Abstract
Treatment of SKH-1 mice orally with caffeine (0.1 mg/ml in the drinking water), voluntary running wheel exercise, or a combination of caffeine and exercise for 2 weeks (i) decreased the weight of the parametrial fat pads by 35, 62, and 77%, respectively; (ii) decreased the thickness of the dermal fat layer by 38, 42, and 68%, respectively; (iii) stimulated the formation of UVB-induced apoptotic sunburn cells in the epidermis by 96, 120, and 376%, respectively; and (iv) stimulated the formation of UVB-induced caspase 3 (active form)-positive cells in the epidermis by 92, 120, and 389%, respectively (average of two experiments). Oral administration of caffeine (0.4 mg/ml in the drinking water) in combination with voluntary exercise was less effective than administration of the low dose of caffeine in combination with exercise in stimulating UVB-induced apoptosis. Although orally administrated caffeine (0.1 mg/ml in the drinking water) or voluntary exercise for 2 weeks caused only a small nonsignificant stimulation of UVB-induced increase in the percentage of phospho-p53 (Ser-15)-positive cells in the epidermis (27 or 18%, respectively), the combination of the two treatments enhanced the UVB-induced increase in phospho-p53 (Ser-15)-positive cells by 99%. The plasma concentration of caffeine in mice ingesting caffeine (0.1–0.4 mg/ml drinking water) is similar to that in the plasma of most coffee drinkers (one to four cups per day). Our studies indicate a greater than additive stimulatory effect of combined voluntary exercise and oral administration of a low dose of caffeine on UVB-induced apoptosis.
Keywords: methylxanthines, physical activity, programmed cell death, skin cancer, sunburn
In earlier studies we demonstrated that oral administration of caffeine or voluntary running wheel (RW) exercise decreased tissue fat (1–3), stimulated UVB light-induced apoptosis (3, 4), and inhibited UVB-induced carcinogenesis in SKH-1 mice (2, 5, 6). These treatments had no effect on body weight. It was of interest that the proapoptotic effects of these treatments were selective because they enhanced apoptosis in UVB-treated epidermis and in UVB-induced tumors, but the treatments had no effect on apoptosis in normal epidermis or in nontumor areas in tumor-bearing mice (1–5). The results of these studies suggest that the proapoptotic effects of caffeine administration and voluntary exercise are important for the inhibitory effects of these regimens on UVB-induced carcinogenesis.
In the present work, we determined the effects of a combination of voluntary RW exercise and oral administration of caffeine on UVB-induced apoptosis in SKH-1 mice. The results indicated a greater than additive stimulatory effect of voluntary RW exercise, when combined with a low dose of caffeine, on UVB-induced apoptosis.
Results
Effects of Voluntary RW Exercise and Oral Administration of Caffeine on Body Weight, Food Consumption, and Fluid Consumption in SKH-1 Mice.
Treatment of female SKH-1 mice with RW, caffeine (0.1 mg/ml in the drinking water), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml) or RW + caffeine (0.4 mg/ml) for 2 weeks did not have a statistically significant effect on body weight or food consumption. In an earlier study, we found that voluntary RW exercise for several months increased both food consumption and fluid intake without having an effect on body weight (2).
In the present study, the average daily fluid consumption ± SE. by control mice or mice treated with RW, caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), or RW + caffeine (0.4 mg/ml) for 2 weeks was 7.97 ± 0.22 ml, 9.66 ± 0.14 ml (21.3% ↑; P = 0.006), 9.76 ± 0.19 ml (22.5% ↑; P = 0.004), 8.53 ± 0.42 ml (7.0% ↑; P > 0.1), 11.45 ± 0.39 ml (43.7% ↑; P < 0.001), or 9.36 ± 0.67 ml (17.4% ↑; P = 0.02), respectively (results from two experiments). The daily dose of caffeine in mice treated with caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), or RW + caffeine (0.4 mg/ml) was 38, 130, 43, or 144 mg/kg, respectively (average of two experiments). There was some variation in RW activity among different mice in the RW group, but the relatively small SE among different mice for tissue fat reduction in the RW group suggests relatively small interindividual differences in RW activity among the different mice.
Although it was difficult to determine the distance run by individual RW mice because there were five mice per cage and sometimes more than one mouse at a time was on the RW, we estimate that the RW mice ran ≈2 miles per day, and this distance was not significantly different from that run by the RW + caffeine (0.1 mg/ml) or from the RW + caffeine (0.4 mg/ml) group (average of two experiments).
Effect of Voluntary RW Exercise in Combination with Oral Administration of Caffeine on Tissue Fat.
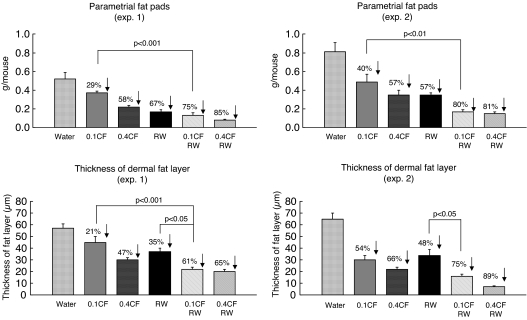
Treatment of SKH-1 mice with RW, caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), or RW + caffeine (0.4 mg/ml) for 2 weeks decreased the weight of the parametrial fat pads by 62%, 35%, 58%, 77%, and 83%, respectively (average of two experiments), and the thickness of the dermal fat layer was decreased by 42%, 38%, 57%, 68%, and 77%, respectively (average of two experiments) (Fig. 1). The results indicate that oral administration of the low dose of caffeine together with RW has a greater effect on tissue fat than either treatment alone.
Fig. 1.
Effect of voluntary RW exercise, oral administration of caffeine, or a combination of exercise and caffeine on tissue fat. SKH-1 mice (10 per group) were treated with RW exercise, oral caffeine [0.1 or 0.4% in the drinking water (0.1 or 0.4 CF)], or a combination of RW and CF for 2 weeks. The weight of the parametrial fat pads and the thickness of the dermal fat layer were determined. Using Tukey's multiple comparison test for parametrial fat pad measurements, we found that the CF (0.1) + RW group was different from the CF (0.1) group in experiments 1 and 2. For the thickness of the dermal fat layer, the CF (0.1) + RW group was different from the CF (0.1) group in experiment 1 and from the RW group in experiments 1 and 2. When we combined data from experiments 1 and 2, the dermal fat thickness from animals treated with CF (0.1) + RW was significantly different from the dermal fat thickness in animals treated with CF (0.1) (P < 0.01).
Effect of Voluntary RW Exercise in Combination with Oral Administration of Caffeine on UVB-Induced Increase in Apoptosis.
A previous study on the time course for UVB-induced apoptosis indicated that peak effects occurred at 6–10 h after UVB, and caffeine administration enhanced apoptosis at 6–10 h after UVB (4). Accordingly, we studied the effects of caffeine administration and RW for 2 weeks on UVB-induced apoptosis at 6 h after UVB.
Treatment of SKH-1 mice with RW, caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), or RW + caffeine (0.4 mg/ml) for 2 weeks stimulated UVB-induced increases in apoptotic sunburn cells by 120%, 96%, 217%, 376%, and 258%, respectively, at 6 h after UVB (average of two experiments), and UVB-induced increases in caspase 3 (active form)-positive cells were enhanced by 120%, 92%, 207%, 389%, and 237%, respectively (average of two experiments) (Fig. 2). The results indicate that the low dose of caffeine in combination with RW had a greater than additive effect in enhancing UVB-induced apoptosis, whereas the high dose of caffeine in combination with RW was less effective than the low dose of caffeine in combination with RW.
Fig. 2.
Effect of voluntary RW exercise, oral administration of caffeine, or a combination of exercise and caffeine on UVB-induced apoptosis in the epidermis. SKH-1 mice (10 per group) were treated with RW exercise, oral caffeine [0.1 or 0.4% in the drinking water (0.1 or 0.4 CF)], or a combination of RW and CF for 2 weeks. Apoptotic sunburn cells and caspase 3 (active form)-positive cells in the epidermis were determined at 6 h after irradiation with UVB (30 mJ/cm2). Using Tukey's multiple comparison test for apoptotic sunburn cell measurements, we found that the CF (0.1) + RW group was different from the CF (0.1) group in experiments 1 and 2 and from the RW group in experiments 1 and 2. For the caspase 3 measurements, the CF (0.1) + RW group was different from the CF (0.1) group in experiments 1 and 2 and from the RW group in experiments 1 and 2.
Effect of Voluntary RW Exercise in Combination with Oral Administration of Caffeine on UVB-Induced Increase in Phospho-p53 (Ser-15).
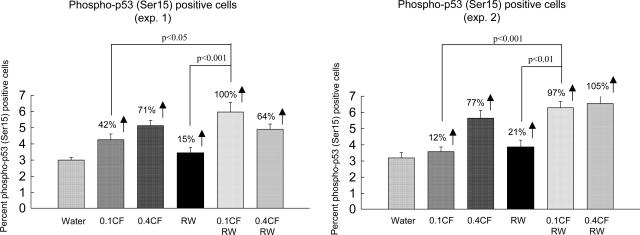
Treatment of SKH-1 mice with RW, caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), or RW + caffeine (0.4 mg/ml) stimulated UVB-induced increases in phospho-p53 (Ser-15) in the epidermis by 18%, 27%, 74%, 99% and 90%, respectively (average of two experiments) (Fig. 3). The results indicate that RW exercise or the low dose of caffeine had only a very small stimulatory effect on UVB-induced increase in phospho-p53 (Ser-15) (not statistically significant), but a combination of the two treatments stimulated the UVB-induced increase by 99%.
Fig. 3.
Effect of voluntary RW exercise, oral administration of caffeine, or a combination of exercise and caffeine on UVB-induced increase in phospho-p53 (Ser-15). SKH-1 mice (10 per group) were treated with RW exercise, oral caffeine [0.1 or 0.4% in the drinking water (0.1 or 0.4 CF)], or a combination of RW and CF for 2 weeks. Phospho-p53 (Ser-15)-positive cells in the epidermis were determined. Using Tukey's multiple comparison test for phospho-p53 (Ser-15)-positive cell measurements, we found that the CF (0.1) + RW group was different from the CF (0.1) group in experiments 1 and 2 and from the RW group in experiments 1 and 2.
Relationship Between the Thickness of the Dermal Fat Layer and UVB-Induced Apoptosis.
In two separate experiments, we used the Pearson correlation coefficient for evaluating the relationship between the thickness of the dermal fat layer and UVB-induced increase in apoptosis in individual control mice and mice treated with RW, caffeine (0.1 mg/ml), caffeine (0.4 mg/ml), RW + caffeine (0.1 mg/ml), and RW + caffeine (0.4 mg/ml) (60 mice). In experiment 1, we found a statistically significant inverse relationship between the dermal fat thickness in individual mice and UVB-induced increase in apoptotic sunburn cells (P < 0.0001; r = −0.50) and for fat thickness vs. caspase 3-positive cells (P = 0.002; r = −0.39). In experiment 2, there was also a significant relationship between dermal fat thickness in individual mice and UVB-induced increase in apoptotic sunburn cells (P = 0.003; r = −0.37) or UVB-induced increase in caspase 3-positive cells (P = 0.003; r = −0.37). Although there was a highly significant inverse relationship between the thickness of the dermal fat layer and UVB-induced apoptosis, the relatively low r value indicates that factors in addition to tissue fat may also play a role in modifying UVB-induced apoptosis.
Discussion
In earlier studies, we showed that voluntary RW exercise or oral administration of caffeine for 2 weeks stimulated UVB-induced apoptosis in SKH-1 mice (3, 4). In the present work, we demonstrated that combined exercise and oral administration of a low dose of caffeine (0.1 mg/ml in the drinking water) for 2 weeks resulted in a greater than additive increase in UVB-induced apoptosis compared with the effects of exercise or caffeine alone (Fig. 2). Although we cannot do a formal evaluation for synergistic effects because there was only one intensity of voluntary exercise, the results suggest that treatment of mice with a combination of RW exercise and an orally administered low dose of caffeine (0.1 mg/ml) may have had a synergistic stimulatory effect on UVB-induced apoptosis. It will be of interest to do additional studies with lower dose levels of caffeine in combination with exercise.
It is difficult to extrapolate the effect of caffeine in mice to that in humans based on dose because of differences in the rates of metabolism of caffeine in mice and humans. Mice metabolize caffeine with a half-life of ≈45 min (7, 8), whereas humans metabolize caffeine with a half-life of ≈5 h (9). Because of these considerations, it is better to compare the action of caffeine in animals and humans based on blood levels rather than on dose.
In an earlier study, we found that oral administration of caffeine (0.4 mg/ml in the drinking water) to SKH-1 mice resulted in an average caffeine plasma concentration of 16 μM (10), which is equivalent to the plasma concentration of caffeine observed in people drinking three to five cups of coffee per day (11). In the present work, oral administration of caffeine (0.1 mg/ml) would be expected to have a plasma concentration similar to that observed in people drinking only one or two cups of coffee per day.
Previously, we showed that the stimulatory effect of caffeine administration on UVB-induced apoptosis was via a p53-dependent as well as a p53-independent pathway (4, 12). In additional studies, we showed that the stimulatory effect of voluntary exercise on UVB-induced apoptosis was predominantly via a p53-independent pathway (3). Although voluntary exercise or oral administration of a low dose of caffeine (0.1 mg/ml in the drinking water) had only a very small nonsignificant stimulatory effect on UVB-induced formation of phospho-p53 (Ser-15), a greater than additive effect was observed in exercising mice that were also treated with a low dose of caffeine (Fig. 3). The mechanism of the greater than additive effect of exercise and caffeine administration on UVB-induced apoptosis is not known.
In recent studies, we found that oral administration of caffeine inhibited the UVB-induced increase in epidermal phospho-Chk1 (Ser-345) and prematurely increased the level of epidermal cyclin B1 as well as the number of mitotic cells with cyclin B1.¶ The time course for these effects was similar to the time course for the effect of caffeine on UVB-induced apoptosis, suggesting that caffeine abrogated the G2/M checkpoint thereby causing lethal mitosis and apoptosis in UVB-treated animals.¶ In the present work, we found that voluntary exercise or oral administration of caffeine (0.1 mg/ml in the drinking water) also prematurely increased the level of cyclin B1 in the epidermis of UVB-treated mice, and a combination of RW exercise and caffeine administration had a greater effect for prematurely increasing the level of epidermal cyclin B1 than exercise or caffeine administration alone (Western blot study; data not shown). These results suggest that both caffeine administration and voluntary exercise may modulate the effect of UVB on the ATR/Chk1/cyclin B1 pathway and override the G2/M checkpoint to cause lethal mitosis and apoptosis. The results of our studies suggest that the greater than additive effects of voluntary exercise and administration of caffeine on UVB-induced apoptosis may result from an increased level of phospho-p53 (Ser-15) combined with a premature increase in cyclin B1 that results in mitotic catastrophe and apoptosis. Further mechanistic studies are needed.
In an earlier study in which we evaluated the effects of administration of regular teas, decaffeinated teas, and caffeine on carcinogenesis in UVB-pretreated high risk mice, we found a significant inverse relationship between the number of tumors per mouse and the thickness of the dermal fat layer in individual mice (n = 179; P = 0.0001; r = 0.34) (1). Mice with a thick dermal fat layer had more tumors than mice with a thin dermal fat layer, suggesting a tumor-promoting effect of tissue fat. In the present work, there was also a significant relationship between the effects of RW exercise and caffeine administration to decrease the thickness of the dermal fat layer and to increase UVB-induced apoptosis in individual mice (described in Results). In a separate study, we examined the effects of tissue fat on UVB-induced apoptosis more directly by surgical removal of the parametrial fat pads, and we found that UVB-induced apoptosis was enhanced in these mice compared with sham-operated animals (3). The results of our studies suggest that tissue fat secretes antiapoptotic substances that inhibit DNA damage-induced apoptosis and possibly apoptosis in tumors. Only moderately strong correlation coefficients were observed for the relationship between tissue fat and carcinogenesis (tumors per mouse) (ref. 1) or for the relationship between tissue fat and UVB-induced apoptosis (described above). These results suggest that although tissue fat is an important modulator of UVB-induced apoptosis and carcinogenesis, other factors are also important.
An obesity epidemic in the United States and elsewhere has important health consequences, including an increased risk for cancer at several organ sites. Possible reasons for the current obesity epidemic are discussed by Potter (13) and Daniels (14). Obesity has been associated with an increased risk of colon cancer (15–19), postmenopausal breast cancer (20–23), pancreas cancer (21), renal cell carcinoma (19), aggressive prostate cancer (24, 25), lower esophageal cancer (19, 26), gastric cardia cancer (26), and melanoma (19, 26, 27), so that decreasing tissue fat may inhibit the formation of these cancers. The results of our studies suggest that lowering the level of tissue fat by exercise, by decreasing dietary fat, by lipectomy, or by administration of caffeine or other substances that decrease tissue fat may inhibit the formation of these cancers by selectively enhancing apoptosis in DNA-damaged cells and in tumors.
The mechanisms by which decreased tissue fat levels enhance apoptosis in DNA-damaged cells remain to be explored but may be related to a decreased serum level of inflammatory cytokines such as TNFα and other substances that are secretory products of fat cells such as leptin or adiponectin or by a decreased level of insulin-like growth factor 1 (IGF-1). It is of interest that exercise stimulates cellular antioxidant effects, has an anti inflammatory effect, suppresses the formation of TNFα, and increases the level of IL-6 (an antiinflammatory cytokine) (28–41). IL-6 is thought to be derived primarily from muscle and has lipolytic activity (41). In accord with the lipolytic activity of this cytokine, IL-6 knockout mice develop late-onset obesity (42). The serum level of leptin is directly related to tissue fat mass, and leptin has been shown to inhibit apoptosis in cultured HT29 colon cancer cells (43) and in human neuroblastoma cells (44). Adiponectin is another fat-derived hormone, and this hormone is decreased in obesity and possesses an antiapoptotic effect in MCF7 breast cancer cells (45). Further studies are needed to determine the effects of voluntary exercise and caffeine administration on the levels of leptin, TNFα, IL-6, adiponectin, and IGF-1 in the serum and epidermis of SKH-1 mice.
Recent studies showed that serum from exercising men or from exercising men on a low-fat diet inhibited the growth of human prostate LNCaP cells and stimulated apoptosis compared with serum from control subjects (46). This effect was associated with decreased serum IGF-1 and increased IGF-binding protein 1 (IGFBP-1) (46). Adding IGF-1 to serum from the low-fat diet/exercising group reversed the effects of the serum on growth and apoptosis in LNCaP cells, and adding IGFBP-1 to serum from control subjects enhanced the ability of the serum to reduce the growth of LNCaP cells and to stimulate apoptosis (47). Whether the stimulatory effects of exercise or administration of caffeine on UVB-induced apoptosis or apoptosis in tumors of tumor-bearing mice is associated with a decreased serum level of IGF-1 remains to be investigated.
Case control or prospective epidemiology studies in humans suggest that increased exercise is associated with a decreased risk of melanoma (48), colon cancer (49, 50), breast cancer (51–53), and advanced prostate cancer (54–56). The effects of exercise and dietary fat on the risk of prostate cancer were recently reviewed (57). In other studies, coffee ingestion, which provides a good source of caffeine, is associated with a decreased risk of nonmelanoma skin cancer (58), liver cancer (59, 60), and breast cancer in BRCA1 and BRCA2 mutation carriers (61), but more detailed confirmatory studies are needed. Although low-dose levels of caffeine or caffeine-containing beverages are believed to be safe, side effects as well as health benefits have been observed with high-dose levels (62, 63).
In summary, the results presented here suggest that oral administration of a low physiologically relevant dose of caffeine (0.1 mg/ml in the drinking water) together with voluntary exercise has a greater than additive stimulatory effect on UVB-induced apoptosis. These results suggest a need for further studies to determine the effects of combinations of voluntary exercise and orally administered low-dose levels of caffeine on UVB-induced carcinogenesis in animal models as well as studies to determine the effects of caffeine or caffeine-containing beverages in combination with exercise on the formation of sunlight-induced actinic keratoses and squamous cell carcinomas in humans.
Materials and Methods
Animals.
Female SKH-1 hairless mice (6–7 weeks old) were purchased from the Charles River Breeding Laboratories (Wilmington, MA), and the animals were kept in our animal facility for at least 1 week before use. Mice were housed in a temperature- and humidity-controlled room with free access to water and a Purina laboratory chow 5001 diet from the Ralston Purina Co. (St. Louis, MO), and they were kept on a 12-h light/12-h dark cycle.
Exposure to UVB.
The UV lamps used (FS72T12-UVB-HO; National Biological Corp., Twinsburg, OH) emitted UVB (280–320 nm; 75–80% of total energy) and UVA (320–375 nm; 20–25% of total energy). The dose of UVB was quantified with a UVB Spectra 305 dosimeter (Daavlin Co., Byran, OH). The radiation was further calibrated with a model IL-1700 research radiometer/photometer (International Light, Inc., Newburyport, MA).
Voluntary Exercise, Caffeine Administration, and the Preparation of Skin Sections.
For studies on the effect of voluntary RW exercise and caffeine administration on UVB-induced apoptosis in the epidermis of SKH-1 mice, female SKH-1 mice (7–8 weeks old, 10 mice per group) were placed in cages with a RW (13.75-cm diameter; 7 cm width) with free access to the wheel 24 h/day for 2 weeks (five mice per cage). Other mice with matched body weights and age were placed in cages without a RW and served as controls. The wheels were attached to a permanent magnetic switch that activated a digital counter to count wheel revolutions. Total wheel revolutions were recorded daily, with total distance run per day determined by multiplying the number of wheel rotations by the circumference of the wheel. A water bottle containing water or caffeine (0.1 or 0.4 mg/ml) was attached at the top of the cage unit. After 2 weeks of voluntary RW exercise with or without caffeine administration, the treated mice and their controls were irradiated once with 30 mJ/cm2 of UVB and killed 6 h later. Our previous studies showed that 6–10 h after a single irradiation with UVB is the peak time for the formation of UVB-induced apoptotic sunburn cells (4, 64). The two parametrial fat pads were removed and weighed. The skin samples (≈2 cm long and 0.5 cm wide), were taken from the middle of the back, stapled flat to a plastic sheet and placed in 10% phosphate-buffered formalin at 4°C for 18–24 h. The skin samples were then dehydrated in ascending concentrations of ethanol (80%, 95%, and 100%), cleared in xylene, and embedded in Paraplast (Oxford Labware, St. Louis, MO). Four-micrometer serial sections of skin containing epidermis and dermis were made, deparaffinized, rehydrated with water, and used for regular H&E or immunohistochemical staining. The thickness of the dermal fat layer was measured by using an ocular micrometer with an Olympus BHTU light microscope (Olympus America, Inc., Melville, NY) under ×100 magnification at 5–10 representative areas per slide and averaged (1). Apoptotic sunburn cells and caspase 3-positive cells labeling were determined as described previously (64).
Measurement of Apoptotic Sunburn Cells.
Identification of apoptotic sunburn cells was based morphologically on cell shrinkage and nuclear condensation as we have done previously (64). Apoptotic sunburn cells were identified by their intensely eosinophilic cytoplasm and small, dense nuclei, which was observed in H&E-stained histological sections of the skin by using light microscopy. The percentage of apoptotic sunburn cells in the epidermis (basal plus suprabasal layers) was calculated from the number of these cells per 100 cells counted from the entire 20-mm length of epidermis for each skin section.
Caspase 3 Immunostaining.
Affinity-purified polyclonal rabbit antibody that reacts with the mouse p20 subunit of caspase 3 but does not react with the precursor form was purchased from R&D Systems, Inc. (Minneapolis, MN). Skin sections used for the measurement of caspase 3 were stained by the horseradish peroxidase-conjugated avidin method as described previously (64). Endogenous peroxidase was blocked by incubating the tissue sections in 3% hydrogen peroxide in methanol for 30 min at room temperature. Sections were then treated with 0.01 M sodium citrate buffer (pH 6.0) in a microwave oven at high temperature for 10 min. The sections were incubated with a protein block (normal goat serum) for 10 min followed by avidin D for 15 min and biotin blocking solution for 15 min (avidin/biotin blocking kit from Vector Laboratories, Burlingame, CA) at room temperature. The sections were incubated with caspase 3 primary antibody (1:2,000 dilution) for 30 min at room temperature followed by incubation with a biotinylated anti-rabbit secondary antibody for 30 min and incubation with conjugated-avidin solution (ABC elite kit purchased from Vector Laboratories) for 30 min. Color development was achieved by incubation with 0.02% 3,3′ diaminobenzidine tetrahydrochloride containing 0.02% hydrogen peroxide for 10 min at room temperature. The slides were then counterstained with hematoxylin and dehydrated and coverslips were added for permanent mounting. A positive reaction was shown as a light brown to dark brown precipitate in the cytoplasm and/or perinuclei of the cells.
The percentage of caspase 3-positive cells in the epidermis was calculated from the number of stained caspase 3-positive cells per 100 epidermal cells counted from the entire 20-mm length of epidermis.
Statistics.
The statistical evaluation of differences between different groups was done with Tukey's multiple comparison test (65).
Acknowledgments
We thank Drs. Weichung Joe Shih and Yong Lin for help with the statistical analysis of the data and Florence Florek for assistance in the preparation of the manuscript. This work was supported in part by National Institutes of Health Grant RO3 CA 121418 (to Y-P.L.).
Abbreviations
- IGF-1
insulin-like growth factor 1
- RW
running wheel.
Footnotes
The authors declare no conflict of interest.
Lu, Y-P., Lou, Y-R., Peng, Q-Y., Xie, J-G., Nghiem, P., and Conney, A. H. (2007) Proc. Am. Assoc. Cancer Res. 48:821 (abstr.).
References
- 1.Lu Y-P, Lou Y-R, Lin Y, Shih WJ, Huang M-T, Yang CS, Conney AH. Cancer Res. 2001;61:5002–5009. [PubMed] [Google Scholar]
- 2.Michna L, Wagner GC, Lou Y-R, Xie J-G, Peng Q-Y, Lin Y, Carlson K, Shih WJ, Conney AH, Lu Y-P. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y-P, Lou Y-R, Nolan B, Peng Q-Y, Xie J-G, Wagner GC, Conney AH. Proc Natl Acad Sci USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y-P, Lou Y-R, Li X-H, Xie J-G, Brash D, Huang M-T, Conney AH. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- 5.Huang M-T, Xie J-G, Wang Z-Y, Ho C-T, Lou Y-R, Wang C-X, Hard GC, Conney AH. Cancer Res. 1997;57:2623–2629. [PubMed] [Google Scholar]
- 6.Lou Y-R, Lu Y-P, Xie J-G, Huang M-T, Conney AH. Nutr Cancer. 1999;33:146–153. doi: 10.1207/S15327914NC330205. [DOI] [PubMed] [Google Scholar]
- 7.Nau H. Environ Health Perspect. 1986;70:113–129. doi: 10.1289/ehp.8670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann M, Czok G. Z Ernahrungswiss. 1980;19:215–227. doi: 10.1007/BF02018787. [DOI] [PubMed] [Google Scholar]
- 9.Gilman AG, Goodman LS, Rall TW, Murad F, editors. Goodman and Gilman's: The Pharmacological Basis of Therapeutics. 7th Ed. New York: Macmillan; 1985. p. 1673. [Google Scholar]
- 10.Conney AH, Zhou S, Lee MJ, Xie J-G, Yang CS, Lou Y-R, Lu Y-P. Toxicol Appl Pharmacol. 2007 doi: 10.1016/j.taap.2006.11.001. in press. [DOI] [PubMed] [Google Scholar]
- 11.de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L, Ghosheh OH, Dwoskin LP, Crooks PA. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:165–171. doi: 10.1016/s0278-5846(02)00348-2. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y-P, Lou Y-R, Peng Q-Y, Xie J-G, Conney AH. Cancer Res. 2004;64:5020–5027. doi: 10.1158/0008-5472.CAN-04-0760. [DOI] [PubMed] [Google Scholar]
- 13.Potter JD. Epidemiology. 2006;17:124–127. doi: 10.1097/01.ede.0000197405.28469.a3. [DOI] [PubMed] [Google Scholar]
- 14.Daniels J. Am J Nurs. 2006;106:40–49. doi: 10.1097/00000446-200601000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, et al. J Natl Cancer Inst. 2006;98:920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 16.Frezza EE, Wachtel MS, Chiriva-Internati M. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacInnis RJ, English DR, Hopper JL, Gertig DM, Haydon AM, Giles GG. Int J Cancer. 2006;118:1496–1500. doi: 10.1002/ijc.21508. [DOI] [PubMed] [Google Scholar]
- 18.Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, Kreger BE. Int J Obesity-Related Metabol Disorders. 2004;28:559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 19.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr Cancer Causes Control. 2006;17:901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 21.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorincz AM, Sukumar S. Endocrine-Related Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 23.Carmichael AR. Int J Obstet Gynaecol. 2006;113:1160–1166. doi: 10.1111/j.1471-0528.2006.01021.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Cancer Epidemiol Biomarkers Prev. 2007;16:63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 25.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, et al. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 26.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 27.Oncology Study Group of the Italian Group for Epidemiologic Research in D. Gallus S, Naldi L, Martin L, Martinelli M, La Vecchia C. Melanoma Res. 2006;16:83–87. doi: 10.1097/01.cmr.0000194429.77643.76. [DOI] [PubMed] [Google Scholar]
- 28.Ji LL. Ann NY Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahima RS, Flier JS. Trends Endocrinol Metabol. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 30.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed-Ali V, Bulmer K, Clarke D, Goodrick S, Coppack SW, Pinkney JH. Int J Obes. 2000;24:S154–S155. doi: 10.1038/sj.ijo.0801311. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 34.Coppack SW. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 35.Hotamisligil GS, Shargill NS, Spiegelman BM. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 36.Fried SK, Bunkin DA, Greenberg AS. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 38.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, Katsilambros N. Metabolism. 1999;48:1332–1335. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 39.Lyngso D, Simonsen L, Bulow J. J Physiol (London) 2002;543:373–378. doi: 10.1113/jphysiol.2002.019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen AM, Pedersen BK. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 41.Petersen AMW, Pedersen BK. J Physiol Pharmacol. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- 42.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 43.Ogunwobi OO, Beales IL. Int J Colorectal Dis. 2007;22:401–409. doi: 10.1007/s00384-006-0181-y. [DOI] [PubMed] [Google Scholar]
- 44.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 45.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Biochem Biophys Res Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 46.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. Prostate. 2003;56:201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 47.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 48.Shors AR, Solomon C, McTiernan A, White E. Cancer Causes Control. 2001;12:599–606. doi: 10.1023/a:1011211615524. [DOI] [PubMed] [Google Scholar]
- 49.Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, Cokkinides VE, Thun MJ. Cancer Epidemiol Biomarkers Prev. 2004;13:2187–2195. [PubMed] [Google Scholar]
- 50.Martinez ME. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 51.Gammon MD, John EM, Britton JA. J Natl Cancer Inst. 1998;90:100–117. doi: 10.1093/jnci/90.2.100. [DOI] [PubMed] [Google Scholar]
- 52.Verloop J, Rookus MA, van der Kooy K, van Leeuwen FE. J Natl Cancer Inst. 2000;92:128–135. doi: 10.1093/jnci/92.2.128. [DOI] [PubMed] [Google Scholar]
- 53.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. Arch Intern Med. 1999;159:2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- 54.Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE. Cancer Epidemiol Biomarkers Prev. 2005;14:275–279. [PubMed] [Google Scholar]
- 55.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 56.Nilsen TI, Romundstad PR, Vatten LJ. Int J Cancer. 2006;119:2943–2947. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 57.Barnard RJ. Evid Based Complement Altern Med. 2004;1:233–239. doi: 10.1093/ecam/neh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobson BK, Bjelke E, Kvåle G, Heuch I. J Natl Cancer Inst. 1986;76:823–831. [PubMed] [Google Scholar]
- 59.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, et al. Br J Cancer. 2005;93:607–610. doi: 10.1038/sj.bjc.6602737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F. J Hepatol. 2005;42:528–534. doi: 10.1016/j.jhep.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Nkondjock A, Ghadirian P, Kotsopoulos J, Lubinski J, Lynch H, Kim-Sing C, Horsman D, Rosen B, Isaacs C, Weber B, et al. Int J Cancer. 2006;118:103–107. doi: 10.1002/ijc.21296. [DOI] [PubMed] [Google Scholar]
- 62.Harv Womens Health Watch. 2004;12:2–4. [PubMed] [Google Scholar]
- 63.Smith BD, Gupta U, Gupta BS, editors. Caffeine and Activation Theory: Effects on Health and Behavior. Boca Raton: CRC; 2007. [Google Scholar]
- 64.Lu Y-P, Lou Y-R, Li X-H, Xie J-G, Lin Y, Weichung JS, Conney AH. Oncol Res. 2002;13:61–70. [PubMed] [Google Scholar]
- 65.Hochberg Y, Tamhane AC. Multiple Comparison Procedures. New York: Wiley; 1987. [Google Scholar]