Abstract
Developmental plasticity in response to environmental cues can take the form of polyphenism, as for the discrete morphs of some insects, or of an apparently continuous spectrum of phenotype, as for most mammalian traits. The metabolic phenotype of adult rats, including the propensity to obesity, hyperinsulinemia, and hyperphagia, shows plasticity in response to prenatal nutrition and to neonatal administration of the adipokine leptin. Here, we report that the effects of neonatal leptin on hepatic gene expression and epigenetic status in adulthood are directionally dependent on the animal's nutritional status in utero. These results demonstrate that, during mammalian development, the direction of the response to one cue can be determined by previous exposure to another, suggesting the potential for a discontinuous distribution of environmentally induced phenotypes, analogous to the phenomenon of polyphenism.
Keywords: developmental plasticity, epigenetics, gene expression, leptin, obesity
Interactions between the developing mammalian embryo or fetus and its environment can be categorized as either disrupting development (e.g., by teratogens) or involving the processes of developmental plasticity (1). This latter set of processes evolved to adjust the pattern of development to produce a phenotype that is matched to the anticipated environment, thus increasing the fitness of the organism (2). Such developmental plasticity can affect multiple traits in an integrated manner, as illustrated by polyphenism in insects (3, 4) or by the pleiotropic and graded responses that effect life history tradeoffs in other taxa, including mammals (5). However, when the prediction of the future environment is inaccurate, or when the environment changes between generations, a mismatched phenotype may result, and then the consequences of plasticity may be maladaptive (6). There are extensive experimental data (7) and robust clinical observations (8) that early life cues have lasting effects on the metabolic phenotype in mammals, and we have suggested that inappropriate anticipatory choices made in early life underlie the relationship between altered fetal development, later metabolic phenotype, and the increased risks of type 2 diabetes, obesity, and cardiovascular disease (9).
Data are now emerging suggesting that alterations in DNA methylation and other epigenetic processes are both central to the processes of developmental plasticity and also underpin the relationships between early life effects and later metabolic status. For example, in offspring of rats given a low protein diet during pregnancy, which later develop abnormalities of metabolic control and cardiovascular function, there are changes in hepatic expression and in gene promoter methylation and histone acetylation of metabolically relevant receptors, the glucocorticoid receptor (GR) and the peroxisome proliferator-activated receptor α (PPARα). These effects are prevented by concurrently supplementing the diet of the pregnant dam with folate, which promotes methyl group provision (10, 11).
Studies in pregnant rodents subject to a variety of dietary challenges or to exposure to glucocorticoids show a relatively consistent outcome for the offspring: abnormalities of insulin secretion and action, sarcopenia, appetite disturbance, obesity, and endothelial dysfunction (7). These features are particularly apparent when the animals are placed on a high-fat diet after weaning (12). We have reported that all of the observed aspects of the induced phenotype after balanced maternal undernutrition are prevented from developing when the female offspring are treated in the neonatal period with recombinant rat leptin (13). We postulated that the leptin administration gave a false developmental cue, signaling adiposity to pups that were actually thin, and that they therefore set their ultimate metabolic phenotype to be more appropriate to a high-nutrition environment (14).
We now report that neonatal leptin treatment not only induces epigenetic and expression changes in specific genes measured in the adult liver, but that the direction of these induced changes depends on the previous environmental exposure of the fetus. Thus, the direction of the developmental response to the second transient cue (neonatal leptin) is influenced by previous environmental history (maternal diet). This finding has implications for understanding mammalian development, because it suggests that processes analogous to those operating in polyphenic species may also operate in mammals.
Results
We studied adult (170-day-old) female rats in which nutritional status had been manipulated in utero by maternal undernutrition; the offspring of ad libitum fed animals served as controls. After birth, a further nutrition-related cue was provided by saline or leptin injections from days 3 to 10 of life, and the animals were then fed a normal or high-fat diet from weaning. We present data for the expression and promoter methylation of genes involved in metabolic regulation and in the effector arm of the glucocorticoid axis.
Bifurcation of Response to Leptin.
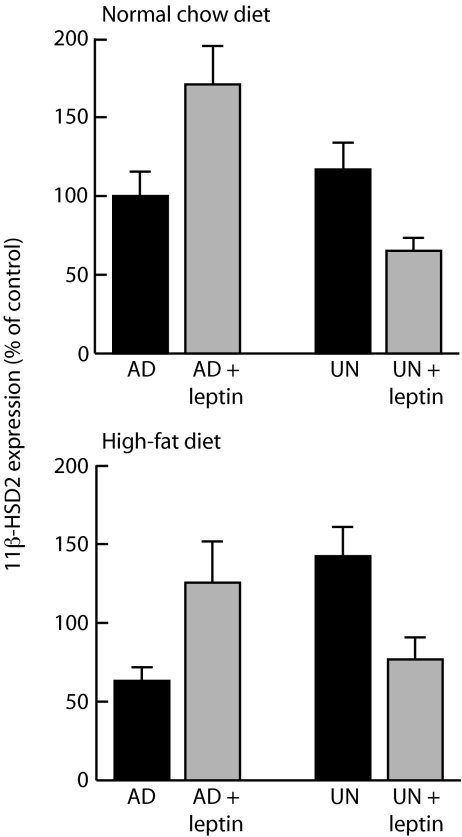
Leptin exposure had bidirectional effects on the expression of hepatic 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), the enzyme that inactivates glucocorticoids, the direction of change in expression being dependent on prior maternal nutritional status (Fig. 1). In offspring of well nourished mothers, neonatal leptin exposure increased 11β-HSD2 expression in adulthood (P < 0.001); conversely, in offspring of undernourished mothers, neonatal leptin exposure decreased hepatic 11β-HSD2 expression in adulthood (P < 0.01). The bidirectional effects were independent of postweaning nutrition (normal chow or high-fat diet). Similar bidirectional effects (P < 0.01) were seen with respect to PPARα expression, except that, in this case, leptin suppressed expression in maternally well nourished offspring and enhanced expression in maternally undernourished offspring (Table 1). For PPARα expression, the effect was obscured by a high-fat diet after weaning. There was a trend to a bidirectional effect on the expression of phosphoenolpyruvate carboxykinase (PEPCK). There were significant interactions between prenatal nutrition and leptin administration for 11β-HSD2 expression (P < 0.0001), PPARα expression (P < 0.0005), and PEPCK expression (P < 0.005).
Fig. 1.
Fetal nutrition induces a bifurcation in the response of 11β-HSD2 gene expression to leptin treatment. We measured mRNA expression of the 11β-HSD2 gene in the livers of adult (170-day-old) female rats exposed in utero to maternal undernutrition (UN) or ad libitum feeding (AD), treated with saline or leptin from days 3–10 of life, and then fed normal chow (Upper) or a high-fat diet (Lower) from weaning. Maternal nutrition altered the direction of the response of 11β-HSD2 expression to neonatal leptin treatment (test for reversal of sign of response, P < 0.01; test for interaction between prenatal nutrition and leptin administration, P < 0.0001); the effect was independent of postweaning nutrition. Data are means ± SEM for n = 8 per group; values are expressed relative to that of normally nourished (maternally ad libitum-fed and postweaning chow-fed) and saline-treated offspring set as 100%.
Table 1.
Changes in gene expression and epigenetic status in response to neonatal leptin are altered in direction by fetal experience
| Group | PPARα expression | PPARα methylation | GR expression | GR methylation | PEPCK expression |
|---|---|---|---|---|---|
| Normal chow diet | |||||
| AD | 100.0 ± 7.4 | 100.0 ± 20.5 | 100.0 ± 15.8 | 100.0 ± 39.1 | 100.0 ± 4.3 |
| AD + leptin | 73.0 ± 7.2 | 131.8 ± 11.3 | 80.7 ± 10.5 | 315.9 ± 38.7 | 84.2 ± 10.7 |
| UN | 39.7 ± 3.9 | 132.7 ± 30.8 | 45.5 ± 4.4 | 156.9 ± 37.3 | 66.6 ± 6.4 |
| UN + leptin | 83.4 ± 10.0 | 110.2 ± 12.7 | 57.4 ± 5.6 | 185.5 ± 42.6 | 99.5 ± 12.0 |
| High-fat diet | |||||
| AD | 77.5 ± 8.0 | 82.4 ± 18.7 | 73.9 ± 9.4 | 219.5 ± 40.7 | 102.6 ± 14.4 |
| AD + leptin | 97.6 ± 6.9 | 133.8 ± 36.1 | 92.9 ± 8.7 | 329.4 ± 44.2 | 92.7 ± 12.3 |
| UN | 69.9 ± 6.8 | 181.8 ± 47.0 | 76.4 ± 10.4 | 208.0 ± 44.2 | 50.5 ± 8.0 |
| UN + leptin | 101.5 ± 9.9 | 79.9 ± 12.4 | 70.2 ± 9.5 | 98.4 ± 46.0 | 77.6 ± 7.7 |
| Test for | |||||
| Reversal in sign of response to leptin | P < 0.01 | P < 0.04 | NS | NS | NS |
| Maternal nutrition × leptin interaction | P < 0.0005 | P < 0.05 | NS | P < 0.005 | P < 0.005 |
The experimental protocol is described in the legend to Fig. 1. There was no correlation between gene expression and weight at killing or weight gain from 30 days of age. Data are means ± SEM for n = 8 per group; values are expressed relative to those of normally nourished (maternally ad libitum-fed and postweaning chow-fed) and saline-treated offspring set as 100%. NS, not significant.
The methylation of the PPARα promoter also exhibited bifurcation (P < 0.04), being elevated (P < 0.04) by leptin in maternally well nourished offspring and suppressed (P < 0.02) by leptin in maternally undernourished offspring (Table 1). GR promoter methylation was elevated (P < 0.008) by leptin in maternally well nourished offspring but was unaffected by leptin in maternally undernourished offspring. There were significant interactions between prenatal nutrition and leptin administration for PPARα methylation (P < 0.05) and GR methylation (P < 0.005). For 11β-HSD2 expression and for PPARα expression and methylation, the response to leptin administration in each condition of prenatal nutrition differed significantly (P < 0.01) from zero, demonstrating a reversal of sign consistent with a switch in the physiological equilibrium of these genes.
No bifurcation was observed in expression of other genes such as acyl-CoA oxidase or lipoprotein lipase (data not shown). The bidirectional effect was tissue specific and was not seen in visceral fat (data not shown).
Normalization of Metabolic Phenotype by Leptin.
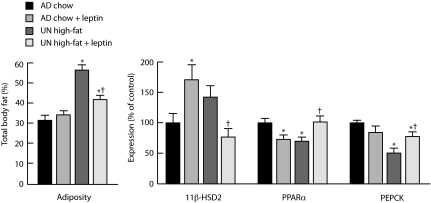
The normalization by leptin of the metabolic phenotype induced by undernutrition in utero followed by hypercaloric nutrition after weaning (13) was paralleled by changes in mRNA expression of several key metabolic genes (Fig. 2).
Fig. 2.
Neonatal leptin treatment normalizes gross phenotype (adiposity) and hepatic gene expression in adult rats exposed to undernutrition in utero followed by hypercaloric nutrition after weaning. We measured total body fat (dual-energy x-ray absorptiometry) (Left) and hepatic mRNA expression (Right) of adult (170-day-old) female rats that had been subject in utero to maternal undernutrition (UN) or ad libitum feeding (AD), treated with saline or leptin from days 3–10 of life, and then fed normal chow or a high-fat diet from weaning. Data are means ± SEM for n = 8 per group; values for gene expression are expressed relative to those of normally nourished (maternally ad libitum-fed and postweaning chow-fed) and saline-treated offspring set as 100%. Data for total body fat are replotted from Vickers et al. (13). *, P < 0.05: significantly different from normally nourished, saline-treated animals. †, P < 0.05: significant effect of leptin treatment in UN high-fat animals.
Discussion
In developmental biology, the reaction norm for a trait or suite of traits describes the range of phenotypes that can develop from a single genotype (15). For some traits in some species, the reaction norm may be discontinuous and these discontinuities are manifest as alternative morphs. Such polyphenism is regulated by environmental cues acting in critical windows. For example, in the honey bee, exclusive exposure to royal jelly during the larval stage induces a queen morph, whereas exposure to other foods induces the worker morph (4). These alternative morphs involve coordinated changes in many traits. However, in mammals, environmentally induced phenotypic variations (e.g., in metabolic set points) are generally considered to be continuously distributed traits. The present observations suggest that a discontinuous range of phenotypes, at least as defined at the molecular level, can be induced by early environmental exposures in mammals. We speculate that prenatal nutrition induced a bifurcation or phase transition in the effects of the second cue on gene expression and methylation, with a significant nonlinear interaction driving the phenomenon. That is, there are at least two distinct stable metabolic set points that the system may attain, and the particular equilibrium depends on the interaction between prenatal nutrition and later metabolic experience.
It is noteworthy that the bifurcation involved lasting effects on expression and/or epigenetic state in a transcription factor involved in metabolic regulation, PPARα, as well as in genes involved in the effector arm of the glucocorticoid axis (11β-HSD2 inactivates glucocorticoids, corticosterone in the rat). These very different responses to the second neonatal stimulus according to prior maternal state suggest that the adult rats have phenotypes resulting from discontinuous equilibria in gene status induced by antenatal nutrition. It is possible that the discontinuous nature of the response at the level of gene expression may not have been recognized previously because metabolic phenotypes are usually described at the level of the whole organism. Furthermore, because developmental cues act across a broad temporal window on different components or pathways, multiple bifurcatory responses could be induced, which, when integrated, could obscure the underlying polyphenic discontinuities.
Because maternal undernutrition has both central and peripheral effects in the offspring, both of which are reversed by neonatal leptin (13), the bifurcation in the response appears to involve multisystem effects. This has analogies to the environmental induction of polyphenism, with a single cue having multisystem effects and presumably operating through an integrated suite of a few regulatory genes. Strikingly, the corrective effect of leptin on obesity was paralleled by effects on gene expression of 11β-HSD2, PPARα, and PEPCK (Fig. 2). These proteins are central to control of lipid metabolism and glucose homoeostasis, modulating or being modulated by an interplay of factors including glucocorticoids, insulin, and nutrient levels (16–18). These and similar genes represent key candidates for further investigation of the molecular mechanisms of metabolic plasticity.
Although the effects of leptin on phenotype and gene expression are consistent with a direct effect of leptin on metabolic parameters in the offspring, it is possible that leptin exerted its actions in part through an effect on the behavior of leptin-treated pups, which was then reflected in level of maternal attention or in her physiology (19). Changes in maternal care are known to affect expression and epigenetic modification of stress-associated genes (20). In the present study, there were no observable differences in maternal behavior toward saline-treated versus leptin-treated pups. However, irrespective of whether the effect of leptin is direct or indirect, the observations support the conclusion that the prior environmental history of the mother/offspring pair can alter the direction of the offspring's response to a later cue. This observation has implications for the design of studies of the long-term effects of developmental stimuli.
Waddington (21) proposed that development proceeds along distinct pathways governed by dynamic equilibrium points that are affected by previous experience, a process he termed “canalization.” In our study, the direction of the response to leptin appears to be dependent on the particular equilibrium point that the system is driven to attain under the influence of the prenatal environment. Thus, we demonstrate developmental bifurcation in the life-long expression of a suite of genes, which is then reflected in a difference in the integrated metabolic phenotype. These different developmental states may be the mammalian equivalent of alternative morphs or polyphenism, although the ability to recognize them requires a detailed assessment of phenotype at the level of gene regulation. Such phenomena may be of broad significance in mammalian developmental biology.
Materials and Methods
Animals and Tissues.
We used a model of developmental programming via maternal undernutrition (12, 13). Virgin Wistar rats (age, 100 ± 5 days) were time mated by using a rat estrous cycle monitor to assess the stage of estrus of the animals before introducing the male. After confirmation of mating, rats were housed individually in standard rat cages with free access to water. All rats were kept in the same room with a constant temperature maintained at 25°C and a 12-h light/12-h darkness cycle. Animals were assigned to one of two nutritional groups: undernutrition (30% of ad libitum) of a standard diet throughout gestation (UN group) or standard diet ad libitum throughout gestation (AD group). Food intake and maternal weights were recorded daily until the end of pregnancy. After birth, pups were weighed and litter size was adjusted to eight pups per litter to ensure adequate and standardized nutrition until weaning. Pups from undernourished mothers were cross-fostered onto dams that had received ad libitum feeding throughout pregnancy. At postnatal day 3, female pups from both groups were randomized to receive either saline or recombinant rat leptin (2.5 μg·g−1·day−1) for 10 days by s.c. injection (n = 16 per group). During and after treatment, all animals were maintained on ad libitum feeding until weaning. At weaning, saline- or leptin-treated offspring were weight-matched and placed on either standard rat chow or a high-fat diet (Research Diets No. 12451; 45% energy as fat). At postnatal day 170, rats were fasted overnight and killed by halothane anesthesia followed by decapitation. Tissues were immediately dissected and snap frozen in liquid nitrogen for molecular analysis. All animal work was approved by the Animal Ethics Committee of The University of Auckland.
Molecular Analyses.
mRNA expression of hepatic genes was measured by real-time PCR. Briefly, total RNA was isolated from cells with TRIzol reagent (Invitrogen, Paisley, Scotland, U.K.), and 1 μg was used as a template to prepare cDNA with 100 units of Moloney murine leukemia virus reverse transcriptase. cDNA was amplified with real-time PCR primers specific for 11β-HSD2, PPARα, PEPCK, GR, and cyclophilin (Table 2). The reaction was performed in a total volume of 25 μl with SYBR Green Jumpstart Ready Mix (Sigma, Poole, Dorset, U.K.) as described by the manufacturer. Samples were analyzed in duplicate, and Ct values were normalized to cyclophilin by the ΔCt method (22).
Table 2.
Primer sequences used in the measurement of mRNA expression by real-time RT-PCR and in methylation-sensitive real-time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| RT-PCR | ||
| 11β-HSD2 | TGGCCACTGTGTTGGATTT | ATCGGCCACTACCATGTTG |
| PPARα | CGGGTCATACTCGCAGGAAAG | TGGCAGCAGTGGAAGAATCG |
| PEPCK | AGCTGCATAATGGTCTGG | GAACCTGGCGTTGAATGC |
| GR | GGAGAATTATGACCACACTCAAC | GCAGTAGGTAAGGACATTCTCAA |
| Cyclophilin | TTGGGTCGCGTCTGCTTCGA | GCCAGGACCTGTATGCTTCA |
| Methylation-sensitive PCR | ||
| PPARα | TGTGTCTCGTTCTGAACCG | TCCACCCACCTCACTGTC |
| GR | CGTCTTGTTCCACCCACT | CCTTGCAGTTGCCGACAG |
| PPARγ2 | GTCTCTGCTCTGGTAATTC | AAGGCTTGTGGTCATTGAG |
The methylation status of GR and PPARα promoters was determined by methylation-sensitive PCR as described (10). Briefly, genomic DNA (5 μg) was isolated from liver by using the Promega Wizard SV Genomic DNA Purification System (Promega, Southampton, Hampshire, U.K.). Genomic DNA (25 ng) was digested with the methylation-sensitive restriction enzymes AciI and HinP1I as recommended by the manufacturer (New England Biolabs, Hitchin, Hertfordshire, U.K.). The resulting DNA was then amplified by real-time PCR (Table 2), which was performed in a total volume of 25 μl with SYBR Green Jumpstart ready mix as described by the manufacturer (Sigma). The promoter region of the rat PPARγ2 gene, which contains no CpG islands and no AciI or HinP1I recognition sites, was used as internal control. All Ct values were normalized to the internal control.
Statistical Approach.
The effects of prenatal nutrition, leptin administration, postnatal diet, and their interactions were tested in an analysis of covariance by using liver weight as a percentage of live weight as a covariate. Because the residuals from PPARα and GR methylation were significantly skewed (nonnormal), the data were log transformed for analysis. The residuals from fitting the log-transformed data were not significantly different from a normal distribution.
To test whether there was a significant reversal in the sign of the response to leptin administration under different levels of prenatal nutrition, the hypothesis that the differences between leptin and saline differed from zero on each level of prenatal nutrition was tested. This test was carried out as a standard multiple comparison within the analysis of variance (23). If this test was significant, and if the signs of the difference were reversed, this was evidence that a significant reverse in the response had occurred as a result of different prenatal nutrition.
Acknowledgments
This work was supported by a grant from the National Research Centre for Growth and Development. M.A.H. and G.C.B. receive salary support from the British Heart Foundation.
Abbreviations
- GR
glucocorticoid receptor
- PPAR
peroxisome proliferator-activated receptor
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- PEPCK
phosphoenolpyruvate carboxykinase
- AD
ad libitum feeding
- UN
undernutrition.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gluckman PD, Hanson MA, Spencer HG, Bateson P. Proc Biol Sci. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 3.Applebaum SW, Heifetz Y. Annu Rev Entomol. 1999;44:317–341. doi: 10.1146/annurev.ento.44.1.317. [DOI] [PubMed] [Google Scholar]
- 4.Evans JD, Wheeler DE. Proc Natl Acad Sci USA. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Beedle AS. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 6.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Mirazon Lahr M, et al. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 7.McMillen IC, Robinson JS. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey K. In: Developmental Origins of Health and Disease. Gluckman PD, Hanson MA, editors. Cambridge, UK: Cambridge Univ Press; 2006. pp. 6–32. [Google Scholar]
- 9.Gluckman PD, Hanson MA. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 10.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 11.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Am J Physiol. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 13.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman PD, Beedle AS, Hanson MA, Vickers MH. Horm Res. 2007;67(Suppl 1):115–120. [Google Scholar]
- 15.Day T, Rowe L. Am Nat. 2002;159:338–350. doi: 10.1086/338989. [DOI] [PubMed] [Google Scholar]
- 16.Desvergne B, Wahli W. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 17.Barthel A, Schmoll D. Am J Physiol. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 18.Seckl JR. Curr Opin Pharmacol. 2004;4:597–602. doi: 10.1016/j.coph.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Oates M, Woodside B, Walker C-D. Horm Behav. 2000;37:366–376. doi: 10.1006/hbeh.2000.1578. [DOI] [PubMed] [Google Scholar]
- 20.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 21.Waddington CH. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology. London: Allen & Unwin; 1957. [Google Scholar]
- 22.Bustin SA. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 23.Snedecor GW, Cochran WG. Statistical Methods. Ames: Iowa State Univ Press; 1969. [Google Scholar]