Abstract
The ever-improving time and space resolution and molecular detection sensitivity of fluorescence microscopy offer unique opportunities to deepen our insights into the function of chemical and biological catalysts. Because single-molecule microscopy allows for counting the turnover events one by one, one can map the distribution of the catalytic activities of different sites in solid heterogeneous catalysts, or one can study time-dependent activity fluctuations of individual sites in enzymes or chemical catalysts. By experimentally monitoring individuals rather than populations, the origin of complex behavior, e.g., in kinetics or in deactivation processes, can be successfully elucidated. Recent progress of temporal and spatial resolution in single-molecule fluorescence microscopy is discussed in light of its impact on catalytic assays. Key concepts are illustrated regarding the use of fluorescent reporters in catalytic reactions. Future challenges comprising the integration of other techniques, such as diffraction, scanning probe, or vibrational methods in single-molecule fluorescence spectroscopy are suggested.
Single-molecule fluorescence spectroscopy (SMFS) has recently developed into a powerful tool for studying biophysical and biochemical phenomena. In studies of enzymatic catalysis, SMFS has revealed that there are large differences between the catalytic activity of individual enzymes within a population (“static disorder”) and that the rate constant (kcat) of an individual enzyme may strongly fluctuate over time (“dynamic disorder”), the latter resulting from conformational changes of the enzyme. The recent application of SMFS to catalysis by solid materials has shown that heterogeneities in kcat also exist between individual catalytic crystals of one powder sample and even between the sites of an individual crystal. In this case, heterogeneity might arise from different chemical environments within the catalyst sample. The observation of heterogeneity in the kcat of those different catalytic systems suggests that the parallel introduction and evolution of SMFS techniques in bio- and chemocatalysis will deepen our insights in almost any type of catalytic conversion. Indeed, the challenge to derive overall kinetics from the contributions of individuals within a population is essentially the same for biological, heterogeneous and even homogeneous systems, as discussed in From Populations to Individuals.
From a technical viewpoint, SMFS requires strongly fluorescent probe molecules. The concepts to use such probes and even the probes themselves can be exchanged freely between heterogeneous, homogeneous, and biocatalysis, as discussed in Probes for SMFS in (Bio)Catalysis. If one wants to map in even more detail the contributions of the individual enzymes or catalytic sites to the overall kinetics, further improvements of the spatial and temporal resolution of SMFS will be required (see Spatial Resolution: Micro- and Nanoscopy and Time Resolution and Dynamics). Finally, to complement the information from SMFS experiments with structural data, an important long-term aim is to integrate SMFS with other in situ techniques (see Integration of Techniques and Perspectives).
From Populations to Individuals
Nonuniformity is an inherent property of almost all catalytic systems. Even if heterogeneities are masked in traditional (bio)-catalyst characterization by averaging over an ensemble of entities, a deeper insight in the origin and nature of nonuniformities is necessary for the design of optimized (bio)catalytic systems.
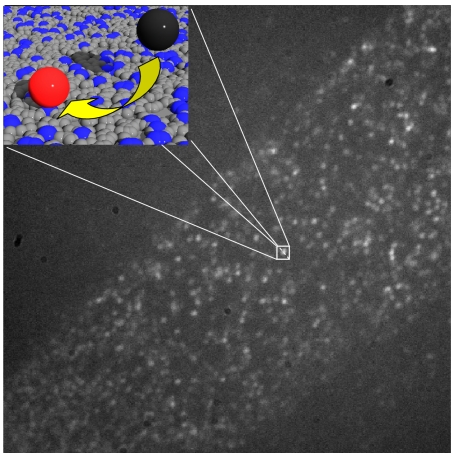
Going down from bulk to the level of individuals has profound implications on the interpretation of the quantitative data. In classical ensemble experiments, a catalytic activity is evaluated by measuring changes in concentrations. When looking at single molecules, turnovers are stochastic events, and kinetics are described by probabilities rather than by concentration changes (1). When activity is monitored at the single-molecule level for a population of identical catalytic sites, the probability of observing a turnover for an individual active site within a short time interval is therefore small (Fig. 1). If one wants to evaluate site diversity, it is therefore necessary to observe not only a sufficiently large number of individual sites but also to observe each individual site over a sufficiently long time.
Fig. 1.
Stochastic nature of turnover events. This wide-field image was recorded during hydrolysis of fluorogenic fluorescein esters on a monolayer containing aminopropyl groups diluted by propyl groups. The amino group density is much higher than the spatial resolution of the fluorescence microscope. However, because each group is only sporadically active, the individual turnover events can be observed as isolated bright spots. (Inset) Density of aminopropyl groups (blue) versus propyl groups (gray) and the conversion of a nonfluorescent substrate (black) into a fluorescent product (red).
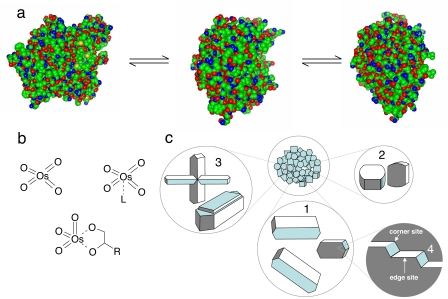
Until now, single-molecule catalytic research has focused mainly on the study of individual enzymes. The results have proven the existence of dynamic disorder, i.e., the fluctuation of activity of an individual enzyme over time, among the individuals of a seemingly homogeneous population (2–4). Dynamic disorder is generally explained as the result of the thermodynamic equilibrium between different conformational states (Fig. 2a). In this case, the ergodic principle holds: the time-averaged activity of one individual is equal to the average activity of the whole enzyme population at a certain point in time. When the disorder is of a static nature, the individuals no longer display the same time-averaged activity. Static disorder in enzymes can have a variety of origins, such as a difference of posttranslational modifications (5), different slowly interchanging conformational substates, or different local environments in immobilized conditions. To account for the disorder inferred from observations of single turnovers, the traditional Michaelis–Menten model needs to be adapted. Therefore an extended two-dimensional Michaelis–Menten model has been proposed by adding a thermodynamic component that describes the interconversion among the several conformational substates of the individual enzymes (6). The thermodynamic and kinetic components of the interconversion may affect each other through memory effects and substrate imprinting (7). With imprinting is meant the conformational change induced by the binding of the substrate that is retained after the enzymatic cycle.
Fig. 2.
Disorder in catalysts. (a) Interconverting enzyme conformations with different activities/selectivities. (b) Some of the Os(VIII) species that can osmylate olefins during asymmetric dihydroxylation. (c) Within a population of crystals, one can distinguish different crystal habitus (1 versus 2), different degrees of intergrowth (3), and on the crystal planes of individual crystals, different sites (4).
In the field of homogeneous chemical catalysis, stereoselectivity and catalyst stability are pursued by designing ligands that strictly define the coordinating atoms and the conformation of the metal complex. Nevertheless, in many cases, the homogeneous catalytic mix contains chemically distinct metal complexes, and each of these subpopulations reacts with different chemo-, regio-, or enantioselectivity. In Wilkinson's mechanism for Rh-catalyzed hydroformylation, HRh(CO)2L2 leads to linear aldehydes, whereas more branched products are formed with HRh(CO)2L (L = triphenylphosphine) (8–10). In Sharpless's ligand-accelerated osmium catalysis, the enantioselectivity is controlled by dynamic ligand association equilibria between OsO4 and OsO4·L species (L = e.g., dihydroquinine) (11, 12) (Fig. 2b). Such activity variations may be caused by dynamic disorder but also by static disorder, for instance, when the ligands of an enantioselective epoxidation catalyst are irreversibly damaged, resulting in a metal species that catalyzes the epoxidation with lower or even no enantiomeric excess (13). Dissolved single metal complexes, if fluorescent, can be studied in fluorescence correlation spectroscopy,¶ or by isolation in femtoliter reactor chambers that contain a discrete number of complexes (0, 1, …) (14). The latter approach was illustrated in the study of inorganic redox catalysis by a discrete number of OsO4 molecules, which catalyze the formation of emissive Ce3+ (15). Alternatively homogeneous catalysts can be observed after isolation on a surface, following the concepts of surface organometallic chemistry (16, 17). Even reversible metal ion complexation dynamics can now be monitored by on/off switching of a fluorescent probe (18), which is an excellent starting point for further SMFS studies in homogeneous catalysis. However, surface immobilization may create additional static disorder.
In heterogeneous catalysts, nonuniformity is intrinsically generated in unit operations such as hydrothermal synthesis, precipitation, catalyst activation, or formulation. In oxides, hydroxides, metals, etc., elemental compositions and structural properties such as porosity or lattice structures vary over different length scales. Both industrially manufactured and research samples of zeolites contain crystals with different dimensions, habitus, and degrees of intergrowth (Fig. 2c). The concept “population” then not only relates to the collection of catalytic crystals but also to the individual active sites within one crystal. High-resolution surface studies, even on single-crystal model catalysts, show that undercoordinated features such as edges, kinks, and defects are preferred reaction sites (19, 20). Even on well defined single crystal surfaces, such as Pt(110) or Pd(100), catalytic reaction induces dynamic reconstructions, resulting in coexistence of different surface structures (21).
Probes for SMFS in (Bio)Catalysis
The potential of an analytical technique in studying catalyst nonuniformity is critically determined by its ability to specifically probe molecular species and to extract information on the local environment. In this section, we will give an overview of the different approaches on how probe molecules can be used to explore bio- as well as chemocatalysts.
SMFS, as such, can easily distinguish between reactants and products of a well chosen catalytic reaction, but the technique is not restricted to merely detecting molecules based on photon flux or intensity. In multiparameter fluorescence detection, other intrinsic chromophore properties can be probed, such as the emission spectrum, the fluorescence lifetime, and the fluorescence anisotropy (22). Although counting of stochastic turnovers is typically based on intensity fluctuations or changes, the other chromophore properties contain supporting information for the physicochemical interpretation of these turnover rates.
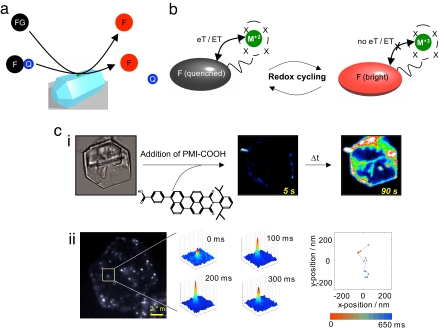
A vast amount of probes that were originally designed for biological research can readily be integrated in different schemes in (bio)catalytic research. First, a fluorogenic substrate can be converted to an emissive product, as in the hydrolysis of fluorescein diacetate to fluorescein. This reaction has been applied to visualizing single turnovers on isolated lipases or to visualizing variations in activity between different facets of inorganic catalytic crystals (23–25). Essentially the same approach has been implemented at the ensemble level for ultrasensitive high-throughput catalyst screening (26, 27). A sufficient bathochromic or hypsochromic shift can also be induced by formation or breakdown of conjugated systems, e.g., in homogeneously catalyzed Heck reactions (26), or in biocatalytic lipid peroxidation (28). Catalytic cleavage of a covalently bound (tethered) quenching group also allows selective detection of a single-product molecule against a background of excess reactant (29) (Fig. 3a).
Fig. 3.
Fluorescence-based visualization of catalytic sites and events. (a) A fluorescent product (F) is formed by transformation of a fluorogenic reactant (FG) or by cleavage of a covalently bound quencher (Q). (b) Cycling of a metal catalyst (M) between two redox states causes quenching and dequenching of a fluorescent reporter (F). (c) Mapping of basic sites on a layered double hydroxide crystal with acid probes: (i) Imaging of time-dependent sorption of a perylene monoimide carboxylic acid. Even at the ensemble level differences between crystal faces can be distinguished (ii). At the single-molecule level, the probe hops between individual basic sites.
Such experiments can be refined beyond simple turnover counting by adding a dimension of chemoselectivity. Substrate regioselectivity can be studied by designing probes in which cleavable and quenched chromophores are incorporated at different loci. Thus, commercial probes for phospholipase activity are available, from which, e.g., bodipy chromophores can be released from the phospholipid backbone by cleavage at the sn-1 or at the sn-2 position (30, 31). Product isomer distributions can be probed locally if the chromophore properties depend on the substitution pattern. For instance, when a monosubstituted aromatic compound is vinylated, o-, m-, and p-isomers of the conjugated product may be formed, each with distinct chromophore properties (lifetimes, spectra, etc.).
In a second widely applicable approach, a fluorescent catalyst is designed in which the fluorescence of a reporter group is switched on and off during each catalytic cycle (Fig. 3b). The early study on cholesterol oxidase by Lu et al. (7) uses the on/off cycle of fluorescent FAD and nonfluorescent FADH2 as an inherent reporter. In an extension of this scheme, the fluorescence of a reporter chromophore in close proximity of a catalytic metal center can be switched on and off by changes of, e.g., the metal valence state or by reversible coordination of a ligand to the metal center (see above and refs. 18, 32, and 33). The latter idea is already used in the design of molecular switches and of fluorescent, cryptand-based indicators for Na+, Ca2+, etc. (34).
In a similar manner, single molecule fluorescence studies provide a toolbox for monitoring a broad range of catalyst properties, particularly in the study of solid materials. For instance, local acidobasicity can be followed with acidic or basic probes (35), which are commercially available in a large variety (36) (Fig. 3c). The acidobasicity of the complete catalyst particle can be mapped after an aliquot of the probe has been equilibrated with the sample, or the chromophore properties can be followed while a single probe is walking over surfaces or through channels by diffusion (Fig. 3c). Analogous probes can be devised for locally reporting redox states, e.g., in a mixed-oxide catalyst, for probing electron-donating or electron-accepting properties of solid surfaces or for locating freely available coordination sites for incoming reagents. Many probes are known to change their spectra, lifetime, or intensity upon changes of the polarity or because of hydrogen bond formation. Finally, use of functional probes with varying dimensions allows the study of the accessibility of enzyme pockets or the access to acid/base functions, metal nanoclusters, etc., in heterogeneous catalysts (37).
Spatial Resolution: Micro- and Nanoscopy
The characteristic lengths for catalysts and catalytic phenomena range from <1 nm to the macroscopic scale. The nanometer is the fundamental length scale to express the size of crystallites in well dispersed supported noble metal catalysts, of cavities in zeolites and metal-organic frameworks, of ligands in coordination complexes, or of active centers of enzymes, which implies that proper characterization techniques should be able to monitor ultralow quantities, preferably with single-molecule sensitivity, with a sufficiently high spatial resolution. Because optical resolution is classically limited by diffraction, the resolving power is of the same order of magnitude as the wavelength used. Subnanometer wavelength techniques, like electron microscopy, generally operate under ex situ conditions and lack the molecular specificity of SMFS. Although SMFS can operate in condensed phase and has the desired single-molecule sensitivity, it makes use of longer, visible wavelengths, which results in a resolution of ≈500 nm in the focal plane and ≈1 μm along the optical axis. The confocal approach facilitates three-dimensional imaging, but it improves the resolution only with a factor of ≈2. Note that the diffraction limit does not impede colocalization of spectrally distinct molecules. However, the crucial challenge in catalytic systems will be to spatially resolve spectrally identical probe molecules interacting with the catalyst, on length scales that are much <200 nm. We will now consider technical evolutions that are expected to be useful in studying structured catalysts.
Although in far-field microscopy the light needs to be focused, this focusing process is no longer necessary in the near-field variant, where ultrasharp tips are used to scan surfaces (38), which allows the localization of objects at subdiffraction resolution. This approach is valuable to study two-dimensional structures, but the depth profile obtained is far more limited than in far-field methods.
A major breach through the diffraction limit was achieved with a nonlinear process, STED (stimulated emission depletion microscopy). In STED, the saturated depletion of the excited state is used to reach macromolecular-scale resolutions, down to 15–20 nm in the focal plane. The excitation spot is overlapped with a doughnut-shaped beam; this second light beam serves as a deexcitation beam forcing the excited molecules back to the ground state, resulting in the fluorescence from a very small central node not covered by the depletion beam. Oversaturating the deexcitation squeezes the fluorescence spot to subdiffraction dimensions (39, 40). Using other reversible saturation processes such as photoswitching can contribute to improving spatial resolution, whereas time resolution is another matter of concern. This approach can, for example, be used to reduce the area over which turnovers are counted.
A recent development in wide-field fluorescence imaging is to combine structured illumination with a nonlinear fluorescence response. Essentially, a high-intensity, sinusoidally patterned illumination results in saturation of the fluorescent molecules in the image except for those in the small nodes of the structured illumination. When scanning the sample, the emission intensity only changes in the zero-intensity regions, so by extracting spatial high-frequency components using Fourier analysis, superresolution is realized. As a proof of concept, individual 50-nm fluorescent beads were distinguished within aggregates (41).
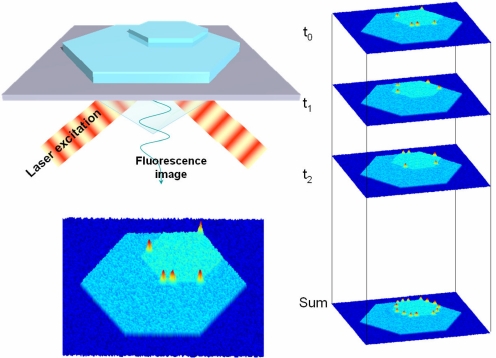
In traditional, linear wide-field fluorescence microscopy, the location of a single emitter can be determined to almost arbitrarily high accuracy if a sufficient signal-to-noise ratio (SNR) can be achieved. By recording the point spread function (PSF) of a single emitter and locating its center by a two-dimensional Gaussian fit, resolutions down to the nanometer scale have been shown (42, 43). However, when multiple emitters are in close proximity, these resolutions cannot be obtained. Recently, a few solutions have been proposed. If nonresolved probe molecules are subject to stepwise bleaching, their relative positions can easily be traced back by consecutive fits of the PSFs (44). Another scheme makes use of the random on/off photoswitching of a fraction of the present molecules, which, combined with reconstruction, yields nanometer resolved images (45, 46). Such a scheme can easily be implemented in the observation of single fluorescent products on the surface of a heterogeneous catalyst. Because reaction at the different sites is a “stochastic” process, recording consecutive frames should allow determining the activity of surface areas down to a few square nanometers (Fig. 4). The required SNR can be obtained by using high excitation powers because bleaching is not an issue. One can think of visualizing differences in activity governed by surface cracks or local defects, or one can now look at nanometer-sized crystals.
Fig. 4.
High-resolution reconstruction of the active-site distribution in a catalyst. The individual frames show stochastic turnover events; superimposition of consecutively recorded frames yields the high-resolution image as in photoactivation high-resolution light microscopy (45).
Next to direct imaging of objects through their fluorescence intensity, indirect methods have been used to study processes at the subnanometer to 10-nm length scale. Fluorescence resonance energy transfer (FRET) between a fluorescent donor and acceptor is routinely used to quantify interactions >1–10 nm; even shorter distances, <1 nm, can be probed by electron transfer between a fluorescent molecule and donors or acceptors in its proximity. By using FRET, folding of RNA during RNase activity has been monitored (47). Electron transfer from tyrosine residues to an isoalloxazine has been used to study conformational dynamics in a flavine reductase at angstrom scale (48). Energy and electron transfer phenomena can also be exploited to study the distances between nanoscale metal particles and the acid sites of the support in bifunctional heterogeneous catalysts. These phenomena could provide a direct experimental verification of Weisz's intimacy criterion, which for hydrocracking reactions empirically describe the maximum distance between reaction sites for (de)hydrogenation and acid-catalyzed reactions such as isomerization and cracking (49, 50).
Time Resolution and Dynamics
Although traditional characterization techniques mainly determine static properties of catalyst populations, probing time-related dynamics of individuals is required for deconvoluting collective reaction rates into contributions of spatially distinguishable, catalytically competent subpopulations. The time scales of interest to a catalytic site can span the whole catalyst lifetime, in the study of catalyst activation or deactivation, or may be shorter than a picosecond if short-living reaction intermediates are to be probed. In many cases SMFS already can give an experimentally justified statistical basis to the kinetics of catalyzed processes.
Single-molecule techniques are highly advantageous for following the course of a catalytic process because there is no need to synchronize the events at individual reaction sites within the population. After statistical analysis of the whole population, the individual components can be assigned to specific subpopulations, which allows study of catalyst populations in which the subgroups are in a dynamic equilibrium with one another (see above). Such information would not be accessible by traditional bulk experiments because of averaging.
For counting individual turnovers, the time resolution achieved in SMFS, e.g., 10−4 s, is sufficient for most catalytic systems. Even for the most active homogeneous catalysts, turnover frequencies (TOFs) seldom exceed 10 s−1; only some enzymes, such as catalase (80,000 s−1), display TOFs beyond the time resolution of current SMFS. To follow turnovers at an individual site over a sufficiently long time, it is preferable to monitor products of a fluorogenic reaction. Other approaches, for instance, using a chromophore with switching fluorescence in a ligand or cofactor, may face the problem of photobleaching, which would prevent observation of sufficiently long time traces. Observation of the dynamic disorder in single enzymes over long time periods has shown that the enzymatic TOF may fluctuate over time scales between 10−3 s and 10 s (3, 23, 24, 51, 52). Although turnover counting by SMFS has not yet been studied extensively in heterogeneous catalysis, it has been used to prove static disorder on either two-dimensionally or three-dimensionally organized catalysts (25, 53).
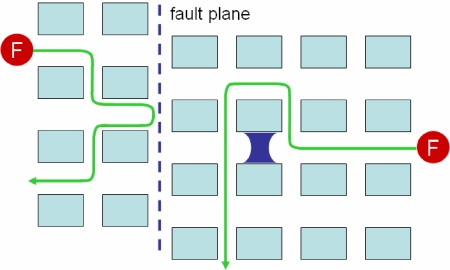
Time-resolved imaging combined with a high degree of spatial resolution fulfills the requirements for investigating diffusion of single molecules in pores of individual catalyst particles (54–56). Even if the channel dimensions in micro- or mesoporous materials are well below the diffraction limit, useful information can be retrieved by analyzing the diffusion trajectories of individual molecules or by studying correlation in a single-point fluorescence time transient (57). For a terrylenediimide dye diffusing in a mesostructured molecular sieve, it was possible to prove the existence of two subpopulations, one containing mobile dye molecules, and a minor fraction of stationary molecules, which could be assigned to “dead ends,” e.g., in collapsed pores, or to strong sorption sites (55). Exchanges between the subpopulations are evidenced by observation of a single molecule that becomes mobile again after a stationary period. In microporous materials such as zeolites, in which (sub)nanometer pores cross micrometer-sized crystals, information on diffusion by SMFS will be invaluable to an understanding of the role of diffusion barriers, either as fault planes or as local obstructions of pores by synthesis debris or collapse (Fig. 5). For hierarchical, bimodal porous materials, which ideally combine improved transport properties in mesopores with shape selectivity in micropores, the dynamic transitions of the molecules between both pore systems should be easy to investigate with SMFS. The results need to be confronted with data from other techniques, such as macroscopic uptake or pulsed-field gradient NMR experiments, which probe diffusion over varying time and length scales (58).
Fig. 5.
Tracking of single fluorescent molecules (F) diffusing in porous materials allows finding of local obstructions or fault planes.
Relative motions of biocatalytic centers and reagents are crucial as well when the enzyme is embedded in a structure, e.g., a natural or artificial membrane, or in an inorganic or polymeric host used for enzyme immobilization. Processive movements of enzymes over substrates have been observed on flat substrates, as for phospholipase-1 action on phospholipid bilayers, or on polymer chains, as for DNA polymerase acting on unwound single-stranded DNA (59, 60). In the case of immobilized enzymes and mobile reagents, SMFS can generate new insights on the diffusion of reagents and products through gel-type or macroreticular resins or through cross-linked enzyme crystals or aggregates, which is crucial in understanding, e.g., product inhibition in industrial biocatalysis.
On top of the study of translational diffusion, SMFS allows us to follow rotational dynamics and orientation of single molecules by monitoring fluctuations in fluorescence polarization. In an experimental catalytic context, it is extremely challenging to attempt to relate catalytic or sorption behavior to local orientation at the single site because doing so requires that the orientation of the surrounding matrix, e.g., the protein structure, or the pore structure of a solid catalyst, can be controlled and measured as a framework of reference. Measurements of average orientation with respect to planar surfaces are possible by using, for instance, second harmonic generation, but clearly such results pertain to ensemble averages (61). For fluorescent single molecules in a polymer film, heterogeneities in rotational dynamics have been observed (62). For adsorption and catalysis in microporous solids, it is important to understand not only the location of adsorbed molecules but also their rotational freedom because bulk equilibrium adsorption data suggest that not only enthalpy but also entropy effects are crucial in determining shape-selective uptake of guests in, for example, molecular sieves (63).
As a long-term goal, increased time resolution is needed for detecting the elementary reactions and intermediates of a catalytic cycle or even the transition states. Attempts have been made recently with pump-probe techniques (64). For photo-induced reactions, the pump pulse can initiate the reaction. The probe pulse, released with an adjustable delay time of a few femtoseconds, can subsequently detect intermediates. However, this experimental scheme requires accumulation of pulse sequences over several milliseconds, thereby significantly lowering the real-time resolution. Because accumulation over an ensemble of molecules, as is done in traditional femtosecond laser spectroscopy, has to be replaced by accumulation of pulse sequences on a single active center, photostability of the probe molecule will be one of the limiting factors in this type of experiment.
Integration of Techniques and Perspectives
Although fluorescence microscopy is able to spatially map inhomogeneous activity in catalytic materials, one needs to couple such data to insights in the nature of the active sites. When the catalytic materials contain sufficiently large crystals, it is straightforward to distinguish the crystallographically different crystal planes or edges, even under an optical microscope. For instance, on a crystalline-layered double hydroxide sample, it is easily recognized that the catalytic properties of the structural OH groups on the basal plane should be different from those of exchanged OH− anions on the crystal fringes (65, 66). However, many catalysts are micro- or nanocrystalline or are even amorphous. Therefore, gaining insight in the elemental composition or in the concentration of active sites with different spectroscopic signature is required. It is preferred to characterize the same catalyst particle simultaneously by SMFS and a combination of other techniques. However, when such an in situ combination is not possible, high-resolution ex situ techniques still can reveal useful information. For instance, extended x-ray absorption fine structure on metal ions or wavelength-dispersive elemental analysis in scanning electron microscopy can be complementary to fluorescence-based activity assays, although spatially overlaying the maps obtained by different techniques remains a potential source of ambiguity.
Fortunately, experimental conditions of SMFS and other techniques increasingly become compatible and show a great potential for future applications. Atomic force microscopy (AFM) is probably the most obvious technique for use in combination with SMFS (67). The possibility to perform AFM measurements in liquid media allows a thorough in situ examination of the surface structure, in particular the exact localization of the catalytically interesting edges, kinks, and defects, while at the same time this information can be coupled to the optically obtained catalytic data. Moreover, the possibility of mapping the chemical composition of a surface by a modified tip makes AFM a very unique tool. It has been shown that local Raman spectra can be recorded with silver- or gold-coated tips (68–70). IR techniques are frequently used to characterize inorganic solid catalysts, but they lack sensitivity and the optical resolution that can be achieved is limited because of the higher wavelengths used (71). Surface-enhanced (resonance) Raman spectroscopy [SE(R)RS] from a metal-coated AFM tip does not suffer from these limitations. Indeed, it has been shown that fingerprint signatures of molecules and functional groups can be obtained with this technique, in the best case with nanometer resolution (72, 73). As illustrated in Fig. 6a, the combination of fluorescence and SER(R)S could give insight into the nature of the active centers responsible for the catalytic activity. For certain catalysts that are conductive (e.g., gold-based catalysts) one can even apply scanning tunneling microscopy (STM) as an angstrom resolution surface characterization technique. Note that besides Raman signals, fluorescence intensity can also be locally enhanced by proper control of the distance between the tip and the fluorescing site or molecule (74, 75).
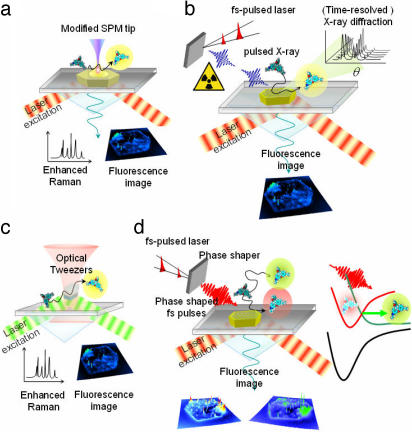
Fig. 6.
Combination of techniques that can be envisioned for in situ characterization of catalytic systems. (a) SMFS and tip-enhanced Raman spectroscopy. Catalytic conversion can be followed by fluorescence while chemical characterization of the active site can be obtained simultaneously. (b) SMFS and (time-resolved) x-ray experiments. This combination allows for simultaneous mapping of catalytic activity and crystallographic data. (c) Optical trapping for immobilizing small catalyst particles in solution. This approach minimizes diffusion resistances. (d) SMFS combined with phase-shaped femtosecond pulses can control the outcome of the catalytic reaction.
When more precise information on the elemental composition and/or crystallographic properties of the inorganic phase is needed, the techniques of choice are x-ray diffraction and electron microscopy. The latter technique, however, has to be applied in a vacuum or at very reduced gas pressure and thus requires ex situ combination with fluorescence observation (45). In situ combination of x-ray diffraction and SMFS seems now within the reach of optical microscopists, thanks to recent developments in benchtop x-ray sources (76–78). Indeed, by focusing femtosecond laser pulses of the type that is generally used in fluorescence microscopy on, for example, aqueous solutions of alkali metal chlorides, femtosecond x-ray pulses of well defined wavelengths are generated. Fiber-assisted “focusing” of these pulses on the sample allows mapping of the crystal properties of the catalysts with a time resolution down to the nanosecond level and a spatial accuracy of down to 1 μm (Fig. 6b). In combination with SMFS activity analysis, these time-resolved diffractograms can reveal local turnover-induced structural transformations in the catalysts (see above) (79). Furthermore, high-resolution optical images obtained by mapping stochastic turnover events (45, 46) can be compared with x-ray diffraction data of the inorganic catalyst, thus directly linking activity to crystal properties.
Although industrial catalysts generally are micrometer-sized to facilitate filtration or recycling, mass transfer limitations are more easily eliminated by scaling down the crystals to submicron dimensions. Suspensions of such catalysts may be studied by combining the fluorescence approach with optical trapping (Fig. 6c). This strategy eliminates the artifacts that arise when a crystal is deposited on a glass slide; in such case, the crystal may be no longer accessible through the face making contact with the substrate. Using optical tweezers to trap a single catalyst in the laser focus removes this problem. Both Raman spectroscopy and fluorescence spectroscopy can be readily combined with optical trapping (80–82).
Until now, possible combinations of SMFS and other in situ characterization techniques have been outlined. As a new challenge, one could envision manipulating the outcome of catalytic processes by applying laser light. Therefore, single-molecule microscopy could be combined with coherent control techniques well established today in femtosecond spectroscopy (83, 84). The use of light to control reaction pathways is one of the most exciting prospects in chemistry. Coherent control aims to steer a molecular system toward a desired outcome by exploiting quantum interference effects. Recent studies have shown that quantum interference between multiple excitation pathways is used to cancel coupling to the undesired channels. To achieve this goal, complex pulse-shaping schemes are being developed. In reactions with potential functionalization at CH3-, -CH2-, or -CH-, such as oxidation reactions, one could envision selectively enhancing the disfavored reaction path, i.e., the reaction at CH3. In the framework of the approach proposed here, the potential product molecules should have different extents of conjugation so that they can be discriminated based on their spectral properties (Fig. 6d). If this scheme can be realized, the chemists' ultimate dream of making molecules at will, one by one, comes within reach.
Acknowledgments
This work was performed within the framework of the Interuniversity Attraction Poles-VI program Functional Supramolecular Systems of the Belgian Federal government and GOA 2006/2 and was supported by the Katholieke Universiteit Leuven in the Centre of Excellence in Catalysis, an Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) fellowship (to M.B.J.R.), and a Fonds Wetenschappelÿk Orderroek (Vlaanderen) fellowship (to G.D.C.).
Abbreviations
- AFM
atomic force microscopy
- SMFS
single-molecule fluorescence spectroscopy.
Footnotes
The authors declare no conflict of interest.
T. Dertinger, I. Gregor, I. von der Hocht, R. Erdmann, B. Kraemer, F. Koberling, R. Hartmann, and J. Enderlein (2006) Progress in Biomedical Optics and Imaging. Proceedings of SPIE 6092:609203.
References
- 1.de Levie R. J Chem Educ. 2000;77:771–774. [Google Scholar]
- 2.Xie XS, Lu HP. J Biol Chem. 1999;274:15967–15970. doi: 10.1074/jbc.274.23.15967. [DOI] [PubMed] [Google Scholar]
- 3.Engelkamp H, Hatzakis NS, Hofkens J, De Schryver FC, Nolte RJM, Rowan AE. Chem Commun. 2006;9:935–940. doi: 10.1039/b516013h. [DOI] [PubMed] [Google Scholar]
- 4.Smiley RD, Hammes GG. Chem Rev. 2006;106:3080–3094. doi: 10.1021/cr0502955. [DOI] [PubMed] [Google Scholar]
- 5.Craig DB, Arriaga EA, Wong JCY, Lu H, Dovichi NJ. J Am Chem Soc. 1996;118:5245–5253. [Google Scholar]
- 6.Min W, English BP, Luo GB, Cherayil BJ, Kou SC, Xie XS. Acc Chem Res. 2005;38:923–931. doi: 10.1021/ar040133f. [DOI] [PubMed] [Google Scholar]
- 7.Lu HP, Xun L, Xie XS. Science. 1998;282:1877–1882. doi: 10.1126/science.282.5395.1877. [DOI] [PubMed] [Google Scholar]
- 8.Evans D, Osborn JA, Wilkinson G. J Chem Soc. 1968:3133–3142. [Google Scholar]
- 9.Frohning CD, Kohlpaintner CW. In: Applied Homogeneous Catalysis with Organometallic Compounds. Cornils B, Herrmann WA, editors. Vol 1. Weinheim: VCH; 1996. pp. 28–104. [Google Scholar]
- 10.Hoegaerts D, Jacobs PA. Tetrahedron-asymmetr. 1999;10:3039–3043. [Google Scholar]
- 11.Berrisford DJ, Bolm C, Sharpless KB. Angew Chem Int Ed. 1995;34:1059–1070. [Google Scholar]
- 12.Sharpless KB. Angew Chem Int Ed. 2002;41:2024–2032. [PubMed] [Google Scholar]
- 13.McGarrigle EM, Gilheany DG. Chem Rev. 2005;105:1563–1602. doi: 10.1021/cr0306945. [DOI] [PubMed] [Google Scholar]
- 14.Rondelez Y, Tresset G, Tabata KV, Arata H, Fujita H, Takechu S, Noji H. Nat Biotechnol. 2005;23:361–365. doi: 10.1038/nbt1072. [DOI] [PubMed] [Google Scholar]
- 15.Tan W, Yeung ES. Anal Chem. 1997;69:4242–4248. [Google Scholar]
- 16.Coperet C, Chabanas M, Saint-Arroman RP, Basset JM. Angew Chem Int Ed. 2003;42:156–181. doi: 10.1002/anie.200390072. [DOI] [PubMed] [Google Scholar]
- 17.De Vos DE, Dams M, Sels BF, Jacobs PA. Chem Rev. 2002;102:3615–3640. doi: 10.1021/cr010368u. [DOI] [PubMed] [Google Scholar]
- 18.Kiel A, Kovacs J, Mokhir A, Krämer R, Herten DP. Angew Chem Int Ed. 2007;46:3158. doi: 10.1002/anie.200604965. [DOI] [PubMed] [Google Scholar]
- 19.Over H, Kim YD, Seitsonen AP, Wendt S, Lundgren E, Schmid M, Varga P, Morgante A, Ertl G. Science. 2000;287:1474–1476. doi: 10.1126/science.287.5457.1474. [DOI] [PubMed] [Google Scholar]
- 20.Lauritsen JV, Vang RT, Besenbacher F. Catal Today. 2006;111:34–43. [Google Scholar]
- 21.Hendriksen BLM, Bobaru SC, Frenken JWM. Top Catal. 2005;36:43–54. [Google Scholar]
- 22.Tinnefeld P, Sauer M. Angew Chem Int Ed. 2005;44:2642–2671. doi: 10.1002/anie.200300647. [DOI] [PubMed] [Google Scholar]
- 23.Flomenbom O, Velonia K, Loos D, Masuo S, Cotlet M, Engelborghs Y, Hofkens J, Rowan AE, Nolte RJM, Van der Auweraer M, et al. Proc Natl Acad Sci USA. 2005;102:2368–2372. doi: 10.1073/pnas.0409039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velonia K, Flomenbom O, Loos D, Masuo S, Cotlet M, Engelborghs Y, Hofkens J, Rowan AE, Klafter J, Nolte RJM, et al. Angew Chem Int Ed. 2005;44:560–564. doi: 10.1002/anie.200460625. [DOI] [PubMed] [Google Scholar]
- 25.Roeffaers MBJ, Sels BF, Uji-i H, De Schryver FC, Jacobs PA, De Vos DE, Hofkens J. Nature. 2006;439:572–575. doi: 10.1038/nature04502. [DOI] [PubMed] [Google Scholar]
- 26.Shaughnessy KH, Kim P, Hartwig JF. J Am Chem Soc. 1999;121:2123–2132. [Google Scholar]
- 27.Su H, Yeung ES. J Am Chem Soc. 2000;122:7422–7423. [Google Scholar]
- 28.Pap EHW, Drummen GPC, Winter VJ, Kooij TWA, Rijken P, Wirtz KWA, Op den Kamp JAF, Hage WJ, Post JA. FEBS Lett. 1999;453:278–282. doi: 10.1016/s0014-5793(99)00696-1. [DOI] [PubMed] [Google Scholar]
- 29.Goddard JP, Reymond JL. Curr Opin Biotech. 2004;15:314–322. doi: 10.1016/j.copbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Meshulam T, Herscovitz H, Casavant D, Bernardo J, Roman R, Haugland RP, Strohmeier GS, Diamond RD, Simons ER. J Biol Chem. 1992;267:21465–21470. [PubMed] [Google Scholar]
- 31.Hendrickson HS, Hendrickson EK, Johnson ID, Farber SA. Anal Biochem. 1999;276:27–35. doi: 10.1006/abio.1999.4280. [DOI] [PubMed] [Google Scholar]
- 32.Fabbrizzi L, Licchelli M, Pallavicini P. Acc Chem Res. 1999;32:846–853. [Google Scholar]
- 33.Rurack K. Spectrochim Acta A. 2001;57:2161–2195. doi: 10.1016/s1386-1425(01)00492-9. [DOI] [PubMed] [Google Scholar]
- 34.Callan JF, de Silva AP, Magri DC. Tetrahedron. 2005;61:8551–8588. [Google Scholar]
- 35.Roeffaers MBJ, Sels BF, Loos D, Kohl C, Müllen K, Jacobs PA, Hofkens J, De Vos DE. Chemphyschem. 2005;6:2295–2299. doi: 10.1002/cphc.200500238. [DOI] [PubMed] [Google Scholar]
- 36.Haugland RP. In: The Handbook: A Guide to Fluorescent Probes and Labeling Technologies. Spence MTZ, editor. Carlsbad, CA: Invitrogen; 2006. [Google Scholar]
- 37.Calzaferri G, Huber S, Maas H, Minkowski C. Angew Chem Int Ed. 2003;42:3732–3758. doi: 10.1002/anie.200300570. [DOI] [PubMed] [Google Scholar]
- 38.Betzig E, Trautman JK. Science. 1992;257:189–195. doi: 10.1126/science.257.5067.189. [DOI] [PubMed] [Google Scholar]
- 39.Hell SW. Nat Biotechnol. 2003;21:1347–1355. doi: 10.1038/nbt895. [DOI] [PubMed] [Google Scholar]
- 40.Donnert G, Keller J, Medda R, Andrei MA, Rizzoli SO, Lurmann R, Jahn R, Eggeling C, Hell SW. Proc Natl Acad Sci USA. 2006;103:11440–11445. doi: 10.1073/pnas.0604965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafsson MGL. Proc Natl Acad Sci USA. 2005;102:13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheezum MK, Walker WV, Guilford WH. Biophys J. 2001;81:2378–2388. doi: 10.1016/S0006-3495(01)75884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yildiz A, Selvin PR. Acc Chem Res. 2005;38:574–582. doi: 10.1021/ar040136s. [DOI] [PubMed] [Google Scholar]
- 44.Muls B, Uji-i H, Melnikov S, Moussa A, Verheijen W, Soumillion JP, Josemon J, Mullen K, Hofkens J. Chemphyschem. 2005;6:2286–2294. doi: 10.1002/cphc.200500235. [DOI] [PubMed] [Google Scholar]
- 45.Betzig E, Patterson GH, Sougrat R, Lindwasser W, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 46.Rust MJ, Bates M, Zhuang XW. Nat Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang X, Kim H, Pereira MJB, Babcock HP, Walter NG, Chu S. Science. 2002;296:1473–1476. doi: 10.1126/science.1069013. [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Luo G, Karnchanaphanurach P, Louie TM, Rech I, Cova S, Xun LY, Xie XS. Science. 2003;302:262–266. doi: 10.1126/science.1086911. [DOI] [PubMed] [Google Scholar]
- 49.Weisz PB. Adv Catal. 1962;13:137–190. [Google Scholar]
- 50.Blomsma E, Martens JA, Jacobs PA. J Catal. 1997;165:241–248. [Google Scholar]
- 51.Xie XS. J Chem Phys. 2002;117:11024–11032. [Google Scholar]
- 52.English BP, Min W, van Oijen AM, Lee KT, Luo GB, Sun HY, Cherayil BJ, Kou SC, Xie XS. Nat Chem Biol. 2006;2:87–94. doi: 10.1038/nchembio759. [DOI] [PubMed] [Google Scholar]
- 53.Roeffaers MBJ, Sels BF, Uji-i H, Blanpain B, L'hoëst P, Jacobs PA, De Schryver FC, Hofkens J, De Vos DE. Angew Chem Int Ed. 2006;46:1706–1709. doi: 10.1002/anie.200604336. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt T, Schütz GJ, Baumgartner W, Gruber HJ, Schindler H. Proc Natl Acad Sci USA. 1996;93:2926–2929. doi: 10.1073/pnas.93.7.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seebacher C, Hellriegel C, Deeg FW, Bräuchle C, Altmaier S, Behrens P, Müllen K. J Phys Chem B. 2002;106:5591–5595. [Google Scholar]
- 56.Kirstein J, Platschek B, Jung C, Brown R, Bein T, Bräuchle C. Nat Mater. 2007;6:303–310. doi: 10.1038/nmat1861. [DOI] [PubMed] [Google Scholar]
- 57.Fu Y, Ye F, Sanders WG, Collinson MM, Higgins DA. J Phys Chem B. 2006;110:9164–9170. doi: 10.1021/jp054178p. [DOI] [PubMed] [Google Scholar]
- 58.Karger J, Ruthven DM. Zeolites. 1989;9:267–281. [Google Scholar]
- 59.Rocha S, Verheijen W, Braeckmans K, Svenson A, Skjøt M, De Schryver FC, Uji-i H, Hofkens J. In: Handai Nanophotonics, Vol 3, Nano Biophotonics. Masuhara H, Kawata S, Tokunaga F, editors. Amsterdam: Elsevier; 2007. pp. 133–141. [Google Scholar]
- 60.Zhang ZQ, Spiering MM, Trakselis MA, Ishmael FT, Xi J, Benkovic SJ, Hammes GG. Proc Natl Acad Sci USA. 2005;102:3254–3259. doi: 10.1073/pnas.0500327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kikteva T, Star D, Zhao ZH, Baisley TL, Leach GW. J Phys Chem Rev B. 1999;103:1124–1133. [Google Scholar]
- 62.Uji-i H, Melnikov SM, Deres A, Bergamini G, De Schryver F, Herrmann A, Mullen K, Enderlein J, Hofkens J. Polymer. 2006;47:2511–2518. [Google Scholar]
- 63.Denayer JFM, Ocakoglu RA, Arik IC, Kirschhock CEA, Martens JA, Baron GV. Angew Chem Int Ed. 2005;44:400–403. doi: 10.1002/anie.200454058. [DOI] [PubMed] [Google Scholar]
- 64.van Dijk EMHP, Hernando J, García-López JJ, Crego-Calama M, Reinhoudt DN, Kuipers L, García-Parajó MF, van Hulst NF. Phys Rev Lett. 2005;94:078302. doi: 10.1103/PhysRevLett.94.078302. [DOI] [PubMed] [Google Scholar]
- 65.Trifirò F, Vaccari A. In: Comprehensive Supramol Chem, Vol 7, Solid-State Supramol Chem: Two- and Three-Dimensional Inorganic Networks. Atwood JL, Davies JE, Macnicol DD, Vögtle F, Lehn JM, Alberti G, Bein T, editors. Oxford: Pergamon; 1996. pp. 251–291. [Google Scholar]
- 66.Sels BF, De Vos DE, Jacobs PA. Catal Rev. 2001;43:443–488. [Google Scholar]
- 67.Kassies R, Van der Werf KO, Lenferink A, Hunter CN, Olsen JD, Subramaniam V, Otto C. J Microsc. 2005;217:109–116. doi: 10.1111/j.0022-2720.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- 68.Stockle RM, Suh YD, Deckert V, Zenobi R. Chem Phys Lett. 2000;318:131–136. [Google Scholar]
- 69.Yeo BS, Zhang WH, Vannier C, Zenobi R. Appl Spectrosc. 2006;60:1142–1147. doi: 10.1366/000370206778664662. [DOI] [PubMed] [Google Scholar]
- 70.Kühn S, Håkanson U, Rogobete L, Sandoghdar V. Phys Rev Lett. 2006;97:017402. doi: 10.1103/PhysRevLett.97.017402. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann E, Chmelik C, Scheidt H, Vasenkov S, Staudte B, Kärger J, Kremer F, Zadrozna G, Kornatowski J. J Am Chem Soc. 2002;124:8690–8692. doi: 10.1021/ja026400z. [DOI] [PubMed] [Google Scholar]
- 72.Hartschuh A, Sanchez EJ, Xie XS, Novotny L. Phys Rev Lett. 2003;90:095503. doi: 10.1103/PhysRevLett.90.095503. [DOI] [PubMed] [Google Scholar]
- 73.Anderson N, Hartschuh A, Cronin S, Novotny L. J Am Chem Soc. 2005;127:2533–2537. doi: 10.1021/ja045190i. [DOI] [PubMed] [Google Scholar]
- 74.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. J Phys Chem B. 2004;108:12568–12574. doi: 10.1021/jp040221h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anger P, Bharadwaj P, Novotny L. Phys Rev Lett. 2006;96:113002. doi: 10.1103/PhysRevLett.96.113002. [DOI] [PubMed] [Google Scholar]
- 76.Hatanaka K, Miura T, Fukumura H. Appl Phys Lett. 2002;80:3925–3927. [Google Scholar]
- 77.Hatanaka K, Miura T, Fukumura H. Chem Phys. 2004;299:265–270. [Google Scholar]
- 78.Bargheer M, Zhavoronkov N, Woerner N, Elsaesser T. Chemphyschem. 2006;7:783–792. doi: 10.1002/cphc.200500591. [DOI] [PubMed] [Google Scholar]
- 79.Hendriksen BLM, Bobaru SC, Frenken JWM. Catal Today. 2005;105:234–243. [Google Scholar]
- 80.Hofkens J, Hotta J, Sasaki K, Masuhara H, Taniguchi T, Miyashita T. J Am Chem Soc. 1997;119:2741–2742. [Google Scholar]
- 81.Tsuboi Y, Nishino M, Sasaki T, Kitamura N. J Phys Chem B. 2005;109:7033–7039. doi: 10.1021/jp044894b. [DOI] [PubMed] [Google Scholar]
- 82.Dol GC, Tsuda K, Weener JW, Bartels MJ, Asavei T, Gensch T, Hofkens J, Latterini L, Schenning APHJ, et al. Angew Chem Int Ed. 2001;40:1710–1714. [PubMed] [Google Scholar]
- 83.Brixner T, Damrauer NH, Niklaus P, Gerber G. Nature. 2001;414:57–60. doi: 10.1038/35102037. [DOI] [PubMed] [Google Scholar]
- 84.Herek JL, Wohlleben W, Cogdell RJ, Zeidler D, Motzkus M. Nature. 2002;417:533–535. doi: 10.1038/417533a. [DOI] [PubMed] [Google Scholar]