Abstract
The FhuA protein in the outer membrane of Escherichia coli actively transports ferrichrome and the antibiotics albomycin and rifamycin CGP 4832 and serves as a receptor for the phages T1, T5, and φ80 and for colicin M and microcin J25. The crystal structure reveals a β-barrel with a globular domain, the cork, which closes the channel formed by the barrel. Genetic deletion of the cork resulted in a β-barrel that displays no FhuA activity. A functional FhuA was obtained by cosynthesis of separately encoded cork and the β-barrel domain, each endowed with a signal sequence, which showed that complementation occurs after secretion of the fragments across the cytoplasmic membrane. Inactive complete mutant FhuA and an FhuA fragment containing 357 N-proximal amino acid residues complemented the separately synthesized wild-type β-barrel to form an active FhuA. Previous claims that the β-barrel is functional as transporter and receptor resulted from complementation by inactive complete FhuA and the 357-residue fragment. No complementation was observed between the wild-type cork and complete but inactive FhuA carrying cork mutations that excluded the exchange of cork domains. The data indicate that active FhuA is reconstituted extracytoplasmically by insertion of separately synthesized cork or cork from complete FhuA into the β-barrel, and they suggest that in wild-type FhuA the β-barrel is formed prior to the insertion of the cork.
The FhuA protein of Escherichia coli K-12 transports ferrichrome, the structurally related antibiotic albomycin, and the unrelated antibiotic rifamycin CGP 4832 across the outer membrane and serves as a receptor for colicin M, microcin J25, and the phages T1, T5, and φ80 (7). Although extensive mutational analyses have been performed (6, 7, 33) and the crystal structure has been determined (15, 30), the molecular mechanism of FhuA transport is unclear.
The protein consists of a β-barrel (residues 161 to 714) composed of 22 antiparallel β-strands that form a channel that is closed by a globular domain (residues 1 to 160), designated the cork or plug, that inserts from the periplasmic side into the β-barrel. Binding of ferrichrome elicits small, 1- to 2-Å movements of the cork domain relative to the β-barrel and a large 17-Å transition of E19 without opening the channel. Release of ferrichrome from the binding site formed by 10 amino acid residues located in the β-barrel and the cork, and opening of the channel, is thought to be triggered by interaction with the TonB protein, which requires the proton motive force of the cytoplasmic membrane to exert its action on FhuA. All FhuA-related activities, except infection by phage T5, depend on TonB.
We previously reported that deletion of the cork (residues 5 to 160) results in a protein (FhuAΔ5-160) that still functions as an active TonB-dependent transporter and receptor (3, 4, 25). These experiments were performed with two fhuA mutant strains; mutant 41/2 contains a number of amino acid replacements and D348 is deleted (26), whereas PCR analysis indicated that mutant H1857 carries a complete deletion of fhuA (31). These results led to the proposal that TonB interacts with the FhuA β-barrel in addition to the TonB box. We have previously shown that mutations in the so-called TonB box, a pentapeptide motif contained in all TonB-dependent proteins (5, 8), inactivate FhuA. FhuA activity is partially restored when the TonB box mutants FhuA(I9P) (isoleucine at position 9 replaced by proline) and FhuA(V11D) are combined with the TonB mutations Q160K and Q160L (39). The same TonB mutations restore the activity of BtuB TonB box mutants (19). The suppression phenotype suggests interaction of the TonB box with region 160 of TonB. This interaction was then biochemically demonstrated by in vivo disulfide cross-linking between genetically inserted cysteine residues into region 160 of TonB and the TonB boxes of BtuB (11) and FecA (35). A subsequent study using the same corkless FhuA but another E. coli fhuA mutant strain provided results that agreed with our data and in addition showed that corkless fepA mutants are sensitive to colicins B and D and display residual ferric enterobactin transport (40). The results obtained with FepA have been questioned, since the same corkless FepA shows no activity in another fepA test strain (47); from these results, it had been suggested that interprotein complementation by two individually nonfunctional FepA proteins, i.e., plasmid-encoded corkless FepA and chromosomally encoded FepA with an unknown mutation, restored FepA activity. The same argument was suggested to explain the activity of corkless FhuA.
Earlier, we found that cosynthesis of the cork with the β-barrel strongly increases the transport activity of corkless FhuA. These results and the discrepancy with the FepA β-barrel activities prompted us to reexamine our data on the activities of FhuAΔ5-160. In the present work, we show that (i) plasmid-encoded corkless FhuA is inactive in a chromosomal mutant lacking the entire FhuA protein, (ii) inactive complete FhuA and C-terminally truncated FhuA complement corkless FhuA, (iii) separately synthesized cork is incorporated in the periplasm into the β-barrel, (iv) the cork is proteolytically degraded, (v) proteolysis of the cork is less pronounced when it is coexpressed with the β-barrel, and (vi) the previously used fhuA deletion mutant H1857 actually carries a 357-residue N-proximal FhuA fragment.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli strains and plasmids used in this study are listed in Table 1. Cells were grown in TY medium (10 g of Bacto tryptone [Difco Laboratories] liter−1, 5 g of yeast extract liter−1, 5 g of NaCl liter−1). Ampicillin (50 μg ml−1) and/or chloramphenicol (40 μg ml−1) was added when required.
TABLE 1.
E. coli strains and plasmids used
| Strain or plasmid | Genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| BL21(DE3) fhuA | F−hsdSB(rB− mB−) gal dcm ompT λ(DE3); T7 polymerase under lacUV5 control; fhuA | 43 and this study |
| H1605 | mcrA hsdR thr ser rpsL aroB zhe-4::Tn10 lac | K. Hantke |
| AB2847 | aroB tsx malT thi | 18 |
| 41/2 | AB2847 cir fepA fhuA | 18 |
| HK99 | AB2847 tonB fhuA | 26 |
| BR158 | AB2847 tonB | 24 |
| H1857 | AB2847 fhuACDB | K. Hantke |
| CH1857 | AB2847 fhuACDB tonB | K. Hantke |
| MB97 | AB2847 ΔfhuA | This study |
| MB99 | BR158 ΔfhuA | This study |
| MB21 | BL21(DE3) ΔfhuA | This study |
| Plasmids | ||
| pT7-6 | Ampr; ColE1 origin | 44 |
| pT7-7 | Ampr; ColE1 origin | 44 |
| pHSG576 | Cmr; pSC101 origin | 45 |
| pSUKS | Cmr; p15A origin | R. Schönherr |
| pHK763 | pT7-6 fhuA wild type | 24 |
| pAB | pT7-6 fhuA with BamHI E159D | 27 |
| pBK7 | pT7-6 fhuAΔ5-160 E3D | 3 |
| pHK570 | pHSG576 fhuA wild type | 28 |
| pBK570 | pHSG576 fhuAΔ5-160 E3D | 28 |
| p76SH15 | pT7-6 fhuA V11D | 13 |
| pFhuA517 | pT7-6 fhuAΔ5-17 E3G | 13 |
| pFE18 | pT7-6 fhuA E522R | 13 |
| pFE22 | pT7-6 fhuA E571R | 13 |
| pFE60 | pT7-6 fhuA T27C P533C | 13 |
| pCEc761 | pT7-6 fhuA 1-160 E159D | This study |
| pCEc762 | pT7-6 fhuA 1-160 Δ5-17 E3G E159D | This study |
| pCEcSU1 | pSUKS fhuA 1-160 E159D | This study |
| pCEc771 | pT7-7 fhuA 1-160 E159D | This study |
| pCEc772 | pT7-7 fhuA 1-160 Δ5-17 E3G E159D | This study |
| pCEc773 | pT7-7 fhuA 1-160 E3D; without its signal sequence | This study |
| pCEcS77 | pT7-7 fhuAΔ5-160 E3D; without its signal sequence | This study |
| pMBlc1 | pHSG576 fhuA 1-160 E159D fhuAΔ5-160 E3D; under control of PT7 gene 10 | This study |
| pMBlc2 | pHSG576 fhuA 1-160 Δ5-17 E3G E159D fhuAΔ5-160 E3D; under control of PT7 gene 10 | This study |
| pMBlc3 | pHSG576 fhuA 1-160 E159D fhuA wild type; under control of PT7 gene 10 | This study |
| pMBlc4 | pHSG576 fhuA 1-160Δ5-17 E3G E159D fhuA wild type; under control of PT7 gene 10 | This study |
| pMBlc5 | pHSG576 fhuA 1-160 T27C E159D fhuAΔ5-160 E3D P533C; under control of PT7 gene 10 | This study |
| pMBlc6 | pHSG576 fhuA 1-160 Δ5-17 E3G E159D T27C fhuAΔ5-160 E3D P533C; under control of PT7 gene 10 | This study |
| pMBlc7 | pHSG576 fhuA 1-357; under control of PT7 gene 10 | This study |
| pMBLc8 | pHSG576 fhuA 1-160 E159D; under control of PT7 gene 10 | This study |
| pMBLc9 | pHSG576 fhuA 1-160 Δ5-17 E3G E159D; under control of PT7 gene 10 | This study |
| pMBFE1 | pHSG576 fhuA T27C P533C; under control of PT7 gene 10 | This study |
| pMBFE2 | pHSG576 fhuAΔ5-17 T27C E3G P533C; under control of PT7 gene 10 | This study |
| pTO4 | pBR322 cma cmi | 36 |
| pTUC203 | pACYC184 mcjABCD | 42 |
Construction of strains.
The chromosomal fhuA gene was deleted by the method described by Datsenko and Wanner (12). This technique uses the highly efficient phage λ Red recombination system encoded on the helper plasmid pKD46 and direct transformation of PCR products consisting of the cat marker gene flanked by homology extensions of 36 to 50 bp to the gene to be deleted.
E. coli MB16051 ΔfhuA::cat was constructed by transforming strain H1605 carrying the Red helper plasmid pKD46 (12) with a PCR product generated by using plasmid pKD3 (12) as the template with primers FhuA_rbs_kd3 (5′-CGTTTACGTTATCATTCACTTTACATCAGAGATATACCAATGGCGCGTGTGTAGGCTGGAGCTGCTTCG-3′) and FhuCstart_kd3_rev (5′-ATTACGCAGTGCAAAAGTGGTATCGGAATGATTCGTGTATTCCTGCATATGAATATCCTCCTTAG-3′). The resulting PCR product carried the cat marker gene flanked by two directly repeated FLP recombinase recognition target (FRT) sites and by an upstream homology extension of 47 bp (positions −39 to +8 of the fhuA gene) and a downstream homology extension of 45 bp (positions +4 to +48 of the fhuC gene). The transformants were selected on TY plates supplemented with chloramphenicol (20 μg ml−1). Replacement of fhuA by the cat gene was verified with PCR. Strains MB101, MB102, and MB22 were obtained by phage P1 transduction of ΔfhuA::cat of strain MB16051 into strains AB2847 (MB101), BR158 (MB102), and BL21(DE3) (MB22), respectively. All transductants were selected on TY agar with chloramphenicol (20 μg ml−1) and tested for loss of phage T5 sensitivity. MB101, MB102, and MB22 were transformed with plasmid pCP20, which has a temperature-sensitive replicon and thermal induction of FLP synthesis (12). Transformants were selected at 30°C, and a few colonies were purified once at 43°C and then tested for loss of all antibiotic resistance markers. The resulting strains MB97 (MB101), MB99 (MB102), and MB21 (MB22) had lost the chromosomal cat insertion and the flanking FRT sites were joined together, thus resulting in disruption of the fhuA gene, as verified by PCR cloning and DNA sequencing. All strains contained fully active FhuCDB transport proteins because the FhuA promoter and a ribosome-binding site inside the “scar” sequence left after disruption of the cat gene (12) were provided on the chromosome.
Construction of plasmids.
Plasmids containing the coding sequence of the wild-type E. coli FhuA cork domain or deletion derivatives of the E. coli FhuA cork domain were constructed by PCR amplification of the cork domain sequence and cloning of the respective fragments into vector pT7-6. The primers used and the constructions are available upon request.
Coexpression and [35S]methionine labeling of FhuA cork domains together with FhuA or the FhuA β-barrel was done with plasmid constructions consisting of the low-copy-number vector pHSG576 with a phage T7 gene 10 promoter and the FhuA cork domain and FhuA or the FhuA β-barrel cloned in tandem. The gene fragments encoding FhuA1-160 and FhuA1-160 Δ5-17 together with the T7 gene 10 promoter were cloned by PCR amplification of a 620-bp fragment of plasmid pCEc771 (FhuA1-160) or pCEc772 (FhuA1-160 Δ5-17).
To investigate the fhuA gene fragment of E. coli H1857, the DNA fragment was cloned into pT7-6 with PCR by using primers FhuA_Hind_for (5′-TCAGTAAGCTTGCGCGTTCCAAAACTGCTCAGCCAAAA-3′) and FhuC_Eco_rev (5′-ATACGTGAATTCACACGAAAGGAGATATTACGCAGTGC-3′) and chromosomal DNA of strain H1857 as the template. The PCR fragment was purified, digested with HindIII and EcoRI, and cloned into HindIII-EcoRI-cleaved pT7-6, resulting in pSKFhuA. Plasmid pMBlc7 was obtained by cloning the 2,000-bp MluI-EcoRI fragment of pSKFhuA into MluI-EcoRI-cleaved pMBlc1.
Recombinant DNA techniques.
Isolation of plasmids, use of restriction enzymes, ligation, agarose gel electrophoresis, and transformation were done by standard techniques (38). All genetic constructions were examined by DNA sequencing by the dideoxy chain termination method with fluorescence-labeled or unlabeled nucleotides (Auto Read sequencing kit; Pharmacia Biotech, Freiburg, Germany) and an A.L.F. sequencer (Pharmacia).
Protein analytical methods.
E. coli BL21 cells (optical density at 578 nm of 0.5) transformed with one of various plasmids were used for labeling the proteins with [35S]methionine and subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously (25).
Cell fractionation.
Cells of E. coli BL21 transformants carrying plasmid pCEc763 or pT7-6 were fractionated as described previously (10).
In vivo cross-linking.
Freshly transformed E. coli BL21 fhuA cells expressing the plasmid-encoded FhuA cork domain and FhuA or FhuAΔ5-160 cloned in tandem were used for site-specific and nonspecific in vivo cross-linking as described previously (35), with the exception that cells were not induced with ferric citrate.
Phenotype assays.
All phenotype assays were performed with freshly transformed E. coli K-12 strains. Sensitivity of cells against the FhuA ligands, growth inhibition by various antibiotics, and growth promotion was tested as previously described (3, 4, 25). The colicin M solution was a crude extract of a strain carrying plasmid pTO4 cma cmi (36), and the microcin J25 solution was a supernatant of a strain carrying plasmid pTUC203 mcjABCD (42).
Phage T5 inactivation assay.
In vitro inactivation of phage T5 by wild-type FhuA and FhuAΔ5-160 was determined with isolated outer membrane fractions. E. coli MB21 ΔfhuA transformed with plasmid pHK763 or pBK7 was grown in 200 ml of TY medium to an optical density at 578 nm of 0.5, and then isopropyl-β-d-thiogalactopyranoside was added to induce synthesis of the chromosomally encoded phage T7 RNA polymerase under lacI control. Outer membrane fractions suspended in 0.5 ml were prepared as previously described (3). Ten microliters of the outer membrane suspension containing wild-type FhuA and 100 μl of the outer membrane fraction containing FhuAΔ5-160 were mixed with buffer (M9 salts, 1 mM MgSO4, 0.1 mM CaCl2) to a final volume of 0.5 ml. To each reaction mixture 50 μl of phage T5 (107 PFU/ml) was added and incubated at 37°C for 15 min, after which 300 μl of the suspension was mixed with 200 μl of ice-cold saline. After centrifugation for 15 min at 4°C, 50 μl portions of 10-fold dilutions of the supernatants were mixed with 108 cells of the indicator strain AB2847 in 3 ml of TY top agar and poured onto a TY plate. After incubation overnight at 37°C, single plaques were counted. The outer membrane fraction of E. coli MB21 ΔfhuA carrying the pT7-6 vector served as a control.
Transport assays.
Transformants of E. coli MB97 ΔfhuA aroB were grown overnight on TY plates and then suspended in M9 salts (32) with 0.4% glucose, washed twice, and used in [55Fe3+]ferrichrome transport assays as described previously (25).
RESULTS
Complementation of corkless FhuA by the separately expressed FhuA cork.
Previously, we measured [55Fe3+]ferrichrome transport activities of FhuAΔ5-160 that amounted to 30 to 60% of that of wild-type FhuA (3, 4). In these experiments, E. coli 41/2, which synthesizes an inactive FhuA protein (26), was used and FhuAΔ5-160 was encoded on the medium-copy-number plasmid pT7-6 under the control of the fhuA promoter. The fhuA mutations exerted no polar effect on the expression of the downstream fhuCDB genes, whose activities are required for transport of ferrichrome across the cytoplasmic membrane. High activity of FhuAΔ5-160 was also obtained in E. coli H1857, for which evidence existed that the fhuACDB genes were deleted (31).
When FhuAΔ5-160 was encoded on the low-copy-number plasmid pHSG576, the rate of transport of ferrichrome by FhuAΔ5-160 into E. coli 41/2 was only 5 to 10% of that by wild-type FhuA, but it was increased 10-fold by cosynthesis of the cork fragment FhuA1-160 cloned on pT7-6 (data not shown, but see Fig. 1, which shows results obtained with the newly constructed strain MB97). The strong increase was unexpected. It was not caused by binding of Fe2+-loaded Fur repressor to the fhuA promoter of the overexpressed gene fragment encoding FhuA1-160, which could have resulted in a higher synthesis of FhuAΔ5-160 through Fur titration. Transformants carrying an fhuA promoter DNA fragment including the binding site of Fe2+-Fur showed no increase of FhuAΔ5-160-mediated ferrichrome transport (data not shown). The specificity of complementation was further revealed by an FhuA1-160 cork fragment in which residues 5 to 17, including the TonB box, were deleted. This cork fragment did not stimulate ferrichrome transport by FhuAΔ5-160 but rather eliminated transport completely in E. coli 41/2 fhuA (data not shown).
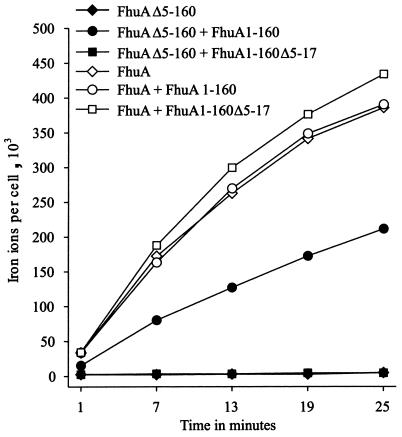
FIG. 1.
Time-dependent transport of [55Fe3+]ferrichrome (1 μM) into E. coli MB97 ΔfhuA aroB expressing plasmid-encoded FhuA or FhuA β-barrel of E. coli together with FhuA cork domain fragments as indicated.
The apparent functional complementation of FhuAΔ5-160 by FhuA1-160 raised the question whether in previous experiments FhuAΔ5-160 was complemented by the cork of inactive chromosomal FhuA either in its free form (arising from proteolytic cleavage) or still linked to the β-barrel. Previously, these possibilities were excluded by using E. coli H1857, which had been characterized by PCR as a mutant with a chromosomal deletion of the entire fhuACDB operon (31). The complementation results prompted a reexamination of the earlier deletion analysis of strain H1857. Cloning and sequencing of the DNA region around fhuA of strain H1857 revealed that after the codon encoding residue 357 of the mature FhuA protein, a frameshift mutation resulted in 27 amino acids unrelated to FhuA, followed by the two stop codons TAA and TAG. Residue 357 is located in the middle of β-strand 8 of the FhuA crystal structure. Either the FhuA1-357 fragment as such or a proteolytic fragment encompassing the cork could have complemented FhuAΔ5-160.
Complementation of the FhuA β-barrel by the cork in an fhuA deletion mutant.
Since E. coli H1857 encoded an N-proximal FhuA fragment, an fhuA deletion mutant that was devoid of the entire FhuA protein was constructed. By using the technique described by Datsenko and Wanner (12), a cat gene flanked by two FRT sites was exchanged for the chromosomal fhuA gene such that the original fhuA gene from bp +8 of fhuA to +3 of fhuC was deleted. The cat gene insertion was then excised by using plasmid-encoded FLP recombinase, which resulted in joint FRT sites. After the excision of the cat gene, the fhuCDB genes downstream of fhuA were transcribed under the control of the original fhuA promoter and a ribosome-binding site together with a start codon that were provided by the remaining “scar” sequence after excision of the cat gene (12). The resulting strain, E. coli MB97 ΔfhuA aroB, showed no negative polar effect on fhuCDB expression.
E. coli MB97 did not transport ferrichrome and remained transport inactive after transformation with plasmid pBK570 derived from the low-copy-number vector pHSG576 and carrying the gene fragment encoding FhuAΔ5-160 (Fig. 1). After additional transformation with the medium-copy-number plasmid pCEc761 carrying the gene fragment encoding FhuA1-160, E. coli MB97 FhuAΔ5-160 FhuA1-160 transported ferrichrome at 45% of the rate of E. coli MB97(pHK570), in which wild-type FhuA was encoded on the same vector used for FhuAΔ5-160. FhuA1-160 Δ5-17, which lacked the TonB box, did not restore transport activity of strain MB97 FhuAΔ5-160 (Fig. 1). A DNA fragment encoding the N terminus of FhuA containing the TonB box (residues 1 to 24) was not sufficient to confer ferrichrome transport (data not shown). FhuA1-160 and FhuA1-160 Δ5-17 coexpressed in E. coli MB97(pHK570) did not affect the transport activity of wild-type FhuA (Fig. 1). Replacement of the FhuA cork by the FepA cork (FepA1-153) or FecA cork (FecA1-223) did not result in transport-competent E. coli MB97 FhuAΔ5-160 transformants (data not shown). The data demonstrate that FhuAΔ5-160 is transport inactive and that the activity is specifically restored by complementation with the missing E. coli wild-type FhuA cork domain.
The ferrichrome transport activities agreed with the receptor activities of FhuAΔ5-160 and FhuAΔ5-160 reconstituted with FhuA1-160. E. coli MB97 FhuAΔ5-160 was resistant to all FhuA ligands and became fully sensitive to all ligands except microcin J25 after in vivo complementation with FhuA1-160 (Table 2). A slight sensitivity towards microcin J25 was restored when the expression levels of FhuAΔ5-160 and FhuA1-160 were increased by using vectors with higher copy numbers (pBK7 and pCEcSU1). The FhuA1-160 Δ5-17 cork lacking the TonB box did not confer TonB-coupled phage and colicin sensitivity to E. coli MB97 FhuAΔ5-160 but restored phage T5 sensitivity (Table 2).
TABLE 2.
Sensitivity of E. coli MB97 ΔfhuA and MB99 ΔfhuA tonB expressing plasmid-encoded FhuA derivativesa
| Strain | Sensitivityb to:
|
||||
|---|---|---|---|---|---|
| T5 | φ80 | Colicin M | Albomycin | Microcin J25 | |
| MB97 ΔfhuA expressing: | |||||
| Wild-type FhuA (pHK570) | 5 (6) | 6 | 4 | 4 (5) | 2 (3) |
| FhuAΔ5-160 (pBK570) | —d | — | — | — | — |
| FhuAΔ5-160 (pBK570) + FhuA1-160 (pCEc761) | 5 (6) | 6 | 3 (4) | 3 (4) | — |
| FhuAΔ5-160 (pBK570) + FhuA1-160 Δ5-17 (pCEc762) | 6 | — | — | — | — |
| FhuAΔ5-160 (pBK570) + FhuA1-160 CPc (pCEc773) | — | — | — | — | — |
| FhuAΔ5-160 (pBK7) + FhuA1-160 (pCEcSU1) | 5 (6) | 6 | 4 | 4 | 0 (1) |
| FhuAΔ5-160 (pBK7) + FhuA1-357 (pMBlc7) | 5 (6) | 6 | 4 | 4 (5) | — |
| FhuAΔ5-160 CP (pMBS77) + FhuA1-160 (pCEcSU1) | — | — | — | — | — |
| FhuA E571R (pFE22) | 1 (2-4) | 0 (1-3) | 0 (1) | — | — |
| FhuA E571R (pFE22) + FhuAΔ5-160 (pBK570) | 5 (6) | 6 | 2 (3) | 1 (2) | — |
| FhuA E522R (pFE18) | 0 (1-3) | (0-3) | (0) | — | — |
| FhuA E522R (pFE18) + FhuAΔ5-160 (pBK570) | 5 (6) | 6 | 2 (3) | 1 (2) | — |
| FhuA V11D (p76SH15) | 5 (6) | (0) | — | — | — |
| FhuA V11D (p76SH15) + FhuA1-160 (pCEcSU1) | 5 (6) | (0) | — | — | — |
| FhuAΔ5-17 (pFhuA517) | 6 | — | — | — | — |
| FhuAΔ5-17 (pFhuA517) + FhuA1-160 (pCEcSU1) | 6 | — | — | — | — |
| MB99 ΔfhuA tonB expressing: | |||||
| FhuAΔ5-160 (pBK570) + FhuA1-160 (pCEc761) | 6 | — | — | — | — |
| FhuAΔ5-160 (pBK570) + FhuA1-160 Δ5-17 (pCEc762) | 6 | — | — | — | — |
Sensitivities to the ligands were tested by using E. coli MB97 ΔfhuA or MB99 ΔfhuA tonB freshly transformed with the indicated plasmids. The sensitivities to phages T1, T5, and φ80 and to colicin M, microcin J25, and albomycin were tested by spotting 3 μl of 10-fold or 3-fold (albomycin and microcin J25) dilutions onto TY agar plates overlaid with TY top agar containing the strain to be tested.
The results are given as the last of a 10-fold or 3-fold dilution series that resulted in a clear zone of growth inhibition. Numbers in parentheses indicate turbid inhibition zones.
CP, cytoplasmic (the protein was expressed without its signal sequence).
—, no growth inhibition and no phage plaques.
FhuAΔ5-160 is incorporated into the outer membrane.
To see whether FhuAΔ5-160 was contained in the outer membrane, an E. coli BL21 derivative carrying the same fhuA deletion as E. coli MB97 (MB21) was transformed with plasmids that encoded FhuAΔ5-160 and, for comparison, wild-type FhuA. The outer membrane fractions were prepared, and the presence of FhuAΔ5-160 was examined by SDS-PAGE. FhuAΔ5-160 was contained in the membrane fraction but in an amount at least 10 times less than that of wild-type FhuA. FhuAΔ5-160, like wild-type FhuA, was largely processed to the mature form, which suggested secretion of FhuAΔ5-160 across the cytoplasmic membrane (SDS-polyacrylamide gel not shown). This result was also obtained previously with an E. coli BL21 derivative carrying an uncharacterized fhuA mutation (3). To exclude the possibility that FhuAΔ5-160 was not incorporated into the outer membrane but aggregated in the periplasm and cofractionated with the outer membrane, the sensitivity of cells to antibiotics for which the outer membrane forms a permeability barrier was tested (34). If FhuAΔ5-160 formed an open channel in the outer membrane, it should increase the antibiotic sensitivity of cells. FhuAΔ5-160 was synthesized in E. coli MB99 fhuA tonB, which was seeded on nutrient agar plates onto which filter paper disks containing various antibiotics were placed. Compared to MB99 synthesizing wild-type FhuA, FhuAΔ5-160 increased sensitivity to erythromycin (molecular weight, 734), rifamycin (molecular weight, 823), and bacitracin (molecular weight, 1,425) (Table 3) but not to the smaller antibiotics novobiocin (molecular weight, 634), neomycin (molecular weight, 614), bekanamycin (molecular weight, 484), and spectinomycin (molecular weight, 405), which may diffuse at a sufficient rate through the porins. Similar results were obtained with strain MB97 synthesizing FhuAΔ5-160 (Table 3). The observed difference between wild-type FhuA and FhuAΔ5-160 was substantial, since there is a logarithmic relationship between the diameter of the inhibition zone and the antibiotic concentration. The results obtained with the antibiotics were supported by the results obtained with ferrichrome. Growth of MB99 (FhuAΔ5-160), whose tonB mutation prevents FhuA-mediated active ferrichrome transport, was supported on NB agar plates on which the available iron was reduced by 0.2 mM dipyridyl. Growth occurred around filter paper disks which were soaked in at least 0.3 mM ferrichrome (Table 3). Ferrichrome passed the outer membrane by diffusion through FhuAΔ5-160, and higher concentrations were required to support growth than when active transport occurred (0.03 mM ferrichrome resulted in a 19-mm-diameter growth zone of MB97 synthesizing wild-type FhuA).
TABLE 3.
Growth promotion by ferrichrome as sole iron source and sensitivity to antibiotics of cells expressing plasmid-encoded FhuAΔ5-160 or wild-type FhuAa
| FhuA derivative on plasmid | Growthb of MB97 ΔfhuA/MB99 ΔfhuA tonB on ferrichrome at:
|
Sensitivityb,c of MB97 ΔfhuA/MB99 ΔfhuA tonB to:
|
|||||
|---|---|---|---|---|---|---|---|
| 0.1 mM | 0.3 mM | 1 mM | 3 mM | Ery (15 μg; 734 Da) | Rif (5 μg; 823 Da) | Bac (30 μg; 1,421 Da) | |
| Wild-type FhuA | 20/— | 24/— | 30/— | 35/— | 9/9 | 12/13 | —/— |
| FhuAΔ5-160 | 8/— | 12/8 | 16/15 | 20/19 | 14/15 | 18/19 | 10 |
| No FhuA | —/— | —/— | —/— | —/— | 9/9 | 12/12 | —/— |
Growth promotion by ferrichrome and sensitivity to antibiotics was determined with E. coli MB97 ΔfhuA and MB99 ΔfhuA tonB expressing plasmid-encoded wild-type FhuA or FhuAΔ5-160. The growth zones with ferrichrome and the inhibition zones with antibiotics around filter paper disks that contained 10 μl of the various solutions with the indicated concentrations and amounts were determined.
The size of the zones (in millimeters are given without subtraction of the paper disk (6 mm). —, no growth or no sensitivity.
The molar masses of the inhibitors are indicated. Ery, erythromycin, Rif, rifamycin, Bac, bacitracin.
To see whether uptake of ferrichrome across the outer membrane was independent of energy, growth promotion by ferrichrome of MB99 tonB was compared with that of MB97 tonB+ expressing FhuAΔ5-160. The growth zones were similar to those obtained with MB99 tonB, but a slight increase in the growth zone of the MB97 transformant was consistently observed. This became particularly apparent at low ferrichrome concentrations (Table 3). The improved uptake seems to be related to FhuAΔ5-160, since untransformed MB99 showed no growth, which excludes another outer membrane protein that transports ferrichrome as a side reaction. It is unclear how TonB accomplishes this low increase of FhuAΔ5-160 uptake activity.
Phage T5, which is inactivated by an isolated outer membrane fraction (48), was used to see whether FhuAΔ5-160 inactivated T5 without necessarily leading to T5 infection of the cells. This assay may also identify binding-competent FhuAΔ5-160 associated with but not incorporated into the outer membrane. Since the outer membrane fractions contained 10 times less FhuAΔ5-160 than wild-type FhuA, inactivation of phage T5 was determined with 10 times more of the FhuAΔ5-160 membrane fraction than of the wild-type FhuA membrane fraction. Under the conditions used, wild-type FhuA reduced the number of phage plaques from 376 (outer membrane fraction lacking FhuA) to 2, in contrast to FhuAΔ5-160, which did not reduce the number of PFU (387).
Identification and localization of the FhuA1-160 cork.
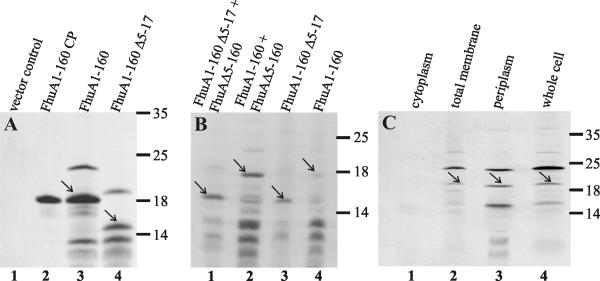
Attempts to identify the cork encoded by the plasmids used for the functional assays by Coomassie blue staining after standard SDS-PAGE failed. Therefore, E. coli BL21 was transformed with the gene fragment encoding FhuA1-160, which was transcribed by the chromosomally encoded T7 RNA polymerase by using the phage T7 gene 10 promoter of plasmid pCEc771. The E. coli RNA polymerase of strain BL21 was inhibited by rifamycin, and the proteins derived from the T7 polymerase transcription were labeled with [35S]methionine. SDS-PAGE revealed several protein bands with cells transformed with plasmid pCEc771 or pCEc772, with electrophoretic mobilities that corresponded to the unprocessed cork with signal sequence, the processed cork, and a degradation product (Fig. 2A, lane 3 and 4). In contrast, cells expressing the cork domain in the cytoplasm without its signal sequence showed only a single protein band at approximately 19 kDa (Fig. 2A, lane 2) The finding that periplasmic but not cytoplasmic FhuA1-160 was degraded indicates that degradation occurred in the periplasm. Coexpression of FhuA1-160 or FhuA1-160 Δ5-17 with FhuAΔ5-160 yielded stronger FhuA1-160 and FhuA1-160 Δ5-17 bands than were observed when the cork domains were synthesized in the absence of FhuAΔ5-160 (Fig. 2B, compare lane 1 with lane 3 and lane 2 with lane 4). FhuA1-160 Δ5-17 lacking the TonB box was degraded to smaller fragments than FhuA1-160 (Fig. 2A, lane 4).
FIG. 2.
(A) Comparison of [35S]methionine-labeled cork domains of FhuA after transformation of E. coli BL21 fhuA with plasmids pT7-7 (lane 1), pCEc773 (lane 2), pCEc771 (lane 3), and pCEc772 (lane 4). The proteins were separated by SDS-PAGE of whole-cell lysates. No other bands were seen outside the gel section presented here. Arrows indicate the mature cork fragments. CP, cytoplasmic (without signal sequence). (B) Autoradiograph after SDS-PAGE with [35S]methionine-labeled proteins of E. coli BL21 fhuA whole-cell lysates after transformation with plasmids pCEc762 and pBK570 (lane 1), pCEc761 and pBK570 (lane 2), pCEc762 and pHSG576 (lane 3), and pCEc761 and pHSG576 (lane 4). Arrows indicate the mature FhuA cork domain proteins. (C) Autoradiograph after SDS-PAGE of different cell fractions of E. coli BL21 fhuA expressing [35S]methionine-labeled FhuA1-160 cork domain of E. coli encoded on plasmid pCEc761. Cytoplasmic fraction (lane 1), total membrane fraction (lane 2), periplasmic fraction (lane 3), and whole-cell lysates (lane 4) were applied. The control strain BL21 fhuA transformed with the pT7-6 vector showed no bands of [35S]methionine-labeled proteins. Arrows indicate the mature cork domains. Numbers on the right of each panel indicate molecular masses in kilodaltons.
Subcellular localization revealed no cork synthesized with signal sequence in the cytoplasm (Fig. 2C, lane 1), mainly two bands in the membrane fraction, and three bands in the periplasmic fraction and the total cell lysate (lanes 3 and 4). The mature protein and the degradation product were contained mainly in the periplasmic fraction; the unprocessed form was mainly in the membrane fraction and the total cell lysate.
Incorporation of the cork into the β-barrel.
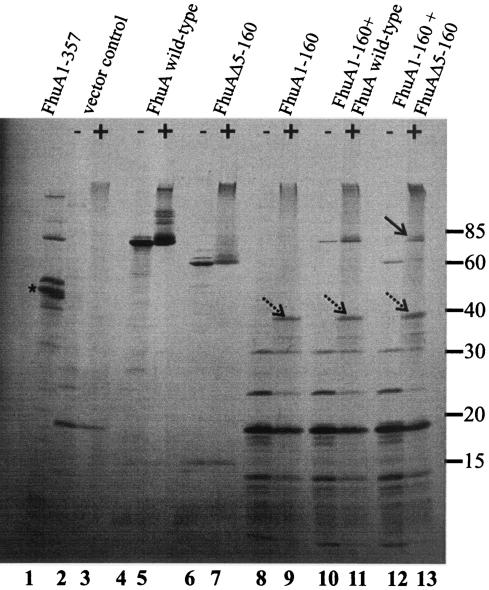
To demonstrate incorporation of FhuA1-160 into FhuAΔ5-160, the genes encoding the FhuA derivatives were cloned in tandem on the low-copy-number plasmid pHSG576, forming pMBlc1. Tandem cloning was necessary because transformants of E. coli BL21 carrying two plasmids with a phage T7 gene 10 promoter, one encoding FhuA1-160 and one encoding FhuAΔ5-160, did not grow in liquid culture. Plasmid pMBlc1 carries a phage T7 gene 10 promoter and the gene for the FhuA 1-160 cork domain, followed by two stop codons and then downstream the gene for FhuAΔ5-160. Both FhuA derivative proteins were specifically transcribed by the T7 RNA polymerase, and the cork domain was synthesized in larger amounts than the FhuA β-barrel, which was caused mainly by the optimal ribosome-binding site upstream of the gene fragment encoding FhuA1-160 and the less ideal fhuA ribosome-binding site upstream of the gene fragment encoding FhuAΔ5-160 (Fig. 3, lanes 10 to 13; compare the 19-kDa band with the 60- and 80-kDa bands). The [35S]methionine-labeled cells were treated for 10 min with 1% formaldehyde to link the two FhuA fragments covalently so that they would stay together in the subsequent SDS-PAGE. In the formaldehyde-treated sample, a protein band with an apparent size of 80 kDa appeared (Fig. 3, lane 13); this band was absent in the untreated sample (Fig. 3, lane 12). The band was at the same position as the wild-type FhuA band (lanes 4, 5, 10, and 11), which indicated incorporation of FhuA1-160 into FhuAΔ5-160. No such band was observed in samples containing only FhuA1-160 (lanes 8 and 9) or FhuAΔ5-160 (lanes 6 and 7).
FIG. 3.
Comparison of [35S]methionine-labeled FhuA proteins after cross-linking with 1% formaldehyde (+ [samples without formaldehyde are marked −]) of FhuA1-160 and FhuAΔ5-160. Cross-linking experiments were performed with E. coli strain BL21 fhuA carrying plasmid pT7-6 (lanes 2 and 3), pHK763 (lanes 4 and 5), pBK7 (lanes 6 and 7), pCEc761 (lanes 8 and 9), pMBlc3 (lanes 10 and 11), or pMBlc1 (lanes 12 and 13). Samples were heated at 65°C for 5 min prior to gel loading. Whole-cell lysate of E. coli BL21 fhuA expressing FhuA1-357 encoded on plasmid pMBlc7 was also applied on the gel (lane 1). The mature protein of the truncated FhuA is marked by an asterisk. The arrow in lane 13 indicates the FhuA1-160 FhuAΔ5-160 cross-link product; arrows with broken lines indicate possible cork dimer products in lanes 9, 11, and 13. Numbers on the right indicate molecular masses in kilodaltons.
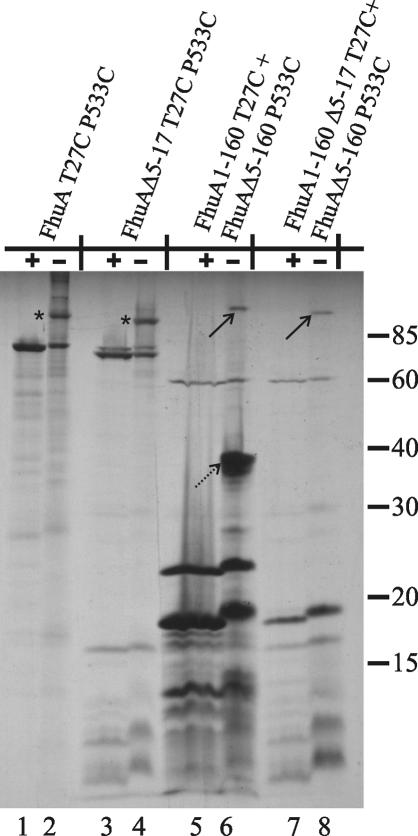
In another approach, T27 of the cork and P533 of the β-barrel were replaced by cysteine. According to the crystal structure, these two residues are close enough to form disulfide bridges. In complete FhuA, these two cysteine residues spontaneously formed disulfide bonds, and the resulting FhuA protein showed a slower electrophoretic mobility than wild-type FhuA (13). FhuA1-160 T27C and FhuAΔ5-160 P533C were cloned in tandem, and transformants were labeled with [35S]methionine. The proteins were separated by SDS-PAGE in the absence or presence of β-mercaptoethanol, which is routinely added to the sample buffers to support unfolding of proteins by cleavage of disulfide bonds.
In the nonreduced sample, a protein band corresponding to a molecular mass of approximately 110 kDa was visible (Fig. 4, lane 6); a similar high-molecular-mass protein with a slightly increased electrophoretic mobility was found in cells that synthesized FhuA1-160 T27C Δ5-17 and FhuAΔ5-160 P533C (Fig. 4, lane 8). The altered electrophoretic mobility indicates the formation of a disulfide bond between T27C of FhuA1-160 (or FhuA1-160 Δ5-17) and P533C of FhuAΔ5-160. Assignment of the 110-kDa band to disulfide-linked cork-β-barrel was supported by the disappearance of the band upon cleavage of the disulfide bond by reduction with β-mercaptoethanol (lanes 5 and 7). Dimer formation between two FhuAΔ5-160 P533C molecules was excluded by the lack of a resulting protein band of approximately 125 kDa and by the orientation of P533 into the barrel interior of the wild-type FhuA crystal structure. However, a strong band of 40 kDa appeared; this band probably represents a dimer of FhuA1-160 T27C (lane 6) and disappeared after reduction (lane 5). Residue C27 is exposed to the periplasm, making it accessible to disulfide formation. In contrast, no dimerization product of FhuA1-160 Δ5-17 T27C was observed (lane 8), which suggests that the Δ5-17 deletion changed the FhuA conformation such that disulfide formation was prevented. In FhuAT27C P533C, only an intramolecular disulfide bridge was formed, as evidenced by the slower mobility of a portion of the protein (lane 2), which upon reduction had the expected mobility (lane 1). Intramolecular disulfide formation also occurred in FhuAΔ5-17 T27C P533C (lane 4). No cross-linked products were observed with proteins in which no cysteine residues were introduced (data not shown). FhuA contains a disulfide bridge between residues 318 and 329 and between residues 692 and 698 (2, 15, 30), which are not essential for FhuA activity (2). Incubation with dithiothreitol (DTT) did not alter the electrophoretic mobility of FhuA.
FIG. 4.
Comparison of [35S]methionine-labeled FhuA proteins after in vivo cysteine cross-linking of FhuA T27C P533C and FhuA1-160 T27C cork mutant with the FhuAΔ5-160 P533C β-barrel mutant. Cross-linking experiments were performed with E. coli strain BL21 fhuA carrying plasmid pMBFE1 (lanes 1 and 2), pMBFE2 (lanes 3 and 4), pMBlc5 (lanes 5 and 6), or pMBlc6 (lanes 7 and 8). Samples were suspended in nonreducing sample buffer (−) or in sample buffer containing 1% β-mercaptoethanol (+) and heated at 90°C for 5 min prior to gel loading. Arrows in lanes 6 and 8 indicate the cross-linked complex between FhuA1-160 T27C and FhuAΔ5-160 P533C. The broken arrow in lane 6 indicates the possible cross-linked FhuA1-160 T27C dimer. Asterisks in lanes 2 and 4 indicate the intraprotein cross-linked complexes of FhuA T27C P533C and FhuAΔ5-17 T27C P533C showing lower electrophoretic mobilities than the un-cross-linked proteins (lanes 1 and 3). Numbers on the right indicate molecular masses in kilodaltons.
Transport of ferrichrome through FhuA1-160 T27C FhuAΔ5-160 P533C.
To determine the activity of the disulfide-cross-linked FhuA1-160 T27C FhuAΔ5-160 P533C protein, E. coli MB97 was transformed with plasmid pMBlc5, and [55Fe3+]ferrichrome transport was measured. The transformants did not transport ferrichrome. Addition of DTT to the transport medium resulted in a transport rate of 236 ions/min. The transport rate of FhuA 1-160 FhuAΔ5-160 without cysteine substitutions was 772 ions/min, which was reduced to 233 ions/min in the presence of DTT. Rather high concentrations of DTT (100 mM) had to be used to reduce the disulfide bonds completely, as shown by the electrophoretic mobility. DTT in such high concentrations probably displayed some cell toxicity, a conclusion that was supported by the same overall transport rate of FhuA1-160 FhuAΔ5-160 and FhuA 1-160 T27C FhuAΔ5-160 P533C upon incubation with DTT. Wild-type FhuA with both substitutions, T27C and P533C, did not transport ferrichrome; transport was restored at the wild-type FhuA rate when 100 mM DTT was added to the transport medium (13). Since covalent fixation of the cork to the β-barrel inactivates the transport function, we conclude that a flexible cork domain is required for transport of ferrichrome.
Extracytoplasmic incorporation of the cork into the β-barrel.
To determine in which cell compartment FhuA1-160 was incorporated into FhuAΔ5-160, the two proteins, one or both of which contained a signal sequence, were cosynthesized. In E. coli MB97, FhuA activity was detected only when both proteins contained a signal sequence. In this case, the sensitivity of cells to phages T5 and φ80, colicin M, and albomycin was comparable to that of E. coli MB97 synthesizing wild-type FhuA (Table 2). Only sensitivity to microcin J25, which requires a high FhuA activity, was not restored. When the amounts of the FhuA derivatives were increased by cloning both proteins on a medium-copy-number vector, a slight sensitivity to microcin J25 was observed (Table 2). The cork with signal sequence did not secrete the β-barrel without signal sequence.
Complementation of FhuAΔ5-160 by FhuA1-357 and inactive complete FhuA.
Cosynthesis of a plasmid-encoded inactive FhuA fragment consisting of 357 N-proximal residues carried by the fhuA mutant H1857 with FhuAΔ5-160 in the fhuA deletion mutant MB97 restored ferrichrome transport to 63% of the wild-type FhuA level (data not shown). FhuA1-357 conferred to E. coli MB97 FhuAΔ5-160 sensitivity to phages T5 and φ80, colicin M, and albomycin to a level that corresponded to the sensitivity of E. coli MB97 synthesizing wild-type FhuA (Table 2). These results indicated that the N-proximal FhuA fragment or a proteolytic degradation product was able to incorporate into FhuAΔ5-160. Attempts to identify the size of the fragment in the FhuAΔ5-160 β-barrel failed. The major product synthesized by pMBlc7 carrying the fragment encoding FhuA1-357 was 43 kDa, which corresponds approximately to a polypeptide of 384 residues (357 residues of FhuA and 27 residues after the frameshift) (Fig. 3, lane 1). A band above the major band was observed in the gel; this band probably was the unprocessed precursor of FhuA1-357. Only very faint bands at the position of the cork (20 kDa) were observed in the gel.
The previously observed complementation of FhuAΔ5-160 by complete mutant FhuA in E. coli 41/2 (3) resulted from complementation between the two proteins. To support this conclusion further, two additional inactive FhuA proteins with single amino acid replacements located in the β-barrel were cosynthesized with FhuAΔ5-160 in E. coli MB97. FhuA(E522R) and FhuA(E571R) alone displayed no or only very low FhuA activities (Table 2) (13). When each of these mutant proteins was combined with FhuAΔ5-160, phage sensitivity was restored to the FhuA wild-type level and colicin M and albomycin sensitivity was regained to a level lower than that obtained with wild-type FhuA (Table 2).
Wild-type cork does not replace mutant cork of FhuA.
Isolated cork and cork contained in FhuA1-357 and full-length but inactive mutant FhuA with a wild-type cork domain and a mutated β-barrel restored activity of FhuAΔ5-160. To examine whether the isolated wild-type cork can replace the mutant cork of full-length FhuA derivatives, FhuA1-160 was cosynthesized with inactive FhuA(V11D) and FhuAΔ5-17. No TonB-coupled activity was restored, and sensitivity to phage T5 remained at the level of FhuA(V11D) and FhuAΔ5-17 (Table 2).
DISCUSSION
FhuAΔ5-160 was incorporated into the outer membrane and increased the permeability for ferrichrome and antibiotics, which suggests that it is folded in a β-barrel that forms a channel. Iron supply by ferrichrome and an increase of sensitivity to antibiotics larger than 700 Da but not to smaller antibiotics demonstrated that these compounds diffused through the FhuAΔ5-160 channel. Previously, in vitro incorporation of FhuAΔ5-160 into an artificial lipid bilayer membrane increased the conductance of KCl across the bilayer (4), which indicated the formation of a channel. FhuAΔ5-160 did not display any FhuA-specific activity, not even the receptor function for phage T5, which does not require TonB. It is therefore not only the loss of the TonB box along with the cork which inactivates FhuA. It is possible that the β-barrel of FhuAΔ5-160 cannot fold exactly like wild-type FhuA. The β-barrel contains the L4 loop, to which binding of phages T5, T1, and φ80 has been demonstrated (29). Phage T5 binding is not affected by TonB-dependent conformational changes, which have to be assumed for binding of phages T1 and φ80 since they bind irreversibly, accompanied by release of DNA from the phage head, only to energized TonB+ cells (17). Phage T5 binds to isolated FhuA and releases DNA (9, 37, 48), in contrast to phages T1 and φ80, which do not bind to isolated FhuA. It is suggested that in corkless FhuA, the conformation of loop 4 is altered such that phage T5 cannot bind and/or that release of DNA from the phage head is not triggered. On the other hand, the cork may not only be required for proper folding of the β-barrel but also serve a role in the receptor activity of FhuA to T5.
The FhuA β-barrel was functionally reconstituted by the FhuA cork, an N-proximal FhuA fragment of 357 residues, and a complete but inactive mutant FhuA, provided that the mutation was located in the β-barrel and the cork was unaltered. A signal sequence on both the cork and the β-barrel was required to restore active FhuA, which makes it likely that complementation occurred in the periplasm, while or after the β-barrel was incorporated into the outer membrane. This result was not unexpected, since presumably unfolded FhuA is translocated across the cytoplasmic membrane, starts to fold in the periplasm, and achieves its final conformation while being incorporated into the outer membrane. The β-barrel might fold independently of the cork, and the cork could then be subsequently incorporated into the β-barrel. In wild-type FhuA the signal sequence attached to the cork serves as the translocation signal for the unfolded cork and the unfolded β-barrel together.
The amounts of reconstituted FhuA derived from FhuAΔ5-160 and FhuA1-160 are not known. It is also not known how many active FhuA molecules are required for the observed transport and receptor activities. It is likely that only a small fraction of the FhuA molecules are active at a given time, since the number of TonB molecules (20) is not sufficient to interact simultaneously with all FhuA molecules and the other TonB-coupled outer membrane transporters (20, 23). SDS-PAGE of specifically radiolabeled proteins had to be used to detect the cork associated with the β-barrel. To prevent dissociation of the cork from the β-barrel, the proteins had to be cross-linked with formaldehyde or disulfide bridges. The formaldehyde-cross-linked products had the expected molecular masses in the range of 80 kDa, whereas the disulfide-cross-linked product displayed a somewhat lower electrophoretic mobility. Disulfide cross-linking prevented ferrichrome transport, which was restored by cleavage of the disulfide bond. A similar result was obtained with FhuAT27C P533C synthesized as a single full-length protein (13). Covalent linkage between T27C of the cork domain and P533C of the β-barrel domain prevented the movement of the cork that accompanies binding of ferrichrome (15, 30). In addition to the restored activity, spontaneous in vivo cross-linking between C27 and C533 demonstrated precisely the same stereochemistry of the β-barrel complemented with the cork as in wild-type FhuA.
Complementation of the β-barrel by inactive complete mutant FhuA with a mutated β-barrel and a wild-type cork domain was unexpected. The restored activity was high if one considers that the inactive complete FhuA was encoded on the chromosome and the amounts synthesized under the iron-sufficient growth conditions were very small. Previously we did not consider the possibility that an N-terminal fragment of FhuA could complement FhuAΔ5-160 if one takes into account the unlikely sequence of reactions which have to occur: (i) secretion of the fragments across the cytoplasmic membrane, (ii) initial folding of the fragments in the periplasm, (iii) incorporation of the cork into the β-barrel, and (iv) incorporation and final folding of the reconstituted protein in the outer membrane. It is also likely that the cork is proteolytically released from only a fraction of the FhuA molecules and that only a fraction of the released corks find their way into the β-barrels of FhuAΔ5-160. Evidence for an incomplete complementation comes from previous experiments which showed that FhuAΔ5-160 increased sensitivity to antibiotics and supported permeation of maltodextrins in a lamB mutant, although those experiments were performed with E. coli 41/2, which expressed an inactive FhuA point mutant and complements FhuAΔ5-160. Since wild-type FhuA fails to increase the permeability of the outer membrane to these compounds, these results indicate that only a portion of FhuAΔ5-160 was complemented by FhuA. Release of the cork may occur prior to the incorporation of the cork into the β-barrel of mutant FhuA, since once incorporated, it is unlikely that the cork leaves the β-barrel because it is fixed by nine salt bridges and over 60 hydrogen bonds (30). This prediction is supported by the failure of wild-type cork to replace mutant cork in inactive full-length FhuA proteins with wild-type β-barrels. However, the resolution of the FhuA crystal structure is too low to exclude water molecules bridging the amino acid residues that are predicted to form direct hydrogen bonds and salt bridges. In this case the energy barrier to release the cork from the barrel would be much lower. For steric reasons, we consider it as unlikely that interprotein complementation occurs in the sense that the mutant β-barrel is inserted into the outer membrane and its wild-type cork, while fixed to the barrel, is incorporated into another cosynthesized wild-type β-barrel. Equally unlikely is a protein hybrid consisting of the wild-type cork incorporated in the wild-type β-barrel, while the mutant FhuA β-barrel remains in a partially folded form in the periplasm. Such a hybrid protein might block access of the TonB protein to the TonB box; access is required for the activity of the complemented protein, as shown by the lack of complementation with a cork with a TonB box deletion. We therefore assume that complementation of the wild-type β-barrel requires proteolytic release of the cork from the mutant β-barrel prior to its incorporation into the wild-type β-barrel.
For FhuA fragments larger than the cork, proteolytic fragmentation to approximately the size of the cork is likely to occur since both domains form very different conformations, and it is frequently observed that the linkage between different structural motifs in proteins are sensitive to proteolytic cleavage. In vivo proteolytic cleavage of the FhuA cork as shown in this work, in contrast to the proteolytic resistance of complete FhuA (21), also shows that the isolated cork assumes one or several conformations with exposed cleavage sites. The DNA fragment that encodes the cork of FepA has been cloned, and the isolated cork has been shown to be predominantly unfolded (46). These results suggest that the cork assumes its conformation while it is being incorporated into the β-barrel. In the FhuA protein, the linkage between the cork and the β-barrel is exposed to the periplasm. Therefore, it is possible that the cork of FhuA fragments larger than the cork, such as the 357-residue fragment of H1857, incorporate into the β-barrel and the rest remains in the periplasm.
A FepA β-barrel was shown to display transport activity, which, however, was much lower [Vmax = 1.4 pmol min−1 (109 cells)−1] than the FhuA β-barrel activity [Vmax = 25 pmol min−1 (109 cells)−1] (40). In light of the FhuA complementation, one may assume that the FepA β-barrel was complemented by the cork domain of a chromosomally encoded FepA fragment. The lower activity of the FepA β-barrel may come from the less frequent reconstitution of FepA compared to FhuA. The interpretation given by Vakharia and Postle (47) that interprotein complementation by two individually nonfunctional proteins restored TonB-dependent activity of FepA is very likely to be true and certainly applies to FhuA.
Complementation was the cause of the β-barrel activity previously described, since those experiments were performed with strains HK97 and H1875 (3, 4, 25). Since only a fraction of FhuAΔ5-160 is complemented by the corks of HK97 and H1857, the data on the diffusion of ferrichrome, antibiotics, and maltodextrins through the channel formed by the FhuAΔ5-160 β-barrel are valid (3, 4, 25). This also holds true for the conductance of isolated FhuAΔ5-160 incorporated into artificial lipid bilayer membranes (25), since only those FhuAΔ5-160 molecules which do not contain the cork increase the membrane conductance for KCl. In addition, FhuAΔ5-160 isolated after SDS-PAGE and therefore free of the cork yielded the same conductance as FhuAΔ5-160 purified via a His tag on an Ni-agarose column (M. Braun, E. Maier, H. Killmann, V. Braun, and R. Benz, unpublished data). The properties of the hybrid proteins constructed by mutual exchange of the β-barrels and the corks of E. coli, Salmonella enterica serovar Typhimurium, S. enterica serovar Paratyphi, and Pantoea agglomerans (25) were not caused by complementation, since the hybrid proteins showed a much higher ferrichrome transport activity than the β-barrels. In addition, as shown in this paper, a cork covalently linked to the β-barrel was not replaced by a separately synthesized cork. Since complementation with an isolated cork is more efficient than that with a cork linked to the β-barrel, complementation of the hybrid proteins can be ruled out. Nevertheless, we tested albomycin sensitivity, which reflects FhuA transport activity of the hybrid proteins consisting of the corks and β-barrels of S. enterica serovar Paratyphi and S. enterica serovar Typhimurium and the barrel of E. coli and the cork of P. agglomerans, in the fhuA deletion mutant MB97 and obtained the same results as in the parallel experiment with the fhuA missense mutant HK97. In a study about TonB regions which may be important for TonB-coupled FhuA activity, TonB point mutants were used and TonB activity was inhibited by synthetic TonB peptides which were transferred into the periplasm through the channel formed by the FhuAΔ322-355 deletion derivative (28). Comparison of wild-type FhuA with FhuAΔ5-160 yielded similar qualitative results, but FhuAΔ5-160 was more strongly affected due to the lower activity of complemented FhuAΔ5-160 by the missense FhuA of HK99. Inhibition of FhuAΔ5-160 activity by the synthetic TonB peptides (28) and the periplasmic TonB portion secreted by the FecA signal sequence (22) does not indicate a β-barrel-related activity of TonB. In addition, tonB of P. agglomerans was sequenced, and since the amino acid sequence deviates from the E. coli TonB sequence (65% identity), its activity was tested in E. coli expressing wild-type FhuA. The ferrichrome transport rate amounted to 40%, whereas TonB of E. coli coupled with FhuA of P. agglomerans was as active as TonB of P. agglomerans coupled with FhuA of P. agglomerans.
The results of this study contribute to the understanding of outer membrane protein assembly. According to current thinking, assembly of outer membrane proteins occurs in several steps. Two models have been discussed: (i) proteins are directly inserted into the outer membrane through contact sites between the cytoplasmic membrane and the outer membrane (1), and (ii) proteins pass through the periplasm on their way into the outer membrane (14, 16, 41). The data of this study favor the latter model, since separately synthesized cork is incorporated into separately synthesized β-barrel, which is most easily envisaged when the unfolded cork diffuses in the periplasm and finds its final conformation while being incorporated into the preformed β-barrel in the periplasm or during or after incorporation of the β-barrel in the outer membrane. Complementation of the β-barrel by full-length mutant FhuA also favors the view that a fragment containing the cork is released in the periplasm and is incorporated into the β-barrel of FhuAΔ5-160. Much evidence exists that folding of outer membrane proteins starts in the periplasm and is completed in the outer membrane (14). Such a model is consistent with the data on FhuA, which may serve as a model system to work out the details of outer membrane protein biogenesis, since the cork and the β-barrel form separate domains and allow the stepwise assembly process to be unraveled.
Acknowledgments
We thank Karen A. Brune for critically reading the manuscript and C. Herrmann for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (BR 330/20-1 and Forschergruppe “Bakterielle Zellhülle: Synthese Funktion und Wirkort”) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Bayer, M. H., G. P. Costello, and M. E. Bayer. 1982. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J. Bacteriol. 149:758-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bös, C., D. Lorenzen, and V. Braun. 1998. Specific in vivo labeling of cell surface-exposed protein loops: reactive cysteines in the predicted gating loop mark a ferrichrome binding site and a ligand-induced conformational change of the Escherichia coli FhuA protein. J. Bacteriol. 180:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, M., H. Killmann, and V. Braun. 1999. The β-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activities of Escherichia coli. Mol. Microbiol. 33:1037-1049. [DOI] [PubMed] [Google Scholar]
- 4.Braun, M., H. Killmann, E. Maier, R. Benz, and V. Braun. 2002. Diffusion through channel derivatives of the Escherichia coli FhuA transport protein. Eur. J. Biochem. 269:4948-4959. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V. 1995. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16:295-307. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., and M. Braun. 2002. Iron transport and signaling in Escherichia coli. FEBS Lett. 529:78-85. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., K. Hantke, and W. Köster. 1998. Bacterial iron transport: mechanisms, genetics, and regulation, p. 67-145. In A. Sigel and H. Sigel (ed.), Metal ions in biological systems, vol. 35. Iron transport and storage in microorganisms. Marcel Dekker, New York, N.Y. [PubMed]
- 8.Braun, V., S. I. Patzer, and K. Hantke. 2002. Ton-dependent colicins and microcins: modular design and evolution. Biochimie 84:365-380. [DOI] [PubMed] [Google Scholar]
- 9.Braun, V., and H. Wolff. 1973. Characterization of the receptor protein for phage T5 and colicin M in the outer membrane of E. coli B. FEBS Lett. 34:77-80. [DOI] [PubMed] [Google Scholar]
- 10.Brutsche, S., and V. Braun. 1997. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol. Gen. Genet. 256:416-425. [DOI] [PubMed] [Google Scholar]
- 11.Cadieux, N., C. Bradbeer, and R. J. Kadner. 2000. Sequence changes in the Ton box region of BtuB affect its transport activities and interaction with TonB protein. J. Bacteriol. 182:5954-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endriss, F., M. Braun, H. Killmann, and V. Braun. 2003. Mutant analysis of the Escherichia coli FhuA protein uncovers sites of FhuA activity. J. Bacteriol. 185:4683-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eppens, E. F., N. Nouwen, and J. Tommassen. 1997. Folding of a bacterial outer membrane protein during passage through the periplasm. EMBO J. 16:4295-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 16.Freudl, R., H. Schwarz, M. Klose, N. R. Movva, and U. Henning. 1985. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 4:3593-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, R. E. W., and V. Braun. 1976. Nature of the energy requirement of the irreversible adsorption of bacteriophages T1 and φ80 to Escherichia coli. J. Bacteriol. 125:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hantke, K., and V. Braun. 1978. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J. Bacteriol. 135:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller, K. J., R. J. Kadner, and K. Gunther. 1988. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene 64:147-153. [DOI] [PubMed] [Google Scholar]
- 20.Higgs, P. I., R. A. Larsen, and K. Postle. 2002. Quantification of known components of the Escherichia coli TonB energy transduction system: TonB, ExbB, ExbD and FepA. Mol. Microbiol. 44:271-281. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, H., E. Fischer, H. Schwarz, and V. Braun. 1986. Overproduction of the proFhuA outer membrane receptor protein of Escherichia coli K-12: isolation, properties, and immunocytochemical localization at the inner side of the cytoplasmic membrane. Arch. Microbiol. 145:334-341. [DOI] [PubMed] [Google Scholar]
- 22.Howard. P. S., C. Herrmann, C. W. Stratilo, and V. Braun. 2001. In vivo synthesis of the periplasmic domain of TonB inhibits transport through FecA and FhuA iron siderophore transporters of Escherichia coli. J. Bacteriol. 183:5885-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadner, R. J., and K. J. Heller. 1995. Mutual inhibition of cobalamin and siderophore uptake systems suggests their competition for TonB function. J. Bacteriol. 177:4829-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killmann, H., R. Benz, and V. Braun. 1993. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 12:3007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killmann, H., M. Braun, C. Herrmann, and V. Braun. 2001. FhuA barrel-cork hybrids are active transporters and receptors. J. Bacteriol. 183:3476-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killmann, H., and V. Braun. 1992. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J. Bacteriol. 174:3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killmann, H., and V. Braun. 1998. Conversion of the coprogen transport protein FhuE and the ferrioxamine B transport protein FoxA into ferrichrome transport proteins. FEMS Microbiol Lett. 161:59-67. [DOI] [PubMed] [Google Scholar]
- 28.Killmann, H., C. Herrmann, A. Torun, G. Jung, and V. Braun. 2002. TonB of Escherichia coli activates FhuA through interaction with the beta-barrel. Microbiology 148:3497-3509. [DOI] [PubMed] [Google Scholar]
- 29.Killmann, H., G. Videnov, G. Jung, H. Schwarz, and V. Braun. 1995. Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and φ80 and colicin M bind to the gating loop of FhuA. J. Bacteriol. 177:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 31.Mademidis, A. 1998. Neue Erkenntnisse zum Eisen(III)-Hydroxamat-Aufnahmesystem von Escherichia coli K12. Doctoral thesis. Universität Tübingen, Tübingen, Germany.
- 32.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Moeck, G. S., and J. W. Coulton. 1998. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol. Microbiol. 28:675-681. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 35.Ogierman, M., and V. Braun. 2003. Interactions between the outer membrane ferric citrate transporter FecA and TonB: studies of the FecA TonB box. J. Bacteriol. 185:1870-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ölschläger, T., E. Schramm, and V. Braun. 1984. Cloning and expression of the activity and immunity genes of colicins B and M on ColBM plasmids. Mol. Gen. Genet. 196:482-487. [DOI] [PubMed] [Google Scholar]
- 37.Plancon, L., M. Chami, and L. Letellier. 1997. Reconstitution of FhuA, an Escherichia coli outer membrane protein, into liposomes. Binding of phage T5 to FhuA triggers the transfer of DNA into the proteoliposomes. J. Biol. Chem. 272:16868-16872. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, N.Y.
- 39.Schöffler, H., and V. Braun. 1989. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol. Gen. Genet. 217:378-383. [DOI] [PubMed] [Google Scholar]
- 40.Scott, D. C., Z. Cao, Z. Qi, M. Bauler, J. D. Igo, S. M. Newton, and P. E. Klebba. 2001. Exchangeability of N termini in the ligand-gated porins of Escherichia coli. J. Biol. Chem. 276:13025-13033. [DOI] [PubMed] [Google Scholar]
- 41.Sen, K., and H. Nikaido. 1990. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solbiati, J. O., M. Ciaccio, R. N. Farias, and R. A. Salomon. 1996. Genetic analysis of plasmid determinants for microcin J25 production and immunity. J. Bacteriol. 178:3661-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 44.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 46.Usher, K. C., E. Ozkan, K. H. Gardner, and J. Deisenhofer. 2001. The plug domain of FepA, a TonB-dependent transport protein from Escherichia coli, binds its siderophore in the absence of the transmembrane barrel domain. Proc. Natl. Acad. Sci. USA 98:10676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vakaria, H. L., and K. Postle. 2002. FepA with globular domain deletions lacks activity. J. Bacteriol. 184:5508-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weidel, W., and E. Kellenberger. 1955. The E. coli B-receptor for the phage T5. Electron microscopic studies. Biochim. Biophys. Acta 17:1-9. [DOI] [PubMed] [Google Scholar]