coli topoisomerase I. Unexpectedly, Ca2+, Cu2+, Co2+, Mn2+, and Ni2+ were all able to replace partially Mg2+ in cleavage reaction, whereas Zn2+ was inactive. The cleavage in the presence of cations other than Mg2+ did not appear to result from contamination of these cations with Mg2+ since it was impossible for the level of the contaminating Mg2+ to be high enough for the amount of cleavage observed in the present study. Our results suggest that Sso Topo III has a relaxed specificity for divalent cations in oligonucleotide cleavage.
In order to study the effect of salt on oligonucleotide cleavage by Sso Topo III, we included an increasing concentration of NaCl in the standard cleavage assay. The enzyme was incapable of detectable cleavage in the absence of NaCl (Fig. 5D). This result provides a possible explanation for the inability of the enzyme to relax DNA in the absence of NaCl. The cleavage was optimal in 250 mM NaCl.
Cleavage specificity.
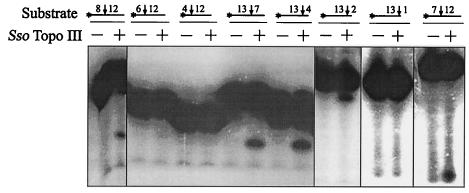
In order to examine the sequence specificity of Sso Topo III in oligonucleotide cleavage, we first investigated the upstream and downstream boundaries of the cleavage site by using two sets of templates derived from the 40-mer. The first set of oligonucleotides contained 12 bases on the 3′ side and 8, 6, or 4 bases on the 5′ side of the cleavage site. The second set had 13 bases on the 5′ side and 7, 4, or 2 bases on the 3′ side of the cleavage site. Sso Topo III was incubated with each of these templates, and the resulting cleavage products were resolved by electrophoresis through polyacrylamide in the presence of urea. As shown in Fig. 6, among the first set of oligonucleotides, the one with 8 bases 5′ of the cleavage site was cleaved, whereas the other two with shortened 5′ sequences were not. On the other hand, all of the templates in the second set were cleaved. In subsequent experiments, we found that the enzyme cleaved a substrate with 7 bases on the 5′ side and 12 bases on the 3′ side of the cleavage point but was unable to cut an oligonucleotide with 1 base on the 3′ side and 13 bases on the 5′ side of the cleavage site (Fig. 6). Therefore, we conclude that the Sso Topo III-catalyzed oligonucleotide cleavage requires 7 bases 5′ and 2 bases 3′ of the cleavage site. The asymmetry of the cleavage sequence with respect to the site of scission has also been reported for other type I topoisomerases (34).
FIG. 6.
Determination of the sequence requirement of the Sso Topo III-catalyzed oligonucleotide cleavage. The 40-mer was successively shortened from both ends to yield eight oligonucleotides. As illustrated at the top of the figure, each oligonucleotide is represented by a horizontal line proportional in length to the size of the oligonucleotide. The point of cleavage is shown by a vertical arrow. The number of bases 5′ or 3′ of a cleavage site is indicated by the number on the left or right, respectively, of the arrow. Each oligonucleotide was incubated at 75°C for 30 min with (+) or without (−) Sso Topo III (2 pmol). The cleavage products of the oligonucleotide containing 13 bases 5′ and 2 bases 3′ of the cleavage site were analyzed by electrophoresis in 25% polyacrylamide containing 7 M urea, whereas those from other oligonucleotides were resolved in 19% polyacrylamide gels.
To examine the cleavage specificity of the enzyme, we employed a 17-nt oligonucleotide corresponding to a portion of the 40-mer (positions −13 to + 4 in relation to the cleavage site) and generated all three possible base substitutions individually at each of the positions from −8 to + 2, a region similar to that delineated in the foregoing analysis of the minimal sequence requirement for Sso Topo III-catalyzed cleavage. Cleavage of these oligonucleotides was quantitated. As shown in Table 1, Sso Topo III was significantly more sensitive to base changes on the 5′ side than to those on the 3′side of the cleavage site. While bases from positions −6 to −3 were generally critical in determining cleavage efficiency, adenine at position −4 and cytosine at position −6 were strictly required for the cleavage. In a short stretch of sequence (−7 to −2), a total of 12 substitutions were found to reduce cleavage to ≤1/4 of their respective controls. These data indicate that Sso Topo III cleaves oligonucleotides in a sequence-specific fashion and that the preferred cleavage site for the enzyme is G(A/T)CA(T)AG(T)G(A)X↓XX.
TABLE 1.
Oligonucleotide cleavage specificity of Sso Topo IIIa
| Base position | Acid
|
|||
|---|---|---|---|---|
| G | A | T | C | |
| −8 | ++++ | ++++ | ++++ | +++ |
| −7 | ++++ | ++ | +++ | + |
| −6 | + | ± | + | ++++ |
| −5 | ± | ++++ | +++ | ± |
| −4 | + | ++++ | ± | + |
| −3 | ++++ | + | +++ | + |
| −2 | ++++ | +++ | ++ | + |
| −1 | ++++ | ++++ | ++++ | +++ |
| +1 | +++ | ++++ | ++++ | ++++ |
| +2 | +++ | +++ | ++++ | +++ |
Three possible substitutions were made individually at each position from −8 to +2 on a labeled 17-base oligonucleotide containing the cleavage site of Sso Topo III. Cleavage of these substrates by Sso Topo III was quantitated. The number of + Signs indicates the relative extent of cleavage for a base at the indicated position in comparison to the maximum cleavage obtained for that position: ±, 0 to 10%; +, 11 to 30%; ++, 31 to 50%; +++, 51 to 70%; and ++++, 71 to 100%.
DISCUSSION
In this study, we have cloned and expressed in E. coli the gene encoding a putative type IA topoisomerase from S. solfataricus. We demonstrate that the recombinant protein is indeed a thermophilic topoisomerase capable of relaxing negatively supercoiled DNA to completion in moderate salt. A revealing observation made in this study is that the S. solfataricus enzyme displayed significant sequence specificity in oligonucleotide cleavage and had a preferred cleavage site similar to those of topoisomerases III. The cleavage required at least 7 bases upstream and 2 bases downstream of the cleavage site. The enzyme was highly selective of bases from positions −2 through −7 and, however, was relaxed toward bases immediately adjacent to the site of strand scission, suggesting an asymmetric binding of the enzyme to the cleavage sequence. An A at position −4 and a T at position −6 were apparently critical to the cleavage activity and therefore are likely to play an important role in the recognition of the substrate by the enzyme. The cleavage sequence is less well conserved at positions −3 and −5. But only a single type of substitution may occur at either position. The base preference of the enzyme at each position over a stretch of 6-bp sequence 5′ to the site of strand scission (−7 to −2) renders the enzyme specific in oligonucleotide cleavage. Among characterized type I topoisomerases, only vaccinia virus topoisomerase I (a Topo IB enzyme) and M. smegmatis topoisomerase I have been shown to be highly specific in DNA cleavage (22, 23). Most type I topoisomerases from prokaryotes are less stringent in cleavage target selection. E. coli, M. luteus topoisomerases I, and Sulfolobus shibatae reverse gyrase require only a C at position −4 for efficient cleavage (16, 23, 27). Because of its sequence specificity, the S. solfataricus topoisomerase presumably acts infrequently on the genomic DNA of S. solfataricus. A strong site (defined as one at which >50% of the maximum cleavage occurs) is found once for every 200 bp, on average, in the S. solfataricus genome. The physiological significance of the relatively high cleavage specificity of the enzyme remains to be understood.
Intriguingly, a strong preference for an A at position −3 or −4 in the cleavage sequence has also been reported for several bacterial and eucaryal topoisomerases III, including E. coli topoisomerase III (24), human topoisomerase IIIα (11), and yeast topoisomerase IIIα (14). By comparison, a conserved T at position −4 was found in the cleavage sequences of E. coli, M. smegmatis, T. maritima topoisomerases I (23, 27, 29), and S. shibatae reverse gyrase (13). These results are consistent with the observation that the S. solfataricus topoisomerase and its archaeal homologues are phylogenetically more closely related to topoisomerases III than to topoisomerases I, whereas reverse gyrases are closely related to bacterial topoisomerases I (4). Based on these data, we suggest that the S. solfataricus enzyme is a topoisomerase III. Likewise, we propose that all putative archaeal topoisomerases I be more appropriately named archaeal topoisomerases III.
S. solfataricus topoisomerase III possesses several unusual properties in catalyzing DNA relaxation. First, the enzyme required a divalent cation not only for DNA relaxation but also for oligonucleotide cleavage. Binding of Mg2+ has been shown to induce conformational changes in E. coli DNA topoisomerase I (36). These conformational changes may be required for one or more steps in the proposed mechanism of DNA relaxation by type IA topoisomerases. Since Mg2+ stimulates but is not indispensable for DNA strand cleavage by E. coli DNA topoisomerase I, the conformational changes in the enzyme as a result of the binding of the cation are thought to be critical to steps following strand passage (6, 36). Unlike the E. coli enzyme, Sso Topo III depended on a metal cofactor for the DNA strand cleavage activity and showed a more relaxed metal cofactor specificity in strand cleavage than in DNA relaxation. It appears, therefore, that a metal cofactor may be involved in strand cleavage as well as steps subsequent to it in DNA relaxation by Sso Topo III. Second, Sso Topo III showed an unusual salt requirement. The enzyme was unable to cleave oligonucleotides or relax supercoiled DNA in the absence of added monovalent salt but was active in both reactions over a wide range of NaCl concentrations (0.1 to 0.6 M). In comparison, E. coli DNA topoisomerase III required low monovalent salt concentrations (<20 mM) for maximal DNA relaxation activity and was inactive in the absence of monovalent salt (24, 35). Human DNA topoisomerase IIIα and Saccharomyces cerevisiae DNA topoisomerase IIIα were inhibited by 100 and 150 mM NaCl, respectively (11, 14). The ability of Sso Topo III to relax DNA supercoils in relatively high salt suggests a high affinity of the enzyme for DNA substrates. Third, spermidine is known to inhibit type IA topoisomerase-catalyzed relaxation of negatively supecoiled DNA by reducing the single-stranded nature of the DNA (25). However, Sso Topo III was not sensitive to inhibition by the polyamine. This property appears to be in keeping with the ability of Sso Topo III to relax negatively supercoiled DNA to completion. It is worth noting that several Sulfolobus strains have been shown to contain high cellular levels of polyamines, including spermidine (15). Therefore, the insensitivity of Sso Topo III to spermidine may be of physiological relevance.
Type IA DNA topoisomerases are omnipresent, and different type IA enzymes may serve distinct functions. E. coli possesses two type IA DNA topoisomerases, topoisomerases I and III. E. coli DNA topoisomerase I has been known to play a role in the regulation of DNA supercoiling. Recently, both E. coli DNA topoisomerases I and III were speculated to be involved in chromosomal decatenation and recombination (31, 37). In Sulfolobus, the genomic DNA is believed to be relaxed or positively supercoiled (19) and bound by small, abundant DNA binding proteins that are capable of constraining DNA in negative supercoils (20, 21, 32). Therefore, the naked region of DNA in the genome, if present, is presumably positively supercoiled. This raises a question about how the two type IA DNA topoisomerases may function in the regulation of the superhelical density of chromosomal DNA in these organisms, since both enzymes only relax negatively supercoiled DNA. It is known that local and transient changes in DNA supercoiling occur constantly in the cell (18). Domains of negatively supercoiled DNA may result from DNA-tracking activities, such as transcription. Both of the type IA topoisomerases are potentially capable of relaxing local negative DNA supercoils, thereby regulating the supercoiling state of the DNA in the cell. However, given its catalytic properties, especially the ability to induce positive supercoils into DNA, reverse gyrase appears to be better suited for this role. If so, what might be the in vivo function of Sulfolobus DNA topoisomerase III? An attractive possibility is that Sso Topo III plays a role in chromosomal segregation and recombination, as suggested for E. coli DNA topoisomerase III. Studies are presently under way to investigate this possibility.
Acknowledgments
This study was supported by grants from the National Science Foundation of China (39925001 and 30030010), the Chinese Academy of Sciences (KSCX2-SW-112 and KSCX2-3-01-02), and the Ministry of Science and Technology of China (2001AA214141) to L.H.
REFERENCES
- 1.Ahumada, A., and Y. C. Tse-Dinh. 2002. The role of the Zn(II) binding domain in the mechanism of E. coli DNA topoisomerase I. BMC Biochem. 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergerat, A., B. de Massy, D. Gadelle, P. C. Varoutas, A. Nicolas, and P. Forterre. 1997. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature 386:414-417. [DOI] [PubMed] [Google Scholar]
- 3.Bhaduri, T., T. K. Bagui, D. Sikder, and V. Nagaraja. 1998. DNA topoisomerase I from Mycobacterium smegmatis. An enzyme with distinct features. J. Biol. Chem. 273:13925-13932. [DOI] [PubMed] [Google Scholar]
- 4.Bouthier de la Tour, C., C. Portemer, H. Kaltoum, and M. Duguet. 1998. Reverse gyrase from the hyperthermophilic bacterium Thermotoga maritima: properties and gene structure. J. Bacteriol. 180:274-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champoux, J. J. 2001. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70:369-413. [DOI] [PubMed] [Google Scholar]
- 6.Chen, S. J., and J. C. Wang. 1998. Identification of active site residues in Escherichia coli DNA topoisomerase I. J. Biol. Chem. 273:6050-6056. [DOI] [PubMed] [Google Scholar]
- 7.Clark, D. J., and G. Felsenfeld. 1991. Formation of nucleosomes on positively supercoiled DNA. EMBO J. 10:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Declais, A. C., C. Bouthier de la Tour, and M. Duguet. 2001. Reverse gyrases from bacteria and archaea. Methods Enzymol. 334:146-162. [DOI] [PubMed] [Google Scholar]
- 9.Domanico, P. L., and Y. C. Tse-Dinh. 1991. Mechanistic studies on E. coli DNA topoisomerase I: divalent ion effects. J. Inorg. Biochem. 42:87-96. [DOI] [PubMed] [Google Scholar]
- 10.Gadelle, D., J. Filee, C. Buhler, and P. Forterre. 2003. Phylogenomics of type II DNA topoisomerases. Bioessays 25:232-242. [DOI] [PubMed] [Google Scholar]
- 11.Goulaouic, H., T. Roulon, O. Flamand, L. Grondard, F. Lavelle, and J. F. Riou. 1999. Purification and characterization of human DNA topoisomerase IIIα. Nucleic Acids Res. 27:2443-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grishin, N. V. 2000. C-terminal domains of Escherichia coli topoisomerase I belong to the zinc-ribbon superfamily. J. Mol. Biol. 299:1165-1177. [DOI] [PubMed] [Google Scholar]
- 13.Jaxel, C., M. Duguet, and M. Nadal. 1999. Analysis of DNA cleavage by reverse gyrase from Sulfolobus shibatae B12. Eur. J. Biochem. 260:103-111. [DOI] [PubMed] [Google Scholar]
- 14.Kim, R. A., and J. C. Wang. 1992. Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. J. Biol. Chem. 267:17178-17185. [PubMed] [Google Scholar]
- 15.Kneifel, H., K. O. Stetter, J. R. Andreesen, J. Wiegel, and S. M. Schoberth. 1986. Distribution of polyamines in representative species of archaebacteria. Syst. Appl. Microbiol. 7:241-245. [Google Scholar]
- 16.Kung, V. T., and J. C. Wang. 1977. Purification and characterization of an omega protein from Micrococcus luteus. J. Biol. Chem. 252:5398-5402. [PubMed] [Google Scholar]
- 17.Lima, C. D., J. C. Wang, and A. Mondragon. 1994. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 367:138-146. [DOI] [PubMed] [Google Scholar]
- 18.Liu, L. F., and J. C. Wang. 1987. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. USA 84:7024-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Garcia, P., and P. Forterre. 1997. DNA topology in hyperthermophilic archaea: reference states and their variation with growth phase, growth temperature, and temperature stresses. Mol. Microbiol. 23:1267-1279. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Garcia, P., S. Knapp, R. Ladenstein, and P. Forterre. 1998. In vitro DNA binding of the archaeal protein Sso7d induces negative supercoiling at temperatures typical for thermophilic growth. Nucleic Acids Res. 26:2322-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai, V. Q., X. Chen, R. Hong, and L. Huang. 1998. Small abundant DNA binding proteins from the thermophilic archaeon Sulfolobus shibatae constrains negative DNA supercoils. J. Bacteriol. 180:2560-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuman, S., and J. Prescott. 1990. Specific DNA cleavage and binding by vaccinia virus DNA topoisomerase I. J. Biol. Chem. 265:17826-17836. [PubMed] [Google Scholar]
- 23.Sikder, D., and V. Nagaraja. 2000. Determination of the recognition sequence of Mycobacterium smegmatis topoisomerase I on mycobacterial genomic sequences. Nucleic Acids Res. 28:1830-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivenugopal, K. S., D. Lockshon, and D. R. Morris. 1984. Escherichia coli DNA topoisomerase III: purification and characterization of a new type I enzyme. Biochemistry 23:1899-1906. [DOI] [PubMed] [Google Scholar]
- 25.Srivenugopal, K. S., and D. R. Morris. 1985. Differential modulation by spermidine of reactions catalyzed by type 1 prokaryotic and eukaryotic topoisomerases. Biochemistry 24:4766-4771. [DOI] [PubMed] [Google Scholar]
- 26.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tse, Y. C., K. Kirkegaard, and J. C. Wang. 1980. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J. Biol. Chem. 255:5560-5565. [PubMed] [Google Scholar]
- 28.Tse-Dinh, Y. C., and R. K. Beran-Steed. 1988. Escherichia coli DNA topoisomerase I is a zinc metalloprotein with three repetitive zinc-binding domains. J. Biol. Chem. 263:15857-15859. [PubMed] [Google Scholar]
- 29.Viard, T., V. Lamour, M. Duguet, and C. Bouthier de la Tour. 2001. Hyperthermophilic topoisomerase I from Thermotoga maritima. A very efficient enzyme that functions independently of zinc binding. J. Biol. Chem. 276:46495-46503. [DOI] [PubMed] [Google Scholar]
- 30.Wang, J. C. 1996. DNA topoisomerases. Annu. Rev. Biochem. 65:635-692. [DOI] [PubMed] [Google Scholar]
- 31.Wang, J. C. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3:430-440. [DOI] [PubMed] [Google Scholar]
- 32.Xue, H., R. Guo, Y. Wen, D. Liu, and L. Huang. 2000. An abundant DNA binding protein from the hyperthermophilic archaeon Sulfolobus shibatae affects DNA supercoiling in a temperature-dependent fashion. J. Bacteriol. 182:3929-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, H. L., and R. J. DiGate. 1994. The carboxyl-terminal residues of Escherichia coli DNA topoisomerase III are involved in substrate binding. J. Biol. Chem. 269:9052-9059. [PubMed] [Google Scholar]
- 34.Zhang, H. L., S. Malpure, and R. J. DiGate. 1995. Escherichia coli DNA topoisomerase III is a site-specific DNA binding protein that binds asymmetrically to its cleavage site. J. Biol. Chem. 270:23700-23705. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, H. L., S. Malpure, Z. Li, H. Hiasa, and R. J. DiGate. 1996. The role of the carboxyl-terminal amino acid residues in Escherichia coli DNA topoisomerase III-mediated catalysis. J. Biol. Chem. 271:9039-9045. [DOI] [PubMed] [Google Scholar]
- 36.Zhu, C. X., C. J. Roche, and Y. C. Tse-Dinh. 1997. Effect of Mg(II) binding on the structure and activity of Escherichia coli DNA topoisomerase I. J. Biol. Chem. 272:16206-16210. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, Q., P. Pongpech, and R. J. DiGate. 2001. Type I topoisomerase activity is required for proper chromosomal segregation in Escherichia coli. Proc. Natl. Acad. Sci. USA 98:9766-9771. [DOI] [PMC free article] [PubMed] [Google Scholar]