Abstract
Introduction
Targeted delivery of thrombolytics to the site of occlusion is an attractive concept, with implications for the treatment of many thrombo-occlusive diseases. Ultrasound enhances thrombolysis, which can be augmented by the addition of a contrast agent. We have previously reported development of echogenic liposomes (ELIP) for targeted highlighting of structures with potential for drug and gene delivery. This study evaluated the potential of ELIP for thrombolytic loading, and the effect of ultrasound exposure of thrombolytic-loaded ELIP on thrombolytic efficacy.
Materials and methods
Tissue-plasminogen activator (tPA) was loaded into ELIP. Echogenicity was assessed and reported as mean grayscale values. Whole porcine clots were treated with plasma, free tPA, tPA+Optison® (echocontrast agent), or tPA-loaded ELIP, with and without ultrasound (1 MHz, continuous wave, 2 W/cm2, for 2 min). Clots were weighed before and after a 30-min treatment period, and results reported as percent clot mass loss.
Results
tPA entrapment into ELIP was feasible with 50% entrapment, and retention of echogenicity. Treatment with tPA-loaded ELIP resulted in effective clot lysis with an effect similar to treatment with free tPA. Ultrasound exposure of tPA-loaded ELIP resulted in enhanced thrombolysis (49.5% relative improvement vs. no ultrasound). Much of the ultrasound effect appeared to be related to drug release from the tPA—ELIP complex.
Conclusions
We have demonstrated entrapment of tPA into ELIP with effective clot lysis and drug release using ultrasound. Our tPA-loaded ELIP has potential for specific highlighting of clots to confirm agent delivery and help focus ultrasound therapy for targeted ultrasound-facilitated thrombolysis.
Keywords: Ultrasound, Thrombolysis, Echogenic, Liposomes, Contrast agent
The acute manifestations of myocardial infarction and stroke often relate to the rupture of an unstable plaque with platelet activation and thrombus formation [1]. The use of thrombolytic agents for the treatment of acute ischemic and neurologic events is limited in many patients by the potential nonspecific activation of plasminogen at sites other than the occluded vessel. Targeted delivery of a thrombolytic agent to the site of occlusion is an attractive concept, with implications for the treatment of many thrombo-occlusive diseases.
Recent clinical studies have demonstrated improved thrombolysis with the concomitant use of focused ultrasound and a thrombolytic agent for stroke and acute myocardial infarction [2—4]. Suggested mechanisms include acoustic streaming which promotes transport of drugs into the thrombus, radiation force which might facilitate reformation and opening of the fibrin matrix enhancing drug diffusion, cleaving of fibrin polymers to extend the surface for drug interaction, and direct effects on binding of the agent to fibrin [5]. Ultrasound can also generate cavitation, which can cause large molecules and particles to penetrate cells (sonoporation) [6,7]; this property is actively being investigated for drug and gene delivery [8—11]. Addition of a contrast agent as a cavitation nucleation agent can lower the threshold for these ultrasound bio-effects [6,12—14].
We have previously reported development of echogenic liposomes (ELIP) for the targeted highlighting of structures [15,16] with potential for drug and gene delivery [17—19]. This study aimed to explore the potential of ELIP for thrombolytic loading, and to evaluate the effect of directed ultrasound exposure of the thrombolytic-loaded ELIP on effecting drug release and promoting thrombolysis. Development of such methodology would represent a novel therapeutic strategy for the local delivery of thrombolytic agents for acute myocardial infarction and stroke.
Materials and methods
Liposome formulation
ELIP were prepared by the sonication—lyophilization—rehydration method as described previously [20]. Liposomal composition used was DPPC/DOPC/ DPPG/Chol in a 46:24:24:6 molar ratio (DPPC=dipalmitoylphosphatidylcholine; DOPC=dioleoylphos-phatidylcholine; DPPG = dipalmitoylphosphatidyl-glycerol; Chol=Cholesterol). The component lipids were dissolved in chloroform and the solvent was allowed to evaporate completely. The resulting lipid film was placed under vacuum for full removal of the solvent and then hydrated with distilled, deionized water. This dispersion was sonicated for 5 min. 0.2 M D-mannitol was added to the liposome suspension and the sample was frozen at −70 °C. The samples were lyophilized for 48 h and resuspended with 0.1 M phosphate-buffered saline (PBS). The final concentration used for dilution was 10 mg lipid/ml PBS.
Drug incorporation
Recombinant tissue-plasminogen activator (tPA) (Activase®, Genentech Inc., San Francisco, CA) was used as the thrombolytic agent. For the drug-loaded ELIP preparation, 1 mg/ml tPA solution was used for the initial rehydration of the lipid film. Entrapped tPA was separated from the free tPA by centrifugation at 16,000 rpm for 10 min at 37 °C. As used by Heeremans et al. [21], “entrapped” tPA in this study refers to both the tPA associated with the lipid bilayer, as well as full encapsulation of the drug within the liposomal aqueous phase. A tPA chromogenic substrate (Sigma, St. Louis, MO) was used to evaluate tPA activity and quantitate drug loading into the ELIP. Briefly, 10 μl of resuspended ELIP were added to 970 μl assay buffer containing 30 mM Tris—HCl (pH 8.4) and 130 nM NaCl, in a cuvette. 50 μl of 4 mM chromogenic substrate solution (Sigma, St. Louis, MO) was then added to the ELIP-assay buffer mixture. Absorbance at 405 nm was measured using a spectrophotometer within 5 min of adding the substrate. Thrombolytic activity was measured before centrifugation ( Fc), as well as before ( Fb) and after ( Fa) the addition of Triton X-100 (a detergent). Total percent tPA entrapment is defined as Fa/Fc ×100%, and percent tPA encapsulation is defined as ( Fa × Fb)/ Fa × 100%.
Echogenicity analysis
The liposomes were imaged with a 20 MHz intravascular ultrasound catheter (SciMed, Inc., Sunnyvale, CA) in a 10-mm inner diameter glass vial, and a 3.5 MHz harmonic transthoracic probe (Acuson, Mountain View, CA) in an anechoic imaging well. Relative echogenicity (apparent brightness) of the liposome formulations was objectively assessed using computer-assisted videodensitometry. This process involves image acquisition, digitization, and grayscale quantification. All image processing and analyses were performed with Image Pro Plus Software (Ver. 1.0, Media Cybernetics, Silver Spring, MD). Images were digitized to 6403480-pixel spatial resolution (approximately 0.045 mm/pixel) and 8-bit (256 level) amplitude resolution. Data are reported as mean grayscale values.
In vitro clot formation
Whole porcine blood clots were used to evaluate thrombolysis [22]. Blood was obtained from non-heparinized Yucatan miniswine. The miniswine were 6—8 weeks old and weighed 28.5 ± 6.5 lb. Baseline blood analysis data, including complete blood count, prothrombin time, activated partial thromboplastin time, D-dimer, and fibrinogen were obtained to ensure normal clotting parameters. Whole blood clots were made by aliquoting 1.5 ml fresh porcine blood into 1.3-cm inner diameter vacutainer tubes; the tubes were incubated in a 37 °C water bath for 3 h. The clots were then stored at 4 °C until use, which ensured complete clot maturation and retraction. This type of clot is fairly similar to physiologic venous clots. Most miniswine used in this study were slightly anemic (hematocrit of 27.8 ± 3.0%). Only donors with values of <250 ng/ ml for the D-dimer test, <15 s for prothrombin time, <18 s for activated partial thromboplastin time, and <300 mg/dl fibrinogen concentration were considered acceptable. The resulting clots were dark red, cylindrical, and weighed 0.47 ± 0.11 g. All unused clots were discarded after 2 weeks.
Thrombolysis studies
Whole porcine blood clots were blotted gently and weighed using an analytical balance. Each clot was placed in an acoustically transparent finger cot from a latex rubber glove containing 10 ml of freshly frozen porcine plasma (Animal Technologies, Inc., Tyler, TX), and placed in a holder within a 37 °C water bath. Ten units of human plasminogen (EMD Biosciences, Inc., La Jolla, CA) were added to each clot holder. Clots were treated with plasma alone (control), ELIP, tPA, or tPA-loaded ELIP. After 30 min, clots were taken out of the holder, blotted gently, and re-weighed. Triton X-100 was added to clots treated with tPA-loaded ELIP to investigate the effect of liposome destruction and total drug release from the tPA—ELIP complex. An echocontrast agent, Optison® (Mallinckrodt Inc., St. Louis, MO), which has been approved for clinical use in echocardiography, was also used to compare the effect of a different contrast agent on ultrasound-facilitated thrombolysis. Data are reported as percent clot mass loss (defined as the difference between the two clot weights obtained divided by the baseline clot weight).
Ultrasound treatment
Porcine clots were treated with 1 MHz continuous wave (CW) ultrasound with an intensity of 2 W/cm2, for 2 min (Sonitron 1000; Rich-Mar). The calculated peak negative acoustic pressure output was 0.12 MPa. Ultrasound was delivered in a water bath from a planar, non-focused, 1 MHz transducer (with an aperture of 1 cm), placed directly next to the finger cot sample holder.
Statistics
Results are reported as mean ± S.D. (n =5). Multiple groups were compared using the analysis of variance (ANOVA) with pairwise multiple comparison performed using the Student—Newman—Keuls method. A p-value of less than 0.05 was considered significant. All analyses were performed using Sigma Stat software.
Results
Entrapment of tPA into ELIP was feasible. A maximum of 200 ± 16 μg of tPA could be loaded per 8.2 ± 0.6 mg of liposomal lipid. Entrapment efficiency was 50%. Of the 50% total tPA entrapped, 15% are fully encapsulated within the ELIP, whereas 35% are associated with the lipid bilayer. The tPA-loaded ELIP were echogenic, with a mean grayscale value of 155 ± 27 (Fig. 1; p <0.001 vs. PBS). However, echogenicity was lower when compared to unloaded ELIP ( p <0.01). Nevertheless, these tPA—ELIP preparations are highly echogenic on ultrasound imaging, both at 20 MHz and 3.5 MHz, as demonstrated in Fig. 1.
Figure 1.
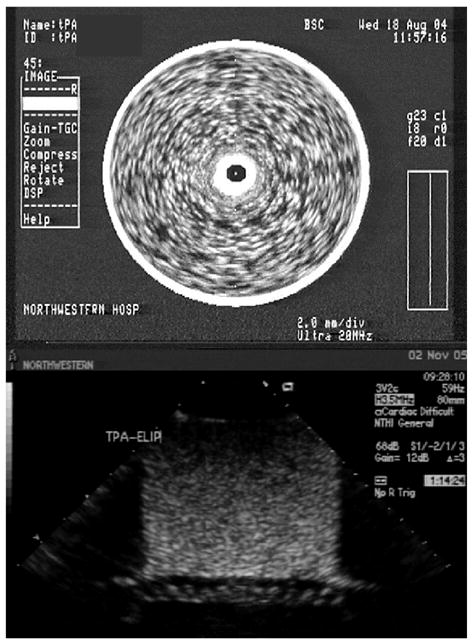
Top: Image of tPA-loaded ELIP in a glass vial imaged with a 20 MHz intravascular ultrasound catheter (25× dilution). The central dark spot corresponds to the imaging catheter. Bottom: Image of tPA-loaded ELIP in an imaging well imaged with a 3.5 MHz harmonic transthoracic probe (100× dilution). The artifact at the bottom of the image corresponds to the Rhocee rubber used as sound-absorbing material.
Fig. 2 illustrates the dose—response curve for the amount of free tPA used and the clot mass loss obtained. Increasing tPA dose resulted in increasing thrombolysis. For all subsequent experiments, 200 μg of tPA per clot experiment was chosen as the benchmark dose.
Figure 2.
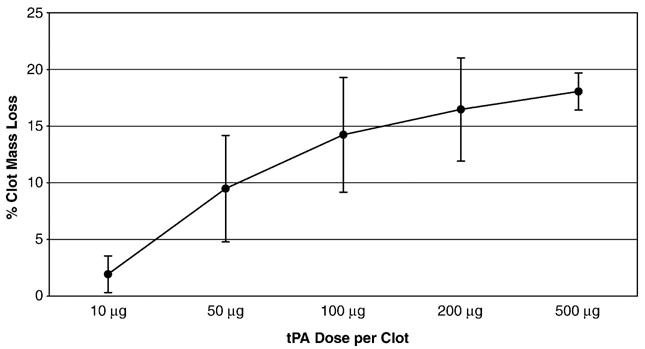
Dose—response graph of amount of free tPA used per clot experiment vs. percent clot mass loss (mean ± S.D., n =5).
For each clot experiment, a total of 200 μg of tPA, 8.2 mg of liposomal lipid, and 1 ml of diluted Optison® were used. The tPA—ELIP complex resulted in effective thrombolysis (Fig. 3), with an effect that is similar to treatment with free tPA ( p > 0.05). There was no significant thrombolysis when unloaded ELIP or Optison® were used without tPA ( p > 0.05 vs. Control). There was enhanced thrombolysis when a 2-min exposure to 1 MHz CW ultrasound was added to the 30-min treatment protocol, but only when tPA-loaded ELIP were present ( p =0.02 vs. no ultrasound; Fig. 3). There was no significant augmentation of thrombolysis when clots were exposed to ultrasound with free tPA or even when Optison® was added to free tPA ( p > 0.05 vs. no ultrasound, Fig. 3). There was a 49.5% relative improvement in tPA-loaded ELIP thrombolytic effect when ultrasound exposure was added to the treatment protocol.
Figure 3.
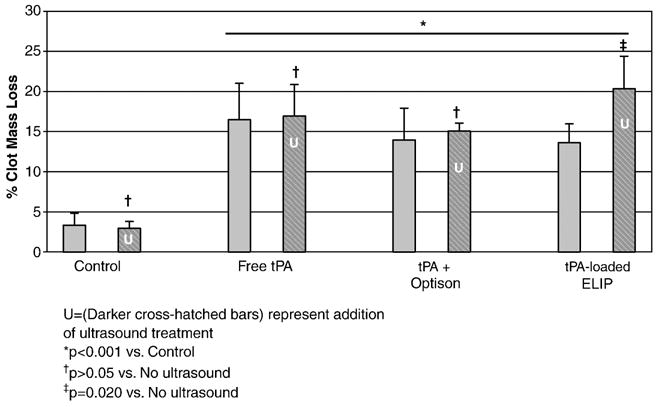
Thrombolytic effect of various treatments compared to plasma (Control) with the concomitant use of ultrasound (mean ± S.D., n =5).
Much of the improvement in ultrasound-facilitated thrombolytic effect seen with the tPA-loaded ELIP appears to be related to drug release from the ELIP. The resultant thrombolysis measured when the detergent Triton X-100 was added to tPA-loaded ELIP treatment (to effect liposome destruction and hence, drug release) was lower although not statistically different from the treatment using tPA-loaded ELIP plus 2-min ultrasound exposure ( p =0.211 vs. tPA—ELIP plus ultrasound; Fig. 4).
Figure 4.
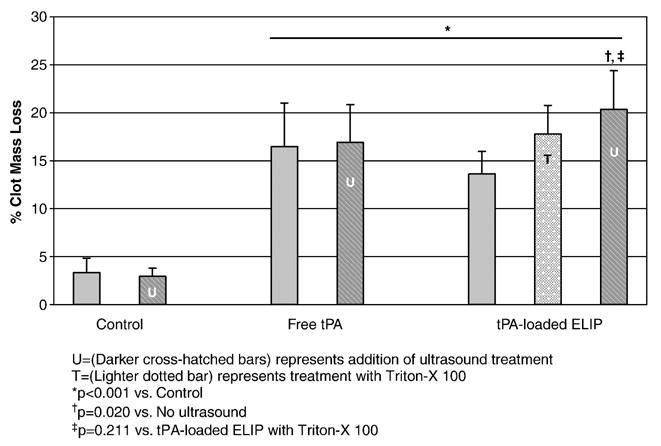
Effect of addition of Triton X-100 (a detergent) on drug release from tPA-loaded ELIP (mean ± S.D., n =5).
Discussion
This study demonstrates ultrasound-facilitated thrombolysis using a novel thrombolytic—contrast agent complex. The novel concept presented involves entrapment of the thrombolytic drug in an acoustically-active agent with demonstration of drug release and enhanced drug effect by ultrasound treatment. This enhanced thrombolytic effect, in the presence of tPA-loaded ELIP, was seen even after only a brief 2-min exposure to 1 MHz CW ultrasound.
Ultrasound has been demonstrated to enhance thrombolysis when used in conjunction with a thrombolytic agent both in vitro and in vivo [23—27]. Ultrasound delivered intravascularly through miniaturized transducers attached to a catheter has demonstrated effective and safe thrombolysis [28]. However, limitations include size of the device such that only proximal vessels can be treated, concern for distal embolization of large thrombus fragments, augmented permeability of distal ischemic vessels that may permit blood extravasation following recanalization in ischemic strokes, and the need for specialized facilities for selective arterial catheterization [29].
The noninvasive use of ultrasound to facilitate clot lysis with tPA was first demonstrated by Kudo and co-workers in 1989 [30]. In a canine model, they demonstrated that application of transcutaneous ultrasound near the site of an occluded femoral artery, when used in conjunction with tPA infusion, resulted in an 80% decrease in time for recanalization [30]. Similarly, in canine models of acute coronary occlusions, transcutaneous ultrasound augmented the efficacy of tPA-facilitated thrombolysis, regardless of whether the anterior or posterior coronary circulations were involved [31,32]. For the clinical application of noninvasive ultrasound therapy, it may be advantageous to determine the exact location of the clot in order to selectively insonify the area of interest, thus limiting potential harmful bioeffects to surrounding tissues.
We have reported a novel technique to generate echogenic liposomes, which can be targeted to specific atheroma components [33]. We have demonstrated specific highlighting of atheroma with intraarterial injection of anti-intercellular adhesion molecule-1-conjugated ELIP [15], as well as targeted highlighting of a left ventricular thrombus with intravenous injection of anti-fibrinogen-conjugated ELIP [16]. We have also reported entrapment of an antibiotic and of reporter genes into these ELIP with demonstration of microbial growth inhibition and gene transfection, respectively, in cultured cells [17,18]. We have reported enhanced gene uptake and transfection with the addition of ultrasound treatment to the gene-bearing ELIP [19]. This study represents our first report of ultrasound-facilitated drug effect using our novel drug-loaded ELIP complex.
Ultrasound-triggered microbubble destruction has been investigated for targeted protein and gene delivery by other investigators [34,35]. The novel concept introduced in this study is the addition of another component to this technique, i.e., the use of a contrast agent as the drug delivery vehicle, with potential for highlighting the target site during drug delivery. Our novel tPA-loaded ELIP area echogenic, and can serve as both a contrast agent to highlight the clot and an ultrasound-releasable thrombolytic delivery agent. Hence, potentially, diagnostic ultrasound can be used to monitor and confirm attachment of the tPA-loaded ELIP to the clot, followed by therapeutic ultrasound pulses to trigger drug delivery.
Ultrasound-mediated thrombolysis may be enhanced by the addition of a contrast agent as cavitation nuclei [36—38]. It is hypothesized that the contrast agent adheres to the clot with resultant shearing effect during bubble destruction [13]. This mechanical erosion of the clot allows more fibrin to be exposed to the lytic agent [13]. The ultrasound-mediated effect improves with longer insonification times and/or use of fresh clots [25,39]. In this study, we chose to use older clots and shorter treatment times to provide more rigorous conditions to demonstrate lytic effect and proof-of-principle regarding the potential of these tPA-loaded ELIP as an ultrasound-releasable drug-delivery/contrast agent. Under these conditions (and the ultrasound parameters chosen), there was no significant enhancement in clot lysis by ultrasound either tPA alone or tPA plus Optison was used.
We hypothesize that much of the ultrasound effect observed in this study was probably due to release of the tPA from the liposomes. However, clot lysis with the use of Triton X-100 to effect liposome destruction was lower (albeit not statistically different) than the clot lysis observed with ultrasound plus tPA-loaded ELIP. This suggests that there may be other ultrasound bioeffects, which could have contributed to the additional clot lysis seen. Devcic-Kuhar et al. showed that ultrasound promoted the penetration of tPA into thrombi hence broadening the zone of lysis [40], while Braaten et al. showed that ultrasound exposure causes reversible disaggregation of uncrosslinked fibrin fibers which may create additional binding sites for fibrinolysis [41]. Perhaps, longer insonification times, or the use of fresh clots would have resulted in even greater ultrasound-mediated thrombolysis through these other bioeffects. Nevertheless, the ultrasound treatment used in this study appeared to be sufficient to effect drug release from the tPA—ELIP complex.
A limitation of this study is the absence of in vivo experiments. This study was designed to provide a proof-of-principle, and lays the groundwork for future animal experiments. We also did not measure fibrin-degradation products in the supernatant. Other investigators have demonstrated good correlation of fibrin-degradation products and percent clot mass loss as outcome endpoints to quantify the amount of thrombolysis [42]. The lowest effective tPA-loaded ELIP dose that results in effective thrombolysis in vivo as well as the ultrasound parameters that are most efficacious for enhanced thrombolysis have yet to be established.
In summary, we have demonstrated entrapment of tPA into a novel contrast agent (i.e., ELIP), with effective clot lysis and drug release using relatively short ultrasound treatment times. Our tPA-loaded ELIP are echogenic, with potential for specific highlighting of the clot to confirm agent delivery and perhaps help focus ultrasound therapy. This study extends the field of ultrasound-facilitated thrombolysis and provides the first report of a tPA-entrapped contrast agent complex with potential for targeted ultrasound-releasable thrombolytic delivery.
Acknowledgments
We would like to acknowledge the invaluable help of Dr. Robert MacDonald for his expertise in liposome development; Bonnie Kane and Janet Martinez for blood draws from our animal models; and Devang Parikh and Kyle Buchanan for imaging of the liposomes. We would also like to acknowledge Sampada Vaidya at the University of Cincinnati for assistance with developing the whole blood porcine clot model.
Abbreviations
- tPA
tissue-plasminogen activator
- ELIP
echogenic liposomes
Footnotes
Supported in part by the National Institutes of Health RO1 HL-059786, HL-74002, and NS-47603, and the Feinberg Cardiovascular Research Institute, Chicago, IL.
Presented in part at the American Heart Association Annual Scientific Sessions, Dallas, TX, November 13—16, 2005.
References
- 1.Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Circulation. 1985;71:669–708. doi: 10.1161/01.cir.71.4.699. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 3.Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MG, Tuero E, Bluguermann J, Kevorkian R, Berrocal O, Carlevaro O, et al. Transcutaneous ultrasound-facilitated coronary thrombolysis during acute myocardial infarction. Am J Cardiol. 2003;92:454–7. doi: 10.1016/s0002-9149(03)00666-0. [DOI] [PubMed] [Google Scholar]
- 5.Daffertshofer M, Hennerici M. Ultrasound in the treatment of ischaemic stroke. Lancet Neurol. 2003;2(5):283–90. doi: 10.1016/s1474-4422(03)00380-6. [DOI] [PubMed] [Google Scholar]
- 6.Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–54. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 7.Koch S, Phol P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther. 1996;7:1339–46. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–9. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana K, Tachibana S. The use of ultrasound for drug delivery. Echocardiography. 2001;18:323–8. doi: 10.1046/j.1540-8175.2001.00323.x. [DOI] [PubMed] [Google Scholar]
- 11.Saad AH, Hahn GM. Ultrasound-enhanced effects of adriamycin against murine tumors. Ultrasound Med Biol. 1992;18:715–23. doi: 10.1016/0301-5629(92)90122-q. [DOI] [PubMed] [Google Scholar]
- 12.Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Green-leaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol. 1998;24:587–95. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li S, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug: recanalization of thrombosed iliofe-moral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–4. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 14.Ward M, Wu J, Chiu JF. Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J Acoust Soc Am. 1999;105:2951–7. doi: 10.1121/1.426908. [DOI] [PubMed] [Google Scholar]
- 15.Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj R, Greene R, et al. In-vivo targeting of acoustically reflective liposomes for intravascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33:867–75. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton A, Huang SL, Warnick D, Stein A, Rabbat M, Madhav T, et al. Left ventricular thrombus enhancement following intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002;105:2772–8. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- 17.Tiukinhoy S, Khan A, Huang S, Klegerman M, MacDonald R, McPherson D. Novel echogenic drug-immunoliposomes for drug delivery. Invest Radiol. 2004;39:104–10. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- 18.Tiukinhoy S, Mahowald M, Shively V, Nagaraj A, Kane B, Klegerman M, et al. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol. 2000;12:732–8. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Huang SL, McPherson DD, MacDonald RC. Ultrasound in conjunction with an ultrasonic-reflective transfection agent enhances gene delivery to cells. [abstr] J Am Coll Cardiol. 2002;39:228A. [Google Scholar]
- 20.Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman DD, McPherson DD, et al. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90(12):1917–26. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- 21.Heeremans JL, Gerritsen HR, Meusen SP, Mijnheer FW, Gangaram Panday RS, Prevost R, et al. The preparation of tissue-type plasminogen activator (tPA) containing liposomes: entrapment efficiency and ultracentrifugation damage. J Drug Target. 1995;3:301–10. doi: 10.3109/10611869509015959. [DOI] [PubMed] [Google Scholar]
- 22.Holland CK, Vaidya SS, Coussios CC, Shaw GJ. Thrombolytic effects of 120 kHz and 1 MHz ultrasound and tissue plasminogen activator on porcine whole blood clots. J Acoust Soc Am. 2002;112:2370. [Google Scholar]
- 23.Tachibana K. Enhancement of fibrinolysis with ultrasound energy. J Vasc Interv Radiol. 1992;3:299–303. doi: 10.1016/s1051-0443(92)72029-6. [DOI] [PubMed] [Google Scholar]
- 24.Behrens S, Spengos K, Daffertshofer M, Schroeck H, Dempfle CE, Hennerici M. Transcranial ultrasound-improved thrombolysis: diagnostic vs. therapeutic ultrasound. Ultrasound Med Biol. 2001;27:1683–9. doi: 10.1016/s0301-5629(01)00481-1. [DOI] [PubMed] [Google Scholar]
- 25.Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–64. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- 26.Olsson SB, Johansson B, Nilsson AM, Olsson C, Rouer A. Enhancement of thrombolysis by ultrasound. Ultrasound Med Biol. 1994;20:375–82. doi: 10.1016/0301-5629(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Nishioka T, Fishbein MC, Cercek B, Forrester JS, Kim CJ, et al. Myocardial ischemia/infarction/thrombolysis: transcutaneous ultrasound augments lysis of arterial thrombi in vivo. Circulation. 1996;94:775–8. doi: 10.1161/01.cir.94.4.775. [DOI] [PubMed] [Google Scholar]
- 28.Hamm CW, Steffen W, Terres W, de Scheerder I, Reimers J, Cumberland D, et al. Intravascular therapeutic ultrasound thrombolysis in acute myocardial infarctions. Circulation. 1997;80:200–4. doi: 10.1016/s0002-9149(97)00318-4. [DOI] [PubMed] [Google Scholar]
- 29.Mahon BR, Nesbit GM, Barnwell SL, Clark W, Marotta TR, Weill A, et al. North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke. Am J Neuroradiol. 2003;24:534–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo S, Furuhata H, Hara M, Maie K, Hamano K, Okamura T. Noninvasive thrombolysis with ultrasound. [abstr] Circulation. 1989;80(Suppl I):I–345. [Google Scholar]
- 31.Siegel RJ, Atar S, Fishbein MC, Brasch AV, Peterson TM, Nagai T, et al. Noninvasive, transthoracic, low-frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction. Circulation. 2000;101(17):2026–9. doi: 10.1161/01.cir.101.17.2026. [DOI] [PubMed] [Google Scholar]
- 32.Jeon DS, Luo H, Fishbein MC, Miyamoto T, Horzewski M, Iwami T, et al. Noninvasive transcutaneous ultrasound augments thrombolysis in the left circumflex artery — an in vivo canine study. Thromb Res. 2003;110:149–58. doi: 10.1016/s0049-3848(03)00335-9. [DOI] [PubMed] [Google Scholar]
- 33.Alkan-Onyuksel H, Murer SE, Lanza GM, Vonesh MJ, Klegerman ME, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996;85:486–90. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 34.Bekeredjian R, Chen S, Grayburn PA, Shohet RV. Augmentation of cardiac protein delivery using ultrasound targeted microbubble destruction. Ultrasound Med Biol. 2005;31(5):687–91. doi: 10.1016/j.ultrasmedbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Pislaru SV, Pislaru C, Kinnick RR, Singh R, Gulati R, Greenleaf JF, et al. Optimization of ultrasound-mediated gene transfer: comparison of contrast agents and ultrasound modalities. Eur Heart J. 2003;24:1690–8. doi: 10.1016/s0195-668x(03)00469-x. [DOI] [PubMed] [Google Scholar]
- 36.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–50. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 37.Porter TR, Le Veen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J. 1996;132:964–8. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka T, Luo H, Fishbein MC, Cercek B, Forrester JS, Kim CJ, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: an in vitro and in vivo study. J Am Coll Cardiol. 1997;30(2):561–8. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- 39.Luo H, Steffen W, Cercek B, Arunasalam S, Maurer G, Siegel RJ. Enhancement of thrombolysis by external ultrasound. Am Heart J. 1993;125(6):1564–9. doi: 10.1016/0002-8703(93)90741-q. [DOI] [PubMed] [Google Scholar]
- 40.Devcic-Kuhar B, Pfaffenberger S, Gherardini L, Mayer C, Groschl M, Kaun C, et al. Ultrasound affects distribution of plasminogen and tissue-type plasminogen activator in whole blood clots in vitro. Thromb Haemost. 2004;92(5):980–5. doi: 10.1160/TH04-02-0119. [DOI] [PubMed] [Google Scholar]
- 41.Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78(3):1063–8. [PubMed] [Google Scholar]
- 42.Kimura M, Iijima S, Kobayashi K, Furuhata H. Evaluation of the thrombolytic effect of tissue type plasminogen activator with ultrasonic irradiation: in vitro experiment involving assay of the fibrin degradation products from the clot. Biol Pharm Bull. 1994;17(1):126–30. doi: 10.1248/bpb.17.126. [DOI] [PubMed] [Google Scholar]


