Abstract
The role of RNA silencing as an antiviral defense mechanism in fungi was examined by testing the effect of dicer gene disruptions on mycovirus infection of the chestnut blight fungus Cryphonectria parasitica. C. parasitica dicer-like genes dcl-1 and dcl-2 were cloned and shown to share a high level of predicted amino acid sequence identity with the corresponding dicer-like genes from Neurospora crassa [Ncdcl-1 (50.5%); Ncdcl-2 (38.0%)] and Magnaporthe oryzae [MDL-1 (45.6%); MDL-2 (38.0%)], respectively. Disruption of dcl-1 and dcl-2 resulted in no observable phenotypic changes relative to wild-type C. parasitica. Infection of Δdcl-1 strains with hypovirus CHV1-EP713 or reovirus MyRV1-Cp9B21 resulted in phenotypic changes that were indistinguishable from that exhibited by wild-type strain C. parasitica EP155 infected with these same viruses. In stark contrast, the Δdcl-2 and Δdcl-1/Δdcl-2 mutant strains were highly susceptible to mycovirus infection, with CHV1-EP713-infected mutant strains becoming severely debilitated. Increased viral RNA levels were observed in the Δdcl-2 mutant strains for a hypovirus CHV1-EP713 mutant lacking the suppressor of RNA silencing p29 and for wild-type reovirus MyRV1-Cp9B21. Complementation of the Δdcl-2 strain with the wild-type dcl-2 gene resulted in reversion to the wild-type response to virus infection. These results provide direct evidence that a fungal dicer-like gene functions to regulate virus infection.
Keywords: Cryphonectria parasitica, Dicer, hypovirus, mycoreovirus, double-stranded RNA
RNA-mediated, sequence-specific suppression of gene expression, termed RNA silencing, has been described as posttranscriptional gene silencing in plants (1, 2), RNA interference (RNAi) in animals (3), and quelling in fungi (4). A common feature of RNA silencing in these different organisms is the processing of structured or dsRNA into small interfering RNAs (siRNAs) of 21–24 nt by RNase III-like endonucleases termed Dicers. These siRNAs then are incorporated into an RNA-induced silencing complex that guides sequence-specific degradation or translational repression of homologous RNA in the cytoplasm or DNA or histone methylation of target sequences in the nucleus (reviewed in refs. 5 and 6).
In plants and animals, the RNA silencing pathway also produces microRNAs (miRNAs) from genome-encoded RNA hairpins that are involved in developmental regulation (reviewed in refs. 7 and 8). miRNAs have not been identified in fungal genomes (9, 10). Thus, RNA silencing in fungi generally is thought to serve primarily as a defense mechanism against invasive nucleic acids and viruses (10). RNA silencing plays a key antiviral defense role in plants (reviewed in refs. 11 and 12) and recently has been demonstrated to influence virus replication in animal cells (reviewed in ref. 13). Although silencing of transposons has been reported in fungi (14), there currently are no reports of RNA silencing functioning as a fungal antiviral defense mechanism.
Mechanisms underlying RNA silencing in fungi have been elucidated primarily through studies with the model fungus Neurospora crassa. Cellular components of RNA silencing in this fungus include the RNA-dependent RNA polymerases QDE-1 and Sad-1, the Argonaute-2 orthologs QDE-2 and Sms-2 that are involved in incorporating siRNA into the RNA-induced silencing complex, a RecQ helicase, QDE-3, and two Dicer orthologs, DCL-1 and DCL-2 (15–20). However, efforts to demonstrate a role for RNA silencing in antiviral defense in N. crassa have been limited by the absence of a well developed mycovirus experimental system.
The chestnut blight fungus Cryphonectria parasitica is phylogenetically related to N. crassa and genetically tractable because of the haploid nature of its genome and the availability of a robust DNA transformation protocol (21, 22). Moreover, C. parasitica has been shown to support the replication of members of five RNA virus families: Hypoviridae, Reoviridae, Narnaviridae, Partitiviridae, and Chrysoviridae (23). A reverse genetics system has been developed for members of the family Hypoviridae (reviewed in ref. 21), and the hypovirus-encoded papain-like protease p29 recently was reported to suppress RNA silencing in both C. parasitica and a heterologous plant system (24). We now report the use of the C. parasitica/mycovirus experimental system to investigate the role of RNA silencing as an antiviral defense pathway in fungi.
Results
Cloning of C. parasitica dicer-like Genes dcl-1 and dcl-2.
RNA silencing is eliminated in N. crassa by disruption of both dicer genes Ncdcl-1 and Ncdcl-2 (16) and in Magnaporthe oryzae by disruption of one of two dicer genes, MDL-2 (25). To examine whether RNA silencing plays a role in antiviral response in C. parasitica, we cloned and disrupted the endogenous dicer-like gene homologues to determine the effect of pathway disruption on mycovirus infection.
Degenerate PCR primers, based on conserved regions in fungal dicer-like proteins from N. crassa (16), M. oryzae (25), and Fusarium graminearum (FG09025.1 and FG04408.1), were used to amplify fragments from C. parasitica genomic DNA, resulting in the identification and characterization of two dicer-like genes. Sequence alignment analysis revealed high levels of deduced amino acid sequence identity of the two C. parasitica dicer-like genes with Ncdcl-1/MDL-1 (50.5%/45.6%) and Ncdcl-2/MDL-2 (38.0%/39.3%) of N. crassa and M. oryzae, respectively, resulting in designations of dcl-1 and dcl-2 for the two C. parasitica genes.
The dcl-1 ORF encodes a protein of 1,548 aa, and the size of the predicted protein encoded by dcl-2 is 1,541 aa. Both DCL-1 and DCL-2 proteins contain domains characteristic of the Dicer protein family (Fig. 1). These include a DEAD box helicase domain near the N terminus followed by a helicase C-terminal domain and a Domain of Unknown Function 283 (DUF), which is found in most Dicer proteins. Two RNase III domains are present in the C-terminal region of both the predicted DCL-1 and DCL-2 sequences. C. parasitica DCL-2 also contains a predicted dsRNA-binding domain at the C terminus that also is found in C-terminal regions of NcDCL-2, MDL-1, and MDL-2 but not in C. parasitica DCL-1 or NcDCL-1 (16, 25).
Fig. 1.
Conserved polypeptide domains for C. parasitica dicer-like proteins DCL-1 and DCL-2. The location of each domain along the predicted amino acid sequence is indicated above the corresponding box representing the domain. The percentage amino acid identity relative to N. crassa DCL-1 and M. oryzae MDL-2 is shown to the right of each domain for C. parasitica DCL-1 and DCL-2, respectively (16, 25). DEAD, DEAD box helicase; HelC, helicase C-terminal domain; DUF238, Domain of Unknown Function 283; RNIIIa and RNIIIb, RNase III a and b domains; dsrm, dsRNA-binding domain.
Disruption of C. parasitica dicer-like Gene dcl-2 but Not dcl-1 Results in Severe Symptoms After Mycovirus Infection.
Strains containing null-mutations of dcl-1, dcl-2, or both genes (Δdcl-1/Δdcl-2) were constructed by homologous recombination. Southern blotting analysis confirmed that the endogenous gene copies were replaced by the disruption construct (Fig. 2), whereas real-time RT-PCR with dcl-1- and dcl-2-specific probes showed the absence of the corresponding gene transcripts in the respective mutant strains (data not shown). Phenotypic analysis of the single and double disruption mutant strains revealed no obvious phenotypic consequences of dicer-like gene disruption. As shown in Fig. 3 Top, the dicer disruption mutants exhibited a colony morphology indistinguishable from the wild-type strain EP155. The mutants were found to be male and female fertile and able to produce viable ascospores (data not shown). Additionally, no differences in virulence on dormant chestnut stems or in the production of asexual spores were found between the dicer mutant and wild-type strains (data not shown).
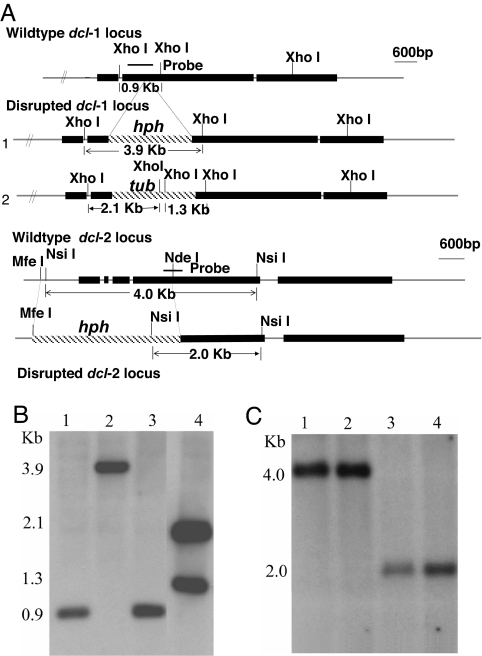
Fig. 2.
Disruption of C. parasitica dicer-like genes dcl-1 and dcl-2. (A) Genomic organization and disruption constructs for C. parasitica dicer-like genes. Disruption of dcl-1 was performed with the PCR-based strategy of Davidson et al. (44) as described by Deng et al. (45). The PCR fragment used for disruption transformation extended 1,020 bp upstream and 1,422 bp downstream of the dcl-1 coding region and contained the hygromycin resistance cassette substituted for a region of the coding region extending from nucleotide 2018 to nucleotide 2024 (construct 1). Double mutants (Δdcl-1/Δdcl-2) were constructed by disrupting dcl-1 in a Δdcl-2 background by using a PCR disruption fragment that contained the benomyl resistance cassette (construct 2). The gene-replacement plasmid construct for C. parasitica dcl-2 was made by using genomic clone dcl-2, which contains the complete dcl-2 ORF with 5′ and 3′ flanking regions of ≈5 and 2 kb, respectively. The 2,690-bp MfeI–NdeI fragment that contains 910 bp of upstream sequence and 1,780 bp of dcl-2 coding sequence was replaced with a cassette conferring hygromycin resistance (46). The resulting dcl-2 gene disruption construct was digested with NotI to release the insert before transformation of wild-type strain EP155. At least two independent deletion mutants were generated and characterized for each dicer-like gene and the double dicer knockout. (B) Southern blotting analysis of C. parasitica dicer-like gene disruption mutants with a probe specific for dcl-1. Genomic DNA, prepared from strain EP155 and mutants Δdcl-1, Δdcl-2, and Δdcl-1/Δdcl-2, was digested with XhoI and hybridized with the dcl-1-specific probe shown in A. Note that the 0.9-kb XhoI fragment in the lanes containing DNA from the wild-type strain EP155 (lane 1) and the Δdcl-2 mutant (lane 3) was replaced by a 3.9-kb band in the Δdcl-1 mutant (lane 2) and two bands of 2.1 and 1.3 kb predicted for disruption of dcl-1 in the double dicer mutant (lane 4) with the benomyl-cassette-containing disruption construct. (C) Southern blotting analysis of disruption mutants with a probe specific for dcl-2. Genomic DNA for strains indicated in B was digested with NsiI and hybridized with the dcl-2-specific probe shown in A. Note that the 4-kb NsiI band present in the lanes containing DNA from strain EP155 (lane 1) and the Δdcl-1 mutant (lane 2) was replaced by a 2-kb band in the lanes containing DNA from the Δdcl-2 (lane 3) and double dicer (lane 4) mutants because of the presence of a NsiI site in the hph cassette of the integrated disruption construct.
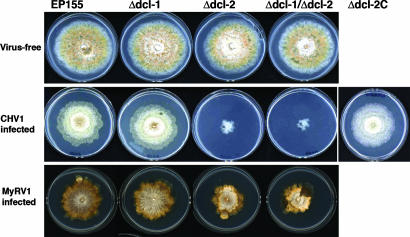
Fig. 3.
Effect of mycovirus infection on C. parasitica dicer gene deletion mutants. (Top) Colony morphology for uninfected wild-type strain EP155, dicer gene disruption mutants Δdcl-1, Δdcl-2, and double mutant Δdcl-1/Δdcl-2 are shown. (Middle) Corresponding strains and a complemented Δdcl-2 strain, Δdcl-2C, infected with hypovirus CHV1-EP713 (marked at left as CHV1 infected) are shown. The Δdcl-2 deletion mutant was complemented with a genomic DNA clone of the dcl-2 coding region cloned into plasmid pCPXNBn1 to generate the complementation plasmid pCDCL2, which contains the benomyl resistance cassette and the C. parasitica glyceraldehyde-3-phosphate dehydrogenase promoter (24) to drive expression of the inserted dcl-2 coding region. Cultures were grown for 7 days on PDA. (Bottom) Corresponding strains infected with reovirus MyRV1–9B21 (marked at left as MyRV1 infected) are shown. Cultures were grown for 9 days on PDA.
To test whether the C. parasitica dicer-like proteins are involved in an antiviral response, the dicer deletion strains were infected independently with hypovirus CHV1-EP713 and reovirus MyRV1-Cp9B21 either by transfection of spheroplasts with viral transcripts (CHV1-EP713) or anastomosis with a virus-infected strain (CHV1-EP713 and MyRV1-Cp9B21). As shown in Fig. 3 Middle, CHV1-EP713 infection of Δdcl-1 resulted in a phenotype indistinguishable from that of CHV1-EP713-infected wild-type strain EP155. In stark contrast, CHV1-EP713-infected Δdcl-2 and Δdcl-1/Δdcl-2 were severely debilitated. Complementation of the Δdcl-2 strain with the wild-type dcl-2 gene, strain Δdcl-2C, resulted in reversion to the wild-type response to CHV1-EP713 infection (Fig. 3 Middle). A similar set of results was obtained for reovirus MyRV1-Cp9B21-infected mutant strains (Fig. 3 Bottom). MyRV1-Cp9B21-infected strains EP155 and Δdcl-1 were indistinguishable, whereas the Δdcl-2- and Δdcl-1/Δdcl-2-infected strains exhibited a reduced growth and altered colony morphology but were not debilitated to the extent observed for the corresponding CHV1-EP713-infected mutant strains (Fig. 3 Middle and Bottom).
Unexpectedly, CHV1-EP713 dsRNA (agarose gel analysis) and total viral RNA [RT-PCR analysis (26)] levels were not found to increase significantly in accumulation in CHV1-EP713-infected Δdcl-2 strains relative to the levels found in the infected control strain EP155 (data not shown). Because deletion of the region encoding the p29 suppressor of RNA silencing in the context of the CHV1-EP713 infectious cDNA clone, virus Δp29, previously was shown to result in a 70–80% reduction in viral RNA accumulation (26), we asked whether Δp29 RNA levels increased in Δdcl-2 mutant strains. Infection of the Δdcl-2 mutant strain with the Δp29 virus resulted in a similar level of debilitation as observed for the CHV1-EP713 parent virus (data not shown), but as shown in Fig. 4, the level of Δp29 viral total and dsRNAs (Fig. 4A) increased to a level approaching that observed for wild-type CHV1-EP713 in wild-type strain EP155, i.e., an ≈4-fold increase. Also consistent with disruption of cellular RNA silencing and increased virus-mediated symptom expression, reovirus MyRV1-Cp9B21 RNA levels were observed to increase on the order of 3- to 4-fold in the Δdcl-2 mutant strains relative to that observed in the EP155 parent stain but not in the Δdcl-1 mutant strain (Fig. 4B).
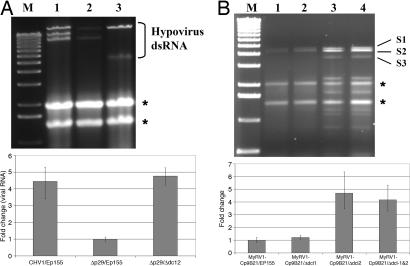
Fig. 4.
Quantitation of mycovirus RNA in dicer mutant C. parasitica strains. (A) Agarose gel analysis of relative dsRNA accumulation for hypovirus CHV1-EP713 and mutant strain Δp29, which lacks suppressor of RNA silencing p29, in wild-type strain EP155 and the Δdcl-2 deletion mutant strain. Lane M, 1-kb DNA ladder size markers; lane 1, CHV1-EP713-infected control strain EP155; lane 2, Δp29-infected strain EP155; lane 3, Δp29-infected Δdcl-2 mutant strain. The migration positions of hypovirus dsRNAs are shown at the right. The slowest migrating band represents full-length viral dsRNA, whereas the faster migrating bands represent internally deleted viral dsRNAs commonly generated by hypoviruses (47). The migration positions of rRNAs are indicated by asterisks. Results of semiquantitative RT-PCR analysis of total viral RNA in the corresponding CHV1-EP713 and Δp29-infected strains relative to 18S rRNA sequences are shown in the chart below the agarose gel. The values are normalized to the viral RNA accumulation in Δp29-infected strain EP155 (set to a value of 1), with the standard deviation based on three independent measurements of two independent RNA preparations indicated by the error bars. (B) Agarose gel analysis of viral dsRNA accumulation in reovirus MyRV1-Cp9B21-infected strain EP155 (lane 1), infected mutant Δdcl-1 (lane 2), infected mutant Δdcl-1 (lane 3), and infected double mutant Δdcl-1/Δdcl-2 (lane 4). Lane M contains 1-kb DNA ladder size markers. The migration position of the three largest MyRV1-Cp9B21 dsRNA segments are shown at the right. Results of semiquantitative RT-PCR analysis of MyRV1-Cp9B21 RNA in the corresponding strains are shown in the chart below the agarose gel. In this case, the relative amount of total viral RNA was estimated by measuring the amount of segment S3-specific RNA relative to 18S rRNA (42). Values were normalized to the amount of S3-specific RNA in strain EP155 (set to a value of 1), with the standard deviation based on three independent measurements of two independent RNA preparations indicated by the error bars.
Discussion
Our previous report that hypovirus CHV1-EP713-encoded protein p29 can suppress RNA silencing (24) provided circumstantial evidence that RNA silencing serves as an antiviral defense mechanism in fungi. The demonstration here that the C. parasitica dcl-2, but not dcl-1, disruption mutants are highly debilitated upon hypovirus infection provides more direct evidence for this conclusion and identifies DCL-2 as a primary component of the antiviral pathway. These results extend the role of fungal dicer-like genes to include protection against virus infection. The enhanced symptoms and increased virus RNA accumulation observed in reovirus MyRV1-Cp9B21-infected Δdcl-2 mutant strains also indicates that the C. parasitica RNA silencing pathway serves as a general antiviral defense response and is not specific for hypovirus infections.
The C. parasitica DCL-2 homologue in M. oryzae, MDL-2, was reported to be responsible for hairpin RNA silencing (25), whereas both Ncdcl-1 and Ncdcl-2 of N. crassa have been reported to be redundantly involved in RNA silencing (16). There was no evidence of redundancy for the C. parasitica dicer genes in antiviral defense. The phenotypic consequences of CHV1-EP713 infection were indistinguishable for wild-type strain EP155 and the Δdcl-1 deletion mutant, whereas the double dicer mutant showed the same level of severe symptoms as the Δdcl-2 single mutant (Fig. 3).
None of the C. parasitica single or double dicer mutants exhibited any detectable phenotypic change from wild type in the absence of virus infection. This differs from the reports that the M. oryza MDL-1 mutant formed abnormal conidia and the MDL-2 mutant showed reduced colony growth (25). Deletion of the dicer-like gene dcp-1 in Mucor circinelloides also was reported to result in reduced growth rate and altered hyphal morphology (27). The phenotypic characteristics of the N. crassa dicer mutants were not described. However, N. crassa dcl-1 has been reported to participate in the N. crassa meiotic silencing pathway (MSUD for meiotic silencing by unpaired DNA) (28).
Disruption of the antiviral RNA silencing pathway in plant or invertebrate systems generally results in an increase in virus titer (29, 30). The observed increase in reovirus MyRV1-Cp9B21 RNA levels in the Δdcl-2 mutant strains (Fig. 4B) was consistent with these reports. Thus, it was surprising, given the severe debilitation observed for the Δdcl-2 mutant strain after CHV1-EP713 infection, not to see a significant increase in hypovirus RNA accumulation in Δdcl-2 cultures grown on potato dextrose agar (PDA) medium. However, a similar result has been reported for flock house virus (FHV). A very modest increase in wild-type FHV RNA was observed in infected dicer-2 mutant Drosophila melanogaster (31, 32), even though the infected flies showed enhanced mortality in response to FHV infection (31). However, a mutant FHV lacking the suppressor of RNA silencing B2 did show a significant increase in RNA accumulation in the dicer-2 mutant animals (31, 32). This last result is the equivalent of the rescue of Δp29 RNA accumulation levels in the C. parasitica Δdcl-2 mutant shown in Fig. 4A and indicates that p29 directly or indirectly counteracts DCL-2 function.
Mycovirus infections are widespread in the Kingdom Fungi (33). The results presented here with the C. parasitica/mycovirus system demonstrate that RNA silencing provides one mechanism for antiviral defense in fungi. However, a number of filamentous fungi (e.g., Candida albicans and Ustilago maydis) appear to lack all or most of the components required for RNA silencing (10), suggesting the possibility of additional, as-yet-undetected, mechanisms used by filamentous fungi to modulate viral infections. In this regard, the products of the SKI genes have been identified as acting as an antiviral system in the yeast Saccharomyces cerevisiae by blocking the translation of viral non-poly(A) mRNAs (34). An understanding of how mycoviruses trigger, suppress, and are regulated by fungal RNA silencing pathways is anticipated to provide new insights into the origin, biological functions, and molecular mechanisms of RNA silencing.
Materials and Methods
Fungal Strains, Growth Conditions, and Transformation.
C. parasitica strains were maintained on Difco PDA as described (35). Cultures used for RNA or protein preparation were grown for 7 days at room temperature under ambient light on PDA or PDA overlaid with cellophane to facilitate harvesting of mycelia. Preparation and transformation of C. parasitica spheroplasts was carried out essentially as described by Churchill et al. (36). Hygromycin (40 μg/ml), benomyl (0.7 μg/ml), or blasticidin (300 μg/ml) was included in the growth medium to provide for selection of transformants. Transfection of fungal spheroplasts with in vitro-transcribed hypovirus RNA transcripts was performed as described (37). Reovirus MyRV1-Cp9B21-infected C. parasitica strain EP155 was provided by Bradley Hillman (Rutgers, The State University of New Jersey, New Brunswick, NJ). Mating assays were performed with deletion strain Δdcl-1 or Δdcl-2 derived from strain EP155 [mating type A or MAT1–2 (38)] as one parent and strain EP146 [mating type a or MAT1–1 (39)] as the second parent, grown on autoclaved American chestnut twigs embedded in 2% water agar, as described by Anagnostakis (40).
Nucleic Acid and Protein Preparation and Analysis.
Fungal genomic DNA was extracted as described by Choi et al. (41). RNA was prepared, and quantitative analysis of RNA accumulation was performed via reverse transcription and quantitative PCR using TaqMan reagents (Applied Biosystems, Foster City, CA) and a GeneAmp 5700 PCR apparatus (Applied Biosystems) as described (26). Hypovirus CHV1-EP713 RNA was quantified as described by Suzuki and Nuss (26), whereas the relative level of MyRV1-Cp9B21 RNA accumulation was determined by quantifying the amount of segment S3-specific RNA (42).
Cloning of C. parasitica dcl-1 and dcl-2 Genes.
Degenerate PCR primers were designed according to conserved regions in dicer-like proteins DCL-1 and DCL-2 from N. crassa (16), MDL-1 and MDL-2 from M. oryzae (24), and FG09025.1 and FG04408.1 from F. graminearum with the program CODEHOP (43): C. parasitica dcl-1 primers (Dcl1-EF1, 5′-AAG TCC ATC GCC GAC GTN TGY GAR GC-3′ and Dcl1-FR1, 5′-GGT TGG AGA CCA TGG CCA TYT TRT GYT C-3′) and dcl-2 primers (Dcl2-AF1, 5′-TCG GCC CCT GGG CNG YNG A-3′ and Dcl2-BR2, 5′-GGG CCC GGC CCC KNC KYT GDA T-3′) and (Dcl2-CF1, 5′-GGT AGA TGT GCC CCA AGA TGB TNG CNG AYG T-3′ and Dcl2-ER1, 5′-AGG ACG GCG TCG CCN ARR AAY TC-3′). The cloned PCR products were sequenced and used to screen a C. parasitica EP155 λ-Bluestar phage (Novagen, San Diego, CA) genomic library, which was constructed from partial Sau3AI-digested genomic DNA according to the manufacturer's instructions. Several independent genomic clones containing the dcl-1 and dcl-2 genes were sequenced by using the ABI-Prism BigDye Terminator Ready-Reaction Cycle Sequencing kit (Applied Biosystems). The 5′ end and 3′ end of the dcl-1 and dcl-2 transcripts were determined by using the FirstChoice RNA Ligase-Mediated Rapid Amplification of cDNA Ends (RLM-RACE) kit (Ambion, Austin, TX) according to the manufacturer's instructions. PCR fragments were amplified from oligo(dT)-primed cDNA with primers located at the transcription start site and polyadenylation sites and sequenced to determine the location of introns. Sequence data for C. parasitica dcl-1 and dcl-2 have been submitted to the GenBank database under accession numbers DQ186989 and DQ186990, respectively.
Acknowledgments
This study was supported in part by Public Health Service Grant GM55981 (to D.L.N.).
Abbreviation
- PDA
potato dextrose agar.
Footnotes
References
- 1.Napoli C, Lemieux C, Jorgensen R. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR. Plant Cell. 1990;2:291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fire A. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 4.Romano N, Macino G. Mol Microbiol. 1992;6:3343–3345. doi: 10.1111/j.1365-2958.1992.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomari Y, Zamore PD. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 6.Zamore PD. Science. 2002;296:1265–1269. doi: 10.1126/science.1072457. [DOI] [PubMed] [Google Scholar]
- 7.Carrington JC, Ambrose V. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 8.Zamore PD, Haley B. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti H, Casas-Mollan JA. Curr Genet. 2006;50:81–99. doi: 10.1007/s00294-006-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayashiki H, Kadotani N, Mayama S. J Mol Evol. 2006;63:127–135. doi: 10.1007/s00239-005-0257-2. [DOI] [PubMed] [Google Scholar]
- 11.Dunoyer P, Voinnet O. Curr Opin Plant Biol. 2005;8:415–423. doi: 10.1016/j.pbi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Voinnet O. Trends Genet. 2001;17:449–459. doi: 10.1016/s0168-9525(01)02367-8. [DOI] [PubMed] [Google Scholar]
- 13.Schultz S, Sarnow P. Virology. 2006;344:151–157. doi: 10.1016/j.virol.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Nolan T, Braccini L, Azzalin G, De Toni A, Macino G, Cogoni C. Nucleic Acids Res. 2005;33:1564–1573. doi: 10.1093/nar/gki300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalanotto C, Azzalin G, Macino G, Cogoni C. Genes Dev. 2002;16:790–795. doi: 10.1101/gad.222402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalanotto C, Pallotta M, ReFalo P, Sachs MS, Vayssie L, Macino G, Cogoni C. Mol Cell Biol. 2004;24:2536–2545. doi: 10.1128/MCB.24.6.2536-2545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cogoni C, Macino G. Proc Natl Acad Sci USA. 1997;94:10233–10238. doi: 10.1073/pnas.94.19.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogoni C, Macino G. Nature. 1999;399:166–169. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, Pratt RJ, McLaughlin M, Aramayo R. Genetics. 2003;164:821–828. doi: 10.1093/genetics/164.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiu PK, Raju NB, Zickler D, Metzenberg RL. Cell. 2001;107:905–916. doi: 10.1016/s0092-8674(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 21.Dawe AL, Nuss DL. Annu Rev Genet. 2001;35:1–29. doi: 10.1146/annurev.genet.35.102401.085929. [DOI] [PubMed] [Google Scholar]
- 22.Dawe AL, McMain VC, Panglao M, Kasahara S, Chen B, Nuss DL. Microbiology. 2003;149:2373–2384. doi: 10.1099/mic.0.26371-0. [DOI] [PubMed] [Google Scholar]
- 23.Hillman BI, Suzuki N. Adv Virus Res. 2004;63:423–472. doi: 10.1016/S0065-3527(04)63007-7. [DOI] [PubMed] [Google Scholar]
- 24.Segers GC, van Wezel R, Zhang X, Hong Y, Nuss DL. Eukaryot Cell. 2006;5:896–904. doi: 10.1128/EC.00373-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadotani N, Nakayashiki H, Tosa H, Mayama S. J Biol Chem. 2004;279:44467–44474. doi: 10.1074/jbc.M408259200. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki N, Nuss DL. J Virol. 2002;76:7747–7759. doi: 10.1128/JVI.76.15.7747-7759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolas FE, de Haro JP, Torres-Martinez S, Ruiz-Vazquez RM. Fungal Genet Biol. 2007;44:504–516. doi: 10.1016/j.fgb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Galagan JE, Calvi SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma L-J, Smirnov S, Purcell S, et al. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 29.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratcliff F, Harrison BD, Baulcombe DC. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 31.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffman JA, Imler J-L. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 32.Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck KW. In: Fungal Virology. Buck KW, editor. Boca Raton, FL: CRC; 1986. pp. 2–84. [Google Scholar]
- 34.Widner WR, Wickner RB. Mol Cell Biol. 1993;13:4331–4341. doi: 10.1128/mcb.13.7.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillman BI, Shipira R, Nuss DL. Phytopathology. 1990;80:850–856. [Google Scholar]
- 36.Churchill ACL, Ciufetti LM, Hansen DR, Van Etten HD, Van Alfen NK. Curr Genet. 1990;17:25–31. [Google Scholar]
- 37.Chen B, Choi GH, Nuss DL. Science. 1994;264:1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- 38.Marra RE, Milgroom MG. Heredity. 2001;86:134–143. doi: 10.1046/j.1365-2540.2001.00784.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Choi GH, Nuss DL. EMBO J. 1993;12:2991–2998. doi: 10.1002/j.1460-2075.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anagnostakis SL. Phytopathology. 1984;74:561–565. [Google Scholar]
- 41.Choi GH, Larson TG, Nuss DL. Mol Plant Microbe Interact. 1992;5:119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Nuss DL, Suzuki S. J Gen Virol. 2006;87:3703–3714. doi: 10.1099/vir.0.82213-0. [DOI] [PubMed] [Google Scholar]
- 43.Rose TM, Henikoff JG, Henikoff S. Nucleic Acids Res. 2003;13:3763–3766. doi: 10.1093/nar/gkg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C. Microbiology. 2002;148:2607–2615. doi: 10.1099/00221287-148-8-2607. [DOI] [PubMed] [Google Scholar]
- 45.Deng F, Allen TD, Nuss DL. Eukaryotic Cell. 2007;6:235–244. doi: 10.1128/EC.00302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cullen D, Leong SA, Wilson LJ, Henner DJ. Gene. 1987;57:21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- 47.Shapira R, Choi GH, Hillman BI, Nuss DL. EMBO J. 1991;10:741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]