Abstract
Vibrio parahaemolyticus, a biofouling marine bacterium and human pathogen, undergoes phase variation displaying translucent (TR) and opaque (OP) colony morphologies. Prior studies demonstrated that OP colonies produce more capsular polysaccharide (CPS) than TR colonies and that opacity is controlled by the Vibrio harveyi LuxR-type transcriptional activator OpaR. CPS has also been shown to be regulated by the scrABC signaling pathway, which involves a GGDEF-EAL motif-containing sensory protein. The present study identifies cps genes and examines their regulation. Transposon insertions in the cps locus, which contains 11 genes, abolished opacity. Such mutants failed to produce CPS and were defective in pellicle formation in microtiter wells and in a biofilm attachment assay. Reporter fusions to cpsA, the first gene in the locus, showed ∼10-fold-enhanced transcription in the OP (opaR+) strain compared to a TR (ΔopaR) strain. Two additional transcriptional regulators were discovered. One potential activator, CpsR, participates in the scrABC GGDEF-EAL-signaling pathway; CpsR was required for the increased cps expression observed in scrA ΔopaR strains. CpsR, which contains a conserved module found in members of the AAA+ superfamily of ATP-interacting proteins, is homologous to Vibrio cholerae VpsR; however, unlike VpsR, CpsR was not essential for cps expression. CpsS, the second newly identified regulator, contains a CsgD-type DNA-binding domain and appears to act as a repressor. Mutants with cpsS defects have greatly elevated cps transcription; their high level of cpsA expression was CpsR dependent in ΤR strains and primarily OpaR dependent in OP strains. Thus, a network of positive and negative regulators modulates CPS production in V. parahaemolyticus.
Vibrio parahaemolyticus is a ubiquitous marine bacterium and human pathogen that can be isolated as a free-living bacterium and from a variety of animate and inanimate surfaces (24, 25, 29). V. parahaemolyticus strains retrieved from these natural sources display heterogeneity in colony morphology. The phase variation is slow and can be reversible, such that opaque (OP) and translucent (TR) clonal dimorphism is generally observed (30). The OP colony is small, mounded, and has a sticky character when touched with a toothpick. The TR colony is large, flat, and not sticky. Colony opacity correlates with increased capsular polysaccharide (CPS) production (18). One locus has been identified as controlling opacity (30). It encodes the transcriptional regulator, OpaR, which is homologous to Vibrio harveyi LuxR. Expression of opaR using an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible vector converts the TR colony to opaque, and mutation of opaR causes an OP strain to become translucent. Multiple mechanisms for this phase variation appear to exist. One mechanism involves spontaneous alterations in the opaR locus (30); many, but not all, TR strains can be converted to OP by using the opaR expression clone.
In addition to the opaR locus, the scr locus is also known to affect CPS production in V. parahaemolyticus. Lesions in the scrABC operon of a TR strain cause a rugose colony morphology that has been shown to be due to an increase in CPS production compared to the parental TR strain (6). The scrABC operon contains three genes that appear to constitute a sensory signaling pathway (6). The ScrA protein has a conserved domain homologous to pyridoxal phosphate-dependent enzymes. ScrB is predicted to be an extracellular solute-binding protein. ScrC has a periplasmic sensing domain and a cytoplasmic domain containing GGDEF and EAL motifs. Since the scrABC operon does not encode a potential transcriptional regulator, the mechanism for increased cps transcription in scr mutants is not apparent.
Here we describe transposon mutagenesis of OP and rugose strains to gain further insight into the role and regulation of CPS in V. parahaemolyticus. A large gene cluster required for CPS production was identified, as well as two unlinked genes encoding transcriptional regulators of cps gene expression.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. All V. parahaemolyticus strains are derivatives of LM5312 (also known as [aka] BB22OP [30]). The translucent strain (LM5674; aka BB22TR) used in this study contains a small deletion in the opaR gene and was spontaneously derived from LM5312 (4, 30). Strain LM5733 was constructed by introducing the polar scrA::Camr allele into the LM5674 background (6). V. parahaemolyticus strains were propagated at 30°C in HI broth (25 g of heart infusion broth [Difco] and 20 g of NaCl per liter) or on HI plates (25 g of heart infusion broth [Difco], 15 g of NaCl, and 20 g of agar per liter). Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; phosphomycin, 60 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 10 μg/ml; tetracycline, 10 μg/ml; gentamicin, 20 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Parent or source |
|---|---|---|
| E. coli | ||
| DH5α | F− λ−recA1 Δ(lacZYA-argF)U169 hsdR17 thi-1 gyrA96 supE44 endA1 relA1 φ80dlacZΔM15 glnV44 | Bethesda Research Labs |
| S17-1 λpir | thi pro hdsR hdsM+ recA, chromosomal insertion of RP4-2 (Tc::Mu Km::Tn7) λpirR6K | de Lorenzo (14) |
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | Wanner (12) |
| V. parahaemolyticus | ||
| LM5312 | Wild type (OP) | aka BB22OP (30) |
| LM5432 | Spontaneous Phosr (OP) | LM5312 |
| LM5674 | ΔopaR (TR) | aka BBTR, derived from BB22OP (30) |
| LM5733 | scrA1::Camr ΔopaR | LM5674 (6) |
| LM5818 | cpsA1::lacZ-Kanr ΔopaR | LM5674 |
| LM5819 | cpsA1::lacZ-Kanr | LM5312 |
| LM5959 | scrA1::CamrcpsD1::Tn5-RL27(Kanr) ΔopaR | LM5733 |
| LM5962 | scrA1::CamrcpsR1::Tn5-RL27(Kanr) ΔopaR | LM5733 |
| LM5964 | scrA1::CamrcpsS1::Tn5-RL27(Kanr) ΔopaR | LM5733 |
| LM5984 | cpsA3::lacZ-Genr ΔopaR | LM5674 |
| LM5985 | cpsA3::lacZ-Genr | LM5312 |
| LM6133 | cpsA3::lacZ-GenrcpsR1::Tn5-RL27(Kanr) ΔopaR | LM5984 |
| LM6134 | cpsA3::lacZ-GenrcpsR1::Tn5-RL27(Kanr) | LM5985 |
| LM6135 | cpsS1::Tn5-RL27(Kanr) ΔopaR | LM5674 |
| LM6136 | cpsS1::Tn5-RL27(Kanr) | LM5312 |
| LM6137 | cpsS1::Tn5-RL27(Kanr) cpsA3::lacZ-Genr ΔopaR | LM6135 |
| LM6138 | cpsS1::Tn5-RL27(Kanr) cpsA3::lacZ-Genr | LM6136 |
| LM6166 | cpsA2::Tn5-RL27(Kanr) | LM5312 |
| LM6239 | cpsA3::lacZ-GenrcpsS1::Tn5-RL27(Kanr) ΔopaR | LM5984 |
| LM6241 | cpsA3::lacZ-GenrscrA1::Camr ΔopaR | LM5984 |
| LM6243 | cpsA3::lacZ-GenrcpsR1::Tn5-RL27(Kanr) scrA1::Camr ΔopaR | LM6133 |
| LM6261 | cpsR2::lacZ-Genr ΔopaR | LM5674 |
| LM6262 | cpsR2::lacZ-Genr | LM5312 |
| LM6263 | scrA1::CamrcpsR2::lacZ-Genr ΔopaR | LM5733 |
| LM6264 | cpsS1::Tn5-RL27(Kanr) cpsR2::lacZ-Genr ΔopaR | LM6135 |
| LM6265 | cpsS1::Tn5-RL27(Kanr) cpsR2::lacZ-Genr | LM6136 |
| LM6296 | cpsA2::Tn5-RL27(Kanr) | LM5432 |
| LM6299 | cpsB1::Tn5-RL27(Kanr) | LM5432 |
| LM6305 | cpsB2::Tn5-RL27(Kanr) | LM5432 |
| LM6306 | cpsF1::Tn5-RL27(Kanr) | LM5432 |
| LM6307 | cpsH1::Tn5-RL27(Kanr) | LM5432 |
| LM6369 | cpsA3::lacZ-GenrcpsS1::Tn5-RL27(Kanr) cpsR3::Camr ΔopaR | LM6239 |
| LM6502 | cpsB1::Tn5-RL27(Kanr) | LM5312 |
| LM6505 | cpsA1::lacZ-KanrcpsR3::Camr ΔopaR | LM5818 |
| LM6654 | cpsA3::lacZ-GenrcpsS1::Tn5-RL27(Kanr) cpsR3::Camr | LM6369 |
| LM6836 | cpsA1::lacZ-Kanr ΔopaR/pLM1877 | LM5818 |
| LM6838 | cpsA1::lacZ-Kanr ΔopaR/pLM2449 | LM5818 |
| LM6841 | cpsA1::lacZ-KanrcpsR3::Camr ΔopaR/pLM2449 | LM6505 |
| Plasmids | ||
| PLAFRII | Tetr, cosmid vector | Friedman (21) |
| pRL27 | Kanr, Tn5-RL27(oriR6K) delivery vector | Metcalf and Larsen (28) |
| pKD46 | Kanr, Red recombinase expression vector | Wanner (12) |
| pHRP314 | Kanr Ampr, lacZ::Kanr | Harwood (36) |
| pUClacZGm | Genr Ampr, lacZ::Genr | Ferrández (19) |
| pLM1877 | Genr, expression vector | McCarter (5) |
| pLM1950 | Tetr, opaR+ | McCarter (30) |
| pLM2102 | Tetr, pLAFRII control cosmid from bank | V. parahaemolyticus cosmid bank |
| pLM2449 | Genr, IPTG-inducible scrC+ | McCarter (6) |
| pLM2846 | Tetr, cosmid containing cpsA locus | V. parahaemolyticus cosmid bank |
| pLM3010 | Kanr, cpsR1::Tn5-RL27(oriR6K) transposon junction plasmid | LM5962 |
| pLM3040 | Kanr, cpsS1::Tn5-RL27(oriR6K) transposon junction plasmid | LM5964 |
| pLM3052 | Tetr, cosmid containing cpsR locus | V. parahaemolyticus cosmid bank |
| pLM3119 | Tetr Kanr, cpsR1::Tn5-RL27 | pLM3052 |
| pLM3120 | Tetr, cosmid containing cpsS locus | V. parahaemolyticus cosmid bank |
| pLM3121 | Tetr Kanr, cpsS1::Tn5-RL27 | pLM3120 |
| pLM3122 | Tetr Genr, cpsA3::lacZ-Genr | pLM2846 |
| pLM3123 | Tetr Kanr, cpsA1::lacZ-Kanr | pLM2846 |
| pLM3131 | Tetr, cosmid containing cpsA locus | V. parahaemolyticus cosmid bank |
| pLM3132 | Tetr Kanr, cpsA2::Tn5-RL27 | pLM2846 |
| pLM3170 | Tetr Camr, cpsR3::Camr | pLM3052 |
| pLM3171 | Tetr Genr, cpsR2::lacZ-Genr | pLM3052 |
| pLM3203 | Tetr Kanr, cpsB1::Tn5-RL27 | pLM3131 |
DNA manipulation, analysis, and reagents.
DNA ligation, gel electrophoresis, Southern analysis, and colony hybridizations were carried out according to Sambrook et al. (40). Oligonucleotides were synthesized by IDT Technologies (Coralville, Iowa). The cps cosmids were retrieved from a cosmid library, described in reference 31, after colony hybridization using arbitrary PCR products obtained from cps mutants. DNA sequencing was performed at the DNA Core Sequencing Facility of the University of Iowa. DNA sequences were assembled using the Genetics Computer Group software package (Madison, Wis.). Homology searches were performed at the National Center for Biotechnology Information with the BLAST network service (2).
Tn5 mutagenesis and pellicle screen for cps mutants.
Tn5 mutagenesis of an OP V. parahaemolyticus strain was performed by using conjugation between the donor Escherichia coli strain bearing pRL27, a Tn5 delivery vector, and the wild-type OP strain, which was made resistant to phosphomycin according to the selection method of Alper and Ames (1) for purposes of counterselection. Inoculated from a single-colony, the freshly grown recipient OP strain (LM5432) at an optical density at 600 nm (OD600) of 0.6 was mixed with an equal volume of an overnight-grown donor strain. The conjugation mix was plated on HI plates that were prepared without adding NaCl, incubated at 37°C overnight, and then suspended in HI broth for plating on HI medium with phosphomycin and kanamycin. After overnight growth at 30°C, mutant colonies were picked on grids of HI plates, and a pellicle assay was subsequently followed to screen for cps mutants in 96-well, tissue culture-treated, nonpyrogenic polystyrene microtiter dishes (no. 3596; Corning Inc.). Cells were first transferred in microtiter wells that were filled with 200 μl of HI medium containing EGTA at 0.1 mM final concentration and statically incubated for 16 h at 30°C. Pellicle formation was inhibited in the presence of EGTA, thus allowing approximately equal amounts of cell transfer for the next set of microtiter wells that were filled with HI medium only. Pellicle formation was scored after 16 h of growth in these cultures. Pellicles were photographed by using a Kodak digital imaging system. Zoom images were taken by using an Olympus SZX12 stereomicroscope.
Identification of the Tn5 insertion site.
The arbitrary PCR protocol and random primers described by O'Toole and Kolter (34) were employed to determine the transposon insertion sites. PCR products were resolved by agarose gel electrophoresis and gel purified by using a gel extraction kit (Qiagen) for sequencing. The nested PCR primers specific for the transposon were KANARB1 (5′-ATTTAAATCTTAGAGTCGACCTGCAGGC-3′) and KANARB2 (5′-GCATGCAAGCTTCAGGGTTGAG-3′), which prime to the right end of the transposon, and KANARB3 (5′-TGACACAGGAACACTTAACGGCTGAC-3′) and KANARB4 (5′-GTACCGAGCTCGAATTCATCGATGATG-3′), which prime to the left end.
Targeted gene disruptions and allelic exchange.
The λ red recombinase technique was adapted from Datsenko and Wanner (12) to introduce mutations onto V. parahaemolyticus cosmid DNA in E. coli. The recombinant cosmids bearing the gene disruptions were then used for allelic replacement in V. parahaemolyticus as previously described (42).
Reconstruction of Tn5 insertions.
Regions encompassing the Tn5 insertions in the cpsA and cpsB genes were amplified from the mutant chromosome by PCR. Primers for cpsA were 5′-CTCTCAGCGTGTTCGATACGCC-3′ (CPSA-F) and 5′-CGTGGCTTGCTTGATGTTTGGG-3′ (CPSA-R). Primers for cpsB were 5′-GAAGACATCACTGAAGGACAAGG-3′ (CPSB-F) and 5′-TAAGGTACTCGGTGAAGACTTG-3′ (CPSB-R). The PCR fragments carrying the Tn5 insertion and flanking DNA sequence for cpsA and cpsB were recombined into cosmids pLM2846 and cosmid pLM3131, respectively, yielding pLM3132 (cpsA2::Tn5) and pLM3203 (cpsB1::Tn5).
The cpsR and cpsS mutations were cloned by taking advantage of the oriR6K contained on the Tn5-RL27. Mutant chromosomal DNA was digested with BamHI and self-ligated. Ligation reactions were transformed into E. coli strain S17-1 λpir, and Kanr transformants were selected. The transposon-bearing plasmids were linearized with BamHI and recombined into cosmids pLM3052 and pLM3120, respectively. The resulting constructs were pLM3119 (cpsR1::Tn5) and pLM3121 (cpsS1::Tn5).
Construction of a transcriptional lacZ fusion to cpsA and cpsR.
The lacZ fusion to cpsA was constructed with lacZ-Kan (Kanr) and lacZ-Gen (Genr) cassettes. Specific activities of the Kan and Gen varieties of the fusions were different, but in all cases the patterns of regulation were similar. A 257-bp fragment from the coding region of cpsA was PCR amplified from the chromosome of the OP strain using CPSA-F and CPSA-R and ligated into HincII-digested pUC19 to make pUCCPS257. pHRP314 (36), containing a lacZ-Kanr cassette, was digested with BamHI, releasing a 5.25-kb BamHI fragment that was ligated into HincII-digested pUCCPS257. The site of HincII cleavage occurred approximately in the middle of the 257-bp insert. The resulting construct was digested with XbaI and PstI, releasing the lacZ-Kanr cassette flanked by a small DNA sequence of cpsA. The fragment was recombined onto the cps cosmid pLM2846 to yield pLM3123 (cpsA1::lacZ-Kanr). The lacZ-Genr fusion to cpsA was similarly constructed. The lacZ-Genr cassette was released by SmaI digestion of pUClacZGm (19) and ligated into HincII-digested pUCCPS257. The resulting construct was digested with XmaI and PstI to release the lacZ-Genr cassette flanked by the cpsA sequence, which was recombined into the cps cosmid, yielding pLM3122 (cpsA3::lacZ-Genr).
For creation of the cpsR::lacZ fusion strain, primers 5′-CGTTGACCTGAGCCATGACGAG-3′ (CPSR-F) and 5′-CGTTGCTCATTCAAAGCGCGGCAG-3′ (CPSR-R) were used to amplify 458 bp from the coding region of cpsR, and the product was cloned into pUC19 at a HincII site, yielding pUCCPSR458. This construct was digested with MscI and ligated with a SmaI fragment containing the lacZ-Genr cassette (19). To make the cpsR::Camr mutation, MscI-linearized pUCCPSR458 was also ligated with a SmaI fragment carrying a gene for chloramphenicol resistance (Camr) derived from p34S-Cm (15). The resulting constructs were digested with XmaI and PstI to release lacZ-Genr or with XbaI and PstI to release the Camr cassette. Recombination onto pLM3052 yielded pLM3171 (cpsR2::lacZ-Genr) and pLM3170 (cpsR3::Camr).
Analysis of CPS by SDS-PAGE.
CPS extracted from plate-grown cells was analyzed in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (18). Briefly, cells were streaked from a single colony onto the entire surface of HI plates and incubated for 16 h at 30°C. Cells were suspended in phosphate-saline buffer, and culture densities were equally adjusted and extracted for CPS. Samples, treated with RNase, DNase I, and proteinase K, were extracted with phenol-chloroform. After ethanol precipitation, samples were suspended in 250 μl of water, and 20 μl of each sample was analyzed by SDS-PAGE. The stacking gel, which was 6.5 cm long, was stained with Stains-All (U.S. Biochemicals, Cleveland, Ohio) to visualize CPS (26).
Quantification of adherence.
Quantification of adherence by V. parahaemolyticus was examined by a crystal violet staining technique as described by O'Toole and Kolter (34). Cells were streaked from a single colony onto the entire surface of an HI plate. After 16 h of growth, cells were harvested in HI broth and the OD600 was adjusted to 0.01. Culture aliquots (200 μl) were added to polystyrene microtiter wells and statically incubated for 12 h at 30°C. The pellicle that formed at the air-liquid interface was removed with a cotton swab applicator. Cells were stained with 1% crystal violet aqueous solution for 15 min. The solution was removed, and the wells were washed with water three times to remove unattached bacteria. Surface-attached bacteria were solubilized with 500 μl of dimethyl sulfoxide, and water was added to bring the final volume to 1 ml. The absorbance (570 nm) of the resulting solution was measured. Measurements were done in triplicate. Experiments were repeated at least twice.
β-Galactosidase assays.
Cells were streaked from a single colony onto the entire surface of an HI plate. After 16 h of growth, cells were harvested in HI broth and the OD600 was adjusted to 0.05. Aliquots of 100 μl of this culture were spread onto HI plates and incubated at 30°C for various times. Cells were harvested from plates in HI broth. Activity assays were performed in triplicate according to Miller (33). Experiments were repeated at least twice.
Nucleotide sequence accession numbers.
The GenBank nucleotide accession numbers are as follows: AY216912 (cpsR locus), AY216913 (cpsS locus), and AY217749 (cpsA locus).
RESULTS
Identification of a V. parahaemolyticus cps locus.
The OP strain was mutagenized to identify genes involved in the production of CPS. Potential mutants that were not opaque were subsequently tested for conversion to OP on the introduction of an opaR-encoding plasmid. Colonies that failed to be converted were candidates for having lesions in cps genes, in contrast to colonies that could be converted to OP by OpaR and were most likely translucent due to phase variation of opaR (30). From this screen of 36,000 transposon mutants, 288 were TR; however, only 1 was not convertible by OpaR. The mutation in this strain causing loss of opacity was identified by sequencing a DNA fragment obtained from an arbitrarily primed PCR. The gene interrupted by the transposon encoded a product homologous to VV21581, a conserved hypothetical protein from Vibrio vulnificus, and VC0935 from Vibrio cholerae, which is found in a cluster of genes that was shown to be responsible for exopolysaccharide production in V. cholerae O1 El Tor (51).
Pellicle defects in microtiter dishes.
The exopolysaccharide mutant, LM6305, displayed a distinctive phenotype: when grown in liquid medium in a test tube, it did not form a pellicle at the air-liquid interface. This observation suggested an additional method for identifying cps mutants, i.e., by screening for pellicle-defective phenotypes. The mutagenesis was repeated, screening first for nonopaque colonies and secondly for pellicle defects in microtiter wells. Ninety-nine out of 14,000 mutants had nonopaque, nonsticky colony morphologies, and 27 of these were pellicle defective. The pellicle-defective mutants were also examined for motility in semisolid motility agar, because flagellar mutants produce pellicle-minus phenotypes (L. McCarter, personal observation). Twenty were nonmotile, and further analysis of these mutants was deferred. Presumably, the majority of the pellicle-defective mutants contained transposon lesions in flagellar genes and had spontaneously converted to the TR colony morphology. We focused on the characterization of the pellicle-minus, motility-plus mutants. None of these mutants could be converted to OP by opaR expression. Southern blot analysis, after probing with the transposon, suggested that three of the seven mutants potentially were siblings.
Transposon insertions in four distinct mutants were identified by sequencing the DNA fragments obtained from arbitrarily primed PCR products. Mutant strain LM6296 had an insertion (cpsA2::Tn5) in a gene encoding a product that was homologous to V. vulnificus VV21582, a predicted sugar transferase gene involved in lipopolysaccharide synthesis (Table 2). Another mutant (LM6299) had an insertion (cpsB1::Tn5) in the VV21581 homolog that was identified in the first mutagenesis screen. The third disrupted gene (cpsF) encoded a potential glycosyltransferase. No conserved domains were found in the fourth predicted gene product (CpsH); however, the product was homologous to VV21577, which has been shown to be essential for cps production in V. vulnificus (43). Additional sequencing on cosmids retrieved from a V. parahaemolyticus cosmid bank showed that all of the mutations were linked in a large cluster of 11 candidate cps genes (Fig. 1). Sequence analysis suggested a potential for an operon-type organization, because there were little or no intergenic spacings between coding regions. One of the cosmids, pLM3131, restored opacity when transferred to a cpsA mutant. To further confirm that mutations causing loss of opacity were due to disruptions in the cps locus, the insertions in the cpsA and cpsB genes were reintroduced into the wild-type OP strain by allelic exchange to make strains LM6166 (cpsA2::Tn5) and LM6502 (cpsB1::Tn5). A transcriptional lacZ fusion to the cpsA gene was also introduced into the OP chromosome to make LM5819 (cpsA1::lacZ-Kanr). The colony morphologies of the reconstructed strains were nonopaque, and opacity was restored in these strains on the introduction of cosmid pLM3131 and not of a control cosmid (pLM2102).
TABLE 2.
Genes in the V. parahaemolyticus cps locus
| Genea | Conserved domain(s)b | Homologous gene, organismc | % Identity/% positive | E value |
|---|---|---|---|---|
| cpsAd | WcaJ, sugar transferase (COG2148) sugar transferase (pfam02397) | VV21582, V. vulnificus | 75/85 | 0.0 |
| cpsB | Uncharacterized, conserved (COG5338) | VV21581, V. vulnificus | 56/70 | e−125 |
| cpsC | Wza, polysaccharide export (COG1596); polysaccharide biosynthesis/export (pfam02563) | VV21580, V. vulnificus | 66/83 | 1e−57 |
| cpsD | GumC, exopolysaccharide synthesis (COG3206); Mrp, ATPase involved in chromosome partitioning (COG0489) | VV21579, V. vulnificus | 65/82 | 0.0 |
| cpsE | No domain | VV21577, V. vulnificus | 27/50 | 5e−21 |
| cpsF | RfaG, glycosyltransferase (COG0438) glycosyltransferase group I (pfam00534) | VV21578, V. vulnificus | 64/78 | e−128 |
| cpsG | No domain | VV21627, V. vulnificus | 36/54 | 4e−64 |
| cpsH | No domain | VV21577, V. vulnificus | 75/86 | e−127 |
| cpsI | RfaG, glycosyltransferase (COG0438) | VV21578, V. vulnificus | 62/78 | e−125 |
| cpsJe | Wzx, flippase (43); RfbX (COG2244) | VV21575, V. vulnificus | 56/74 | e−102 |
| cpsKf | No domain | VV21574, V. vulnificus | 48/60 | 2e−13 |
Genes highlighted in bold were isolated from mutagenesis of the OP or scr strains.
Conserved domains were identified by a BLAST search at NCBI Conserved Domain Search.
Deduced amino acid sequence of an entire ORF was used in a BLAST homology search. Highest-scored gene and organism were reported.
A coding region upstream of cpsA (<1..564) encodes a potential protein that resembles MalK, the maltose/maltodextrin ABC transporter of many bacteria.
Sequence analysis was terminated within the cpsJ coding region (148 aa); however, the recently published sequence of the genome of V. parahaemolyticus RIMD 2210633 predicts an encoding 435 aa.
The cpsK sequence (VPA1413) was retrieved from the genome sequence of V. parahaemolyticus RIMD 2210633.
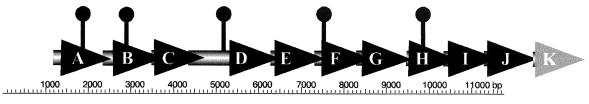
FIG. 1.
Physical map of the cpsA locus and location of transposons causing loss of opacity. Open reading frames and direction of transcription are depicted by arrows. Transposon insertions are indicated as balloon drawings and map at 1,946 bp (LM6296), 2,991 bp (LM6299), 5,337 bp (LM5959), 7,552 bp (LM6306), and 9,853 bp (LM6307). The 11,750 bp that were sequenced in this study (GenBank accession no. AY217749) are 98% identical with the sequenced V. parahaemolyticus strain RIMD2210633. Existence of cpsK is inferred from the genome database (GenBank accession no. AP005088).
Nonopaque mutants fail to produce CPS and display attachment defects.
CPS production was examined by extracting plate-grown OP, TR, and mutant strains. Mutants with the original Tn5 insertions in cpsA and cpsB as well as the reconstructed mutants, which were made by allelic exchange in unmutagenized backgrounds, failed to produce extracellular material that was detectable using Stains-All, a cationic carbocyanine dye that stains acidic polysaccharides (26). Figure 2 shows the SDS-PAGE profile of extracts prepared from the reconstructed mutants with and without the complementing cosmid, pLM3131. Similar to the TR strain (Fig. 2, lane 2), mutant strains LM6166 (cpsA2::Tn5) and LM6502 (cpsB1::Tn5) did not produce detectable CPS material (Fig. 2, lanes 3 and 5); however, the mutant strains bearing cosmid pLM3131 (lanes 4 and 6) produced CPS equivalent to the OP strain (lane 1). Thus, the cpsA and cpsB genes are required for CPS production, and the cosmid pLM3131 carries sufficient DNA to complement the lack of CPS production in the cpsA and cpsB mutant strains.
FIG. 2.
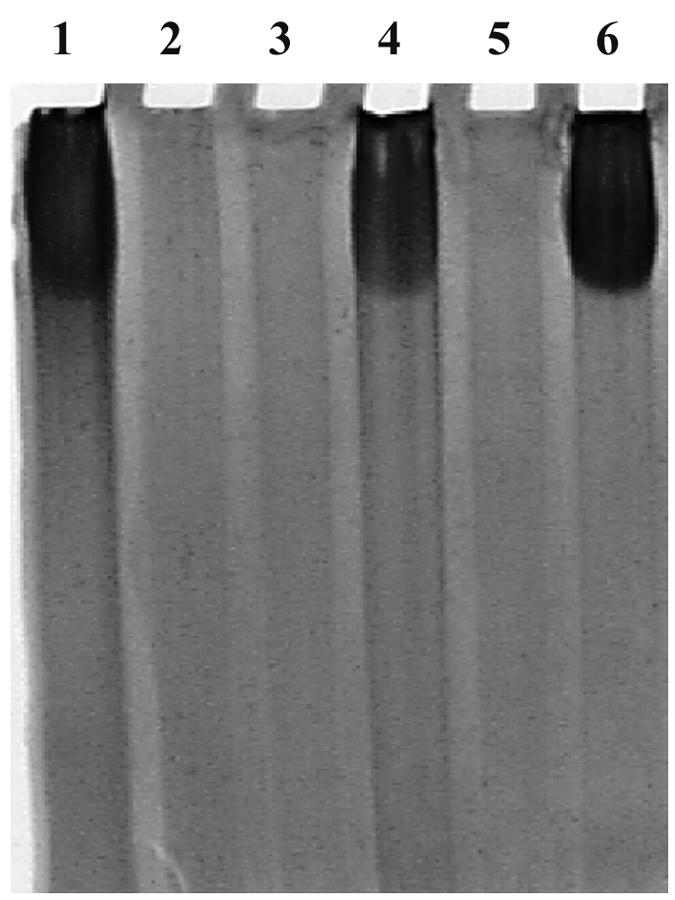
SDS-PAGE analysis of CPS extracted from the OP, TR, and cps mutant strains. Gel was stained with Stains-All to visualize the polysaccharide. Lanes: 1, LM5312 (OP strain); 2, LM5674 (TR strain; ΔopaR); 3, LM6166 (cpsA2::Tn5); 4, LM6166/pLM3131 (cpsA2::Tn5/CPS cosmid); 5, LM6502 (cpsB1::Tn5); and 6, LM6502/pLM3131 (cpsB1::Tn5/CPS cosmid).
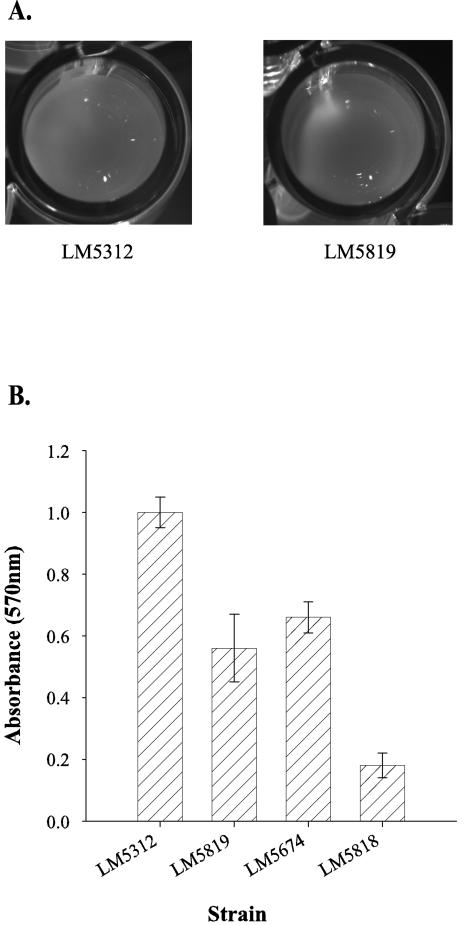
Similar to the original mutants, the reconstructed mutant strains were defective in pellicle formation. Figure 3A shows the pellicle-minus phenotype of the strain LM5819 (cpsA1::lacZ-Kanr) and the pellicle-plus phenotype of the OP strain. Both strains were statically grown in HI in polystyrene microtiter wells. The wild-type strain formed a pellicle at the air-liquid interface (obscuring the bottom of the microtiter well), whereas the cpsA mutant did not form a pellicle (allowing visualization of the bottom of the well), even after 16 h of incubation. Furthermore, the adherence properties of the cps mutants were altered. Attachment to the polystyrene wells was measured by performing a crystal violet staining assay (Fig. 3B). Measurements taken after 12 h of growth showed that adherence was 44% reduced in a cpsA1::lacZ-Kanr mutant compared to that of the OP parent strain, indicating that the cpsA gene contributes to, but is not solely responsible for, the adherence properties of surface-attached V. parahaemolyticus cells. Attachment was also affected in the TR strain; surface attachment by the ΔopaR strain (LM5674) was 34% less than that with the OP (opaR+) strain, and it was further reduced by the cpsA1::lacZ-Kanr mutation (strain LM5818) (Fig. 3B).
FIG. 3.
Pellicle and adherence phenotype of the cpsA mutant. (A) Pellicle formation by the LM5312 (OP) and LM5819 (cpsA1::lacZ-Kan) strains in polystyrene plastic microtiter wells. Cells inoculated in HI were statically grown for 16 h. Pictures show a view of the wells from above. (B) Crystal violet staining assay to quantitate attachment to a polystyrene plastic surface by LM5312 (OP), LM5819 (cpsA1::lacZ), LM5674 (TR; ΔopaR), and LM5818 (cpsA1::lacZ ΔopaR) strains after 12 h of growth at 30°C.
Analysis of cpsA::lacZ expression in TR and OP strains.
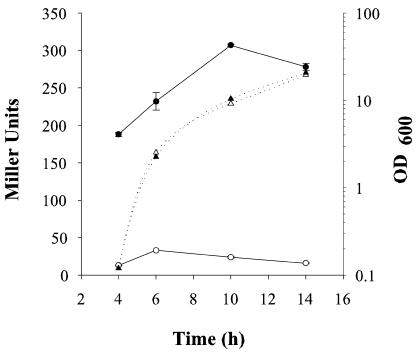
Colony opacity is correlated with CPS production in V. parahaemolyticus. The amount of CPS extracted from plate-grown OP cells is significantly more than that of TR strains (18). To examine whether this difference in CPS production is due to transcriptional regulation of gene expression, β-galactosidase activities were monitored in strains carrying a lacZ transcriptional fusion to cpsA introduced into the chromosome of the OP (opaR+) and TR (ΔopaR) strains. Strain LM5819 (opaR+) produced about 10-fold more β-galactosidase activity than LM5818 (ΔopaR), which had a low level of expression throughout growth (Fig. 4). These data are consistent with the 12-fold difference in total carbohydrate measured using the phenol-sulfuric acid method (18) and suggest that the difference in CPS production between opaR+ and opaR mutant strains is the result of transcriptional regulation.
FIG. 4.
cpsA::lacZ expression in TR and OP strains. β-Galactosidase activities (solid lines) were monitored over time from cells grown on plates. Culture turbidity is shown with dotted lines. Open symbols represent LM5818 (TR; ΔopaR cpsA1::lacZ). Filled symbols represent LM5819 (OP; cpsA1::lacZ).
Identification of cpsR and cpsS, additional potential regulatory genes controlling cps gene expression.
In addition to opaR, the scrABC operon is another locus affecting CPS production in translucent V. parahaemolyticus strains (6). Mutations in the scrABC operon cause a rough colony morphology due to overproduction of CPS. To learn more about the regulatory pathway by which the scrABC locus influences cps transcription, strain LM5733, containing a polar scrA insertion, was mutagenized. Colony morphologies for 8,000 Kanr transposon mutants were examined, and six mutants possessing phenotypes different from the rugose parental colony were identified. Five colonies were smooth, and one was super rugose.
Among the smooth mutants, two were characterized in this study; the other smooth mutants remain to be analyzed. One smooth mutation mapped in the cpsD gene (Table 2), demonstrating the role of CPS in the scr phenotype. Another mutation was found in an unlinked locus that was named cpsR. The deduced amino acid sequence of CpsR (444 amino acids [aa]) strongly suggested that it was a transcriptional regulator, showing significant conserved domain alignments with COG2204 (AtoC), COG3829 (RocR), and COG3283 (TyrR). CpsR yielded 78% identity and 90% positives with VpsR of V. cholerae in a BLAST comparison analysis (E = 0.0; 711 bits). VpsR is required for exopolysaccharide production in V. cholerae (50). Although CpsR resembles a number of transcriptional regulators similar to NtrC, it does not appear to require σ54. Introduction of an rpoN mutation into the rough scr strain did not convert the strain to smooth, unlike the cpsR1::Tn5 allele (data not shown).
The transposon mutation causing the superrugose colony morphology was found in an open reading frame coding for another potential transcription regulator named CpsS. The deduced amino acid sequence of CpsS (223 aa) contained a conserved helix-turn-helix DNA-binding domain (smart00421; HTH_LuxR) and showed homology to a number of known and potential transcriptional regulators, including CsgD of Salmonella enterica serovar Typhimurium (36% identity and 56% positives; 112 bits; E = 3e−24). CsgD is a positive transcriptional regulator of the thin aggregative fibers known as curli (38).
CpsR mediates rugose colony morphology of scr mutants, but it is not required for CPS production in TR or OP strains.
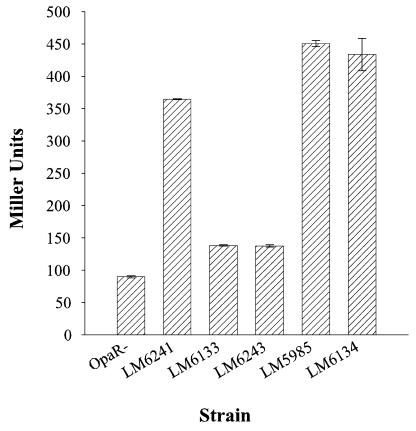
Reconstruction experiments confirmed that introduction of the cpsR1::Tn5 mutation into an scrA ΔopaR mutant strain converted the rugose colony morphology to smooth. To probe the role of cpsR in the regulation of cps gene expression, the cpsR1::Tn5 mutation was also introduced into various cpsA::lacZ fusion strains (Fig. 5). In the TR background (i.e., ΔopaR), an scr mutation increased β-galactosidase activity fourfold (compare strain LM6241 with LM5984); however, the increased cps gene expression was abolished on the introduction of the cpsR1::Tn5 allele into strain LM6241 (to make strain LM6243). Levels of β-galactosidase activity in the scrA cpsR mutant LM6243 were similar to the level of cpsA::lacZ activity observed for the TR strain (LM5984). Thus, loss of function of cpsR was dominant to the scrA1::Camr allele.
FIG. 5.
A cpsR lesion affects cpsA::lacZ expression in a scrA::Camr ΔopaR mutant but not in OP or TR (ΔopaR) strains. β-Galactosidase activities were measured from cells grown on plates for 24 h. Strains: LM5984 (TR; ΔopaR); LM6241 (scrA1::Camr ΔopaR); LM6133 (cpsR1::Tn5 ΔopaR); LM6243 (scrA1::Camr cpsR1::Tn5 ΔopaR); LM5985 (OP); LM6134 (cpsR1::Tn5).
Prior work demonstrated that overexpression of scrC caused increased expression of cpsA (6). To further analyze the relationship of CpsR and the scrABC signaling pathway, the effect of overexpression of scrC on the regulation of cpsA::lacZ was compared in cpsR+ and cpsR-deficient backgrounds. Reporter strains were harvested after 24 h of incubation on plates supplemented with 0.1 mM IPTG and gentamicin. Strain LM6838 harboring the scrC expression plasmid pLM2449 produced 833 ± 14 Miller units of β-galactosidase activity; in comparison strain LM6836, containing only the vector, produced 112 ± 2 Miller units. However, the cpsR mutant strain LM6841, which also contained plasmid pLM2449, produced 173 ± 2 Miller units. Therefore, the level of reporter activity in strain LM6841 was comparable to the level measured in the absence of ScrC overproduction. This result is consistent with a dominant role for cpsR in the scr signaling pathway: CpsR was required for the activation of cpsA::lacZ that is induced by scrC overexpression.
Introduction of the cpsR mutation into the TR strain (to make LM6133) caused no alteration in the levels of β-galactosidase, indicating that CpsR was not required for the cps expression observed in the TR strain (LM5984). Furthermore, CpsR was not required for CPS production in OP strains. Introduction of cpsR1::Tn5 into the OP strain (to make LM6134) had no effect on the high levels of β-galactosidase activity produced by the parental OP strain (LM5985). So, although CpsR was required for cps expression in the scr mutant strain, it was not required for the basal level of expression in the TR strain or the higher level of expression in the OP strain.
Disruption of cpsS results in high-level cps gene expression in TR and OP strains.
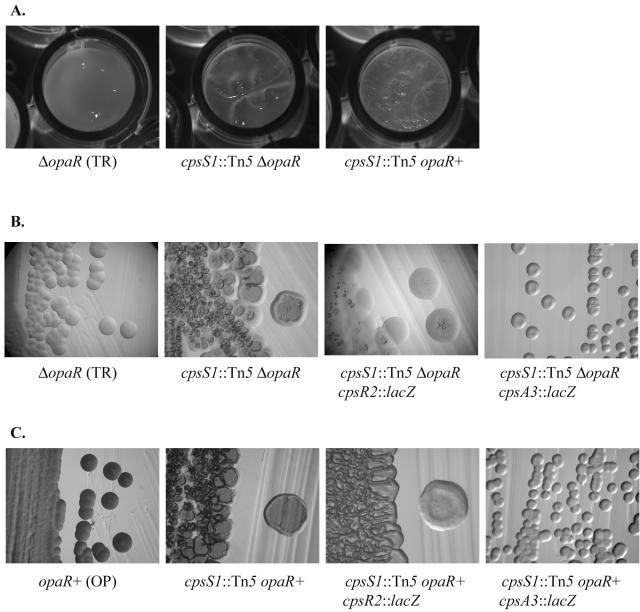
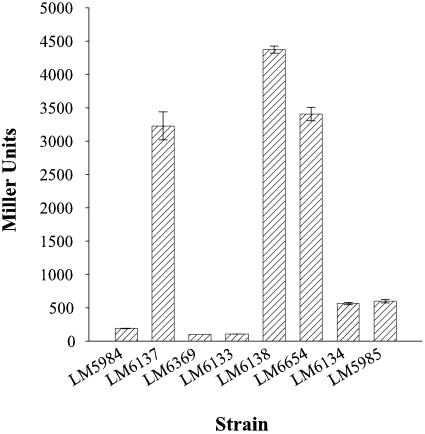
To assess the role of CpsS, the second newly discovered regulator, the cpsS1::Tn5 insertion was introduced into the TR strain. The resultant strain (LM6135) possessed crinkly pellicle and superrugose colony morphologies compared to that of the parent strain (LM5674), which had smooth pellicle and colony morphologies (Fig. 6A and B). This indicated that a lesion in cpsS was sufficient to induce changes in colony and pellicle morphologies and was not dependent on the scr signaling pathway. Introduction of cpsS1::Tn5 into the OP strain (to make LM6136) also produced crinkly pellicle and superrugose colony morphotypes (Fig. 6A and C). To examine whether these phenotypes were due to changes in cps expression, cpsA3::lacZ-Genr reporter fusion was monitored. In the TR background, the cpsS mutant strain LM6137 produced about 17-fold more β-galactosidase than its parental strain, LM5984 (3,225 and 186 Miller units, respectively [Fig. 7]). Significant derepression of cpsA gene expression was also observed on the introduction of the cpsS allele into the OP background (to make strain LM6138 [Fig. 7]). Furthermore, introduction of the cpsA3::lacZ allele, which prevented CPS biosynthesis, into the TR and OP cpsS mutants diminished the rough colony phenotypes (Fig. 6B and C).
FIG. 6.
The cpsS phenotype. (A) Pellicle; (B and C) colony morphologies. Strains: LM5674 (ΔopaR); LM6135 (cpsS1::Tn5 ΔopaR); LM6264 (cpsS1::Tn5 cpsR2::lacZ ΔopaR); LM6137 (cpsS1::Tn5 cpsA3::lacZ ΔopaR); LM5312 (OP); LM6136 (cpsS1::Tn5); LM6265 (cpsS1::Tn5 cpsR2::lacZ); LM6138 (cpsS1::Tn5 cpsA3::lacZ).
FIG. 7.
A lesion in cpsS increases cpsA::lacZ expression. β-Galactosidase activities were measured from cells grown on plates for 24 h. Strains containing cpsA1::lacZ were as follows: LM5984 (TR; ΔopaR); LM6137 (cpsS1::Tn5 ΔopaR); LM6369 (cpsS1::Tn5 cpsR3::Camr ΔopaR); LM6133 (cpsR1::Tn5 ΔopaR); LM6138 (cpsS1::Tn5); LM6654 (cpsS1::Tn5 cpsR3::Camr); LM6134 (cpsR1::Tn5); LM5985 (OP).
β-Galactosidase activity was examined in a TR strain that contained lesions in both regulatory genes, cpsR and cpsS (LM6369) (Fig. 7). The high level of reporter gene expression observed in the cpsS mutant strain LM6137 was dependent on CpsR; activity was reduced in strain LM6369 to 97 Miller units, similar to the basal levels observed in the TR strain LM5984 or cpsR-defective strain LM6133. Heightened cpsA expression in the cpsS1::Tn5 opaR+ strain LM6138 was not solely dependent on CpsR; the activity of the reporter gene in the cpsR cpsS strain LM6654 was only slightly reduced compared to that of LM6138 and about sixfold higher than that of the OP strain LM5985 or cpsR strain LM6134 (Fig. 7). Thus, loss of function of cpsS resulted in high-level expression of cps genes that was dependent on CpsR in the ΔopaR strain but mainly dependent on OpaR in the opaR+ strain.
The combination of these alleles (cpsS1::Tn5 and cpsR2::lacZ) in the cps+ strain (LM6264) in the ΔopaR background produced a smoother colony (Fig. 6B); however, the colony morphology was slightly irregular and not equivalent to the smooth colonies of the TR (LM5674) strain. These results suggest cpsS may control expression of additional genes. In the opaR+ background, the rough colony morphology of the cpsS cpsR mutant (LM6265 [Fig. 6C]) was also somewhat reduced compared to the cpsS mutant, but the morphology was still quite rough compared to the OP strain (LM5312). Therefore, the colony morphologies of the cpsS cpsR strains correlated with the levels of cps expression measured in the reporter strains.
Analysis of cpsR::lacZ expression.
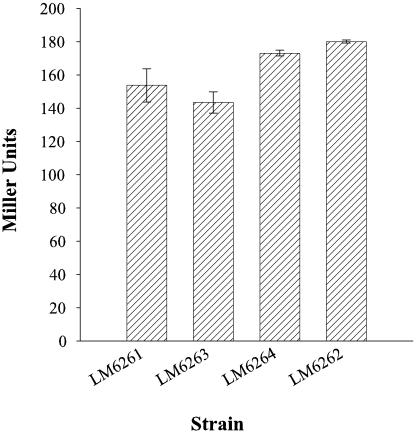
To understand more about CpsR-mediated cps expression, we examined cpsR::lacZ expression in strains with altered CPS production. A cpsR2::lacZ-Genr fusion was introduced to make the following strains: LM6261(ΔopaR), LM6263 (scrA1::Camr ΔopaR), LM6264 (cpsS1::Tn5 ΔopaR), and LM6262 (opaR+). All strains produced similar levels of β-galactosidase (Fig. 8), suggesting that cpsR was constitutively expressed in the strains tested. The difference in levels of cps expression between opaR+ and opaR-deficient strains cannot be attributed to fluctuating levels of CpsR and is consistent with the lack of effect on cps expression on the disruption of cpsR in the OP or TR (ΔopaR) strains. Furthermore, although the increased cps expression observed in scrA mutants was dependent on cpsR, it was not the result of increased cpsR transcription, suggesting that regulation of cpsR occurs posttranscriptionally.
FIG. 8.
cpsR::lacZ expression. β-Galactosidase activities were measured from cells grown on plates for 24 h. Strains containing cpsR::lacZ were as follows: LM6261 (TR; ΔopaR); LM6263 (scrA1::Camr ΔopaR); LM6264 (cpsS1::Tn5 ΔopaR); LM6262 (OP).
DISCUSSION
In order to survive under changing environmental conditions and compete for available nutrients, microorganisms have developed many strategies for adaptation and survival. V. parahaemolyticus undergoes a reversible phase variation between TR and OP colony morphologies that is thought to serve as an adaptive mechanism (30). Known differences between OP and TR include the production of copious CPS by OP but not TR colonies and proficient swarming by TR but not OP colonies. We suggest that at times it may be advantageous for the organism to produce extracellular polysaccharide, while at other times it may be useful to be less adhesive and more mobile. For many organisms, exopolysaccharide has been shown to aid in adhesion and the establishment and protection of multicellular communities known as biofilms (10, 13, 46, 51). Perhaps conversion to the TR cell type permits a mechanism of detachment or dispersal from the biofilm community. Other vibrios are also known to display reversible phase variation of CPS. For V. vulnificus, the OP-to-TR switch is characterized by a reduction in CPS production and loss of virulence in a mouse model (48, 49). For V. cholerae O1 El Tor, a phase transition between a smooth and rugose, or rough, colony type has been characterized; the rugose colonial variant produces a larger amount of exopolysaccharide and forms a thicker and more differentiated biofilm than the smooth variant (51).
This study describes the identification and characterization of a V. parahaemolyticus locus encoding CPS. Transposon mutagenesis of the OP strain identified a large gene cluster required for CPS production and colony opacity. Our sequencing efforts identified 10 genes, cpsA to -J (Table 2). The recently sequenced V. parahaemolyticus genome places this locus on the small chromosome and reveals one additional open reading frame, which occurs at the end of a potential operon and encodes a small hypothetical protein (133 aa). The cpsA locus, which contains coding regions for a number of glycosyl transferases and Wz*-type proteins believed to participate in export, is quite similar to a cps region recently identified in V. vulnificus (43, 48). The transposon mutagenesis was not saturating, and we have most certainly not identified all genes required for CPS biosynthesis.
OP and TR V. parahaemolyticus cultures form robust pellicles, or mats, at the air-liquid interface when grown in test tubes, even when shaken at high speeds. The TR strain used in these studies is stably translucent due to a deletion in the regulatory gene opaR. OP and TR pellicles are so cohesive that they can be lifted intact by using a toothpick or cotton swab. A pellicle-defective phenotype was discovered for the cps mutants during the course of the experiments, and this phenotype served as an easy and powerful way to screen for potential cps mutants. Strain reconstruction experiments confirmed that introduction of a cpsA defect into the OP strain affected pellicle formation; furthermore, the mutant allele also impaired pellicle formation by the TR strain. These results suggest that CPS plays a significant role in pellicle formation for both OP and TR strains. Direct measurements of CPS material (18) and cps::lacZ gene expression are compatible with such an idea, namely, that the TR strain produces a significant but low level of CPS compared to the higher levels produced by the OP strain. Adherence to a surface such as polystyrene, measured in the crystal violet assay, is often used as an indicator of the capacity to form biofilms (35). The capacity to adhere was greater for the OP strain than the TR strain and decreased in both strains on the elimination of CPS production. Thus, as has been found in other systems (10, 50), exopolysaccharide contributes to but is not completely required for attachment.
Introduction of the cpsA allele into the OP strain abolished pellicle formation and decreased adherence to microtiter wells; however, it did not eliminate adherence or even decrease adherence to the level observed for the cpsA-defective TR strain. This suggests that there are other differences, in addition to CPS production, between OP and TR cells, i.e., that OpaR is a global regulator controlling additional factors pertinent to colonization of surfaces. Further support for such an idea is derived from scrutiny of the colony morphology of cps mutants in the OP background. Although the colonies were no longer opaque and sticky when touched with a toothpick, they were not identical to the TR colonies, having a darker brown translucence and retaining the mounded shape.
In addition to OpaR, another potential positive regulator of cps gene expression is CpsR. CpsR is homologous to V. cholerae VpsR, and like vpsR it was discovered by mutagenizing a rugose strain to isolate smooth mutants. Introduction of a vpsR null mutation converted a rugose colony to smooth, eliminated production of exopolysaccharide, and decreased the capacity for adherence and biofilm formation (50). Introduction of the cpsR1::Tn5 allele into the rugose scrA mutant caused similar changes. However, unlike VpsR, the V. parahaemolyticus regulator is not absolutely required for CPS production; in fact, introduction of a cpsR lesion into the OP or TR strains had no effect on levels of cps gene expression. CpsR somehow participates in the scr signaling network, which influences swarming and CPS production. Mutants in the TR (ΔopaR) background with defects in the scrABC operon are defective for swarming and activated for cps expression about three- to fourfold over that of the TR strain. Thus, ΔopaR scrA mutants express cps genes to an intermediate level between that of TR and OP. As a result, colony morphology is also an intermediate type, i.e., it is slightly rough (or rugose). The ΔopaR scrA strain containing a cpsR defect loses the elevated expression of cpsA::lacZ, and β-galactosidase activity is equivalent to that of the ΔopaR strain; however, swarming is not restored. Thus, CpsR appears to act as a cps-specific regulator in this signaling pathway.
Recently, a new rugose mutant was identified in V. cholerae, and the lesion was in a gene encoding a GGDEF-EAL-type protein (called MbaA) with similarities in domain architecture to ScrC (6, 7). Thus, V. cholerae also seems to have a GGDEF-signaling pathway that influences extracellular matrix production, although ScrC and MbaA are not orthologs, based on sequence alignment and operon organization. Manifestation of the mbaA phenotype requires VpsR. MbaA, which is postulated to be involved in the maintenance of biofilm architecture, and ScrC belong to a growing family of GGDEF-type proteins controlling extracellular surface properties and multicellular behavior; other examples include AdrA of S. enterica serovar Typhimurium (39), CelR2 of Rhizobium trifolii (3), and WspR homologs in Pseudomonas species (11, 44). The most direct evidence for the role of these proteins comes from Gluconoacetobacter xylinus (47). In this organism, multiple GGDEF- and EAL-containing proteins act as diguanylate cyclases or phosphodiesterases to modulate the level of cyclic di-GMP, which activates synthesis of cellulose.
Although CpsR and VpsR resemble NtrC and are annotated in their respective genome databases as σ54-dependent transcriptional activators (VP0514 and VC0665, respectively), we emphasize that they are not σ54-interacting regulatory proteins. Rather, the significant identity may be as members of the AAA+ superfamily. Members of this family use ATP hydrolysis to mediate protein-protein or protein-DNA interactions (17). For V. cholerae, VpsR activation of exopolysaccharide production was demonstrated to be independent of σ54 (50). Similarly, we have introduced an rpoN mutation into rugose scr mutants with no effect on colony morphology (data not shown). Reporter fusion analysis suggests that cpsR is not regulated at the level of transcription, and thus it seems likely that cpsR mRNA is posttranscriptionally regulated or CpsR activity is somehow modulated. How this occurs remains to be determined. Although it has been suggested that VpsR acts as a response regulator (50), alternate mechanisms for modulating activity might occur. Based on multiple sequence alignments, CpsR lacks three of the four amino acid residues considered essential for phosphorylation of receiver domains, corresponding to D12, D13, and K109 of NtrC (45). Perhaps like TyrR, which is a member of the AAA+ superfamily (16), determination of CpsR activity is mediated in part by ATP-dependent oligomerization.
V. parahaemolyticus has a much greater capacity for CPS production than is observed under typical laboratory conditions. CpsS acts as a negative regulator of cps gene expression. Mutants with cpsS defects form superrugose colonies and crinkly pellicles at air-liquid interfaces. Reporter gene analysis showed that cps gene expression was greatly increased at least 17-fold in the TR strain and 8-fold in the OP strain compared to that of the parental strains. In TR (ΔopaR) strains, increased CPS production is mediated through CpsR, as introduction of a cpsR lesion into a cpsS strain eliminated the extremely high level of reporter gene expression and altered the superrugose colony morphology. However, in opaR+ strains, cpsA::lacZ expression was only slightly reduced in the cpsS cpsR strain compared to the cpsS strain. Moreover, the colonies of the cps+ strain were still quite rough, correlating with the high level of cps expression. These findings indicate that (i) expression of cps is controlled mainly by the negative regulator CpsS; and (ii) in the absence of CpsS, OpaR and CpsR control cps gene expression in a positive manner and the presence of either regulator is sufficient for cps transcription. Whether these regulators act directly or indirectly to modulate expression of the cps genes remains to be elucidated.
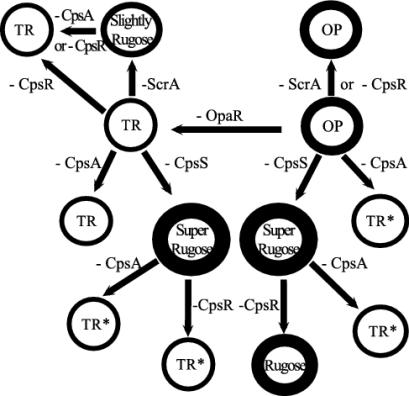
In summary, we have identified a locus required for CPS synthesis in V. parahaemolyticus. Reporter gene analysis suggests that the organism has a great capacity for modulating levels of cps gene expression. The roles of three regulatory genes have been examined, opaR, cpsS, and cpsR, and are depicted in Fig. 9. Homologs of these regulators control multiple cell surface properties and multicellular phenotypes in other bacteria, although the specific target genes and regulatory circuits seem particular for each organism (9, 20, 27, 32, 37, 39, 41, 52). For example, loss of function of OpaR in V. parahaemolyticus causes decreased production of CPS, whereas loss of function of the OpaR homolog HapR in V. cholerae causes a rugose colony phenotype (23). In S. enterica and E. coli, the positive activator CsgD, a CpsS homolog, is required for development of the multicellular morphotype, including production of curli and cellulose synthesis (8, 22, 38), whereas CpsS acts negatively to repress CPS in V. parahaemolyticus. The regulatory networks in which these controlling elements participate, as well as the signaling inputs that stimulate the circuitry and the additional target genes of the pathways, should prove important for understanding bacterial survival strategies. In particular, regulation of CPS production and other cell surface properties may be significant with respect to promoting dispersal or transitions between multicellular communities and planktonic cells or between different kinds of biofilms that may require more or less extracellular matrix or other alterations in cell surface characteristics.
FIG. 9.
Positive and negative modulators of CPS production in V. parahaemolyticus. Mutation of opaR converts the OP to the TR colony morphology and causes decreased CPS production (indicated by thickness of circle). Mutation of the scrA operon converts the opaR-defective TR strain to a slightly rough (or rugose) colony type as a result of production of an intermediate level of CPS (between levels of OP and TR strains). The positive regulator CpsR mediates enhanced transcription of the cpsA biosynthesis gene in the opaR scrA mutant but not the opaR+ scrA-deficient strain. Furthermore, introduction of the cpsR lesion has no effect on colony morphology or CPS production in the OP or the opaR-deficient TR strains. Introduction of a mutation in the negative regulatory gene cpsS causes derepression of cps biosynthetic gene expression and superrugose colonies, irrespective of the status of the opaR allele. In the opaR-deficient cpsS-deficient strain, derepression of cpsA gene expression is mediated through CpsR, whereas the significant portion of the cps derepression in the opaR+ cpsS-deficient strain is independent of CpsR. OpaR, CpsR, and CpsS may be global regulators of gene expression affecting other cell surface genes in addition to cps genes, because introduction of the cpsA lesion, which abolishes CPS production, does not completely reverse the mutant colony types (hence the “*” designation).
Acknowledgments
We thank Jeff Valiga for help in the OP mutagenesis and Blaise Boles for performing scr mutagenesis experiments.
This work was supported by National Science Foundation research grant MCB-0077327.
REFERENCES
- 1.Alper, M. D., and B. N. Ames. 1978. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J. Bacteriol. 133:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausmees, N., R. Mayer, H. Weinhouse, G. Volman, D. Amikam, M. Benziman, and M. Lindberg. 2001. Genetic data indicate that proteins containing the GGDEF domain possess diguanylate cyclase activity. FEMS Microbiol. Lett. 204:163-167. [DOI] [PubMed] [Google Scholar]
- 4.Belas, R., M. Simon, and M. Silverman. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boles, B. R., and L. L. McCarter. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 182:1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boles, B. R., and L. L. McCarter. 2002. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J. Bacteriol. 184:5946-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomchil, N., P. Watnick, and R. Kolter. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirwa, N. T., and M. B. Herrington. 2003. CsgD, a regulator of curli and cellulose synthesis, also regulates serine hydroxymethyltransferase synthesis in Escherichia coli K-12. Microbiology 149:525-535. [DOI] [PubMed] [Google Scholar]
- 9.Croxatto, A., V. J. Chalker, J. Lauritz, J. Jass, A. Hardman, P. Williams, M. Camara, and D. L. Milton. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184:1617-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, D. G., A. M. Chakrabarty, and G. G. Geesey. 1993. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 59:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 15.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon, M. P., R. N. Pau, G. J. Howlett, D. E. Dunstan, W. H. Sawyer, and B. E. Davidson. 2002. The central domain of Escherichia coli TyrR is responsible for hexamerization associated with tyrosine-mediated repression of gene expression. J. Biol. Chem. 277:23186-23192. [DOI] [PubMed] [Google Scholar]
- 17.Dougan, D. A., A. Mogk, K. Zeth, K. Turgay, and B. Bukau. 2002. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Lett. 529:6-10. [DOI] [PubMed] [Google Scholar]
- 18.Enos-Berlage, J. L., and L. L. McCarter. 2000. Relation of capsular polysaccharide production and colonial cell organization to colony morphology in Vibrio parahaemolyticus. J. Bacteriol. 182:5513-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrandez, A., A. C. Hawkins, D. T. Summerfield, and C. S. Harwood. 2002. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J. Bacteriol. 184:4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidopiastis, P. M., C. M. Miyamoto, M. G. Jobling, E. A. Meighen, and E. G. Ruby. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131-143. [DOI] [PubMed] [Google Scholar]
- 21.Friedman, A. M., S. R. Long, S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 22.Hammar, M., A. Arnqvist, Z. Bian, A. Olsen, and S. Normark. 1995. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18:661-670. [DOI] [PubMed] [Google Scholar]
- 23.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko, T., and R. R. Colwell. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl. Microbiol. 29:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko, T., and R. R. Colwell. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley, J. T., and C. D. Parker. 1981. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J. Bacteriol. 145:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1347. [DOI] [PubMed] [Google Scholar]
- 28.Larsen, R. A., M. M. Wilson, A. M. Guss, and W. W. Metcalf. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178:193-201. [DOI] [PubMed] [Google Scholar]
- 29.McCarter, L. 1999. The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1:51-57. [PubMed] [Google Scholar]
- 30.McCarter, L. L. 1998. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J. Bacteriol. 180:3166-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarter, L. L., and M. Silverman. 1987. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J. Bacteriol. 169:3441-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDougald, D., S. A. Rice, and S. Kjelleberg. 2001. SmcR-dependent regulation of adaptive phenotypes in Vibrio vulnificus. J. Bacteriol. 183:758-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 35.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 36.Parales, R. E., and C. S. Harwood. 1993. Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J. Bacteriol. 175:5829-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinkoster. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Shao, C. P., and L. I. Hor. 2001. Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. J. Bacteriol. 183:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverman, M., R. Showalter, and L. McCarter. 1991. Genetic analysis in Vibrio. Methods Enzymol. 204:515-536. [DOI] [PubMed] [Google Scholar]
- 43.Smith, A. B., and R. J. Siebeling. 2003. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect. Immun. 71:1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiers, A. J., S. G. Kahn, J. Bohannon, M. Travisano, and P. B. Rainey. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stock, J. B., M. Surette, M. Levit, and P. Park. 1995. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis, p. 25-51. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 46.Sutherland, I. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 47.Tal, R., H. C. Wong, R. Calhoon, D. Gelfand, A. L. Fear, G. Volman, R. Mayer, P. Ross, D. Amikam, H. Weinhouse, A. Cohen, S. Sapir, P. Ohana, and M. Benziman. 1998. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180:4416-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright, A. C., J. L. Powell, M. K. Tanner, L. A. Ensor, A. B. Karpas, J. G. Morris, Jr., and M. B. Sztein. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS(ETr)-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]