Abstract
A unique 16-year time series of deep video surveys in Monterey Bay reveals that the Humboldt squid, Dosidicus gigas, has substantially expanded its perennial geographic range in the eastern North Pacific by invading the waters off central California. This sustained range expansion coincides with changes in climate-linked oceanographic conditions and a reduction in competing top predators. It is also coincident with a decline in the abundance of Pacific hake, the most important commercial groundfish species off western North America. Recognizing the interactive effects of multiple changes in the environment is an issue of growing concern in ocean conservation and sustainability research.
Keywords: invasive species, top predators, top-down forcing
One of the greatest challenges in contemporary ocean science is that of predicting how oceanic communities will respond to impending changes, such as climatic warming and the removal of top predators (1). Interactive changes are of particular concern, but little information is available on the collateral effects of multiple factors. Rising temperatures have been implicated in shifting the geographical distribution patterns of fishes and plankton (2, 3) and in the disruption of plankton communities (4, 5). Removing top predators from an ecosystem can result in a cascade of effects that restructures the food web at lower trophic levels (6, 7) as well as at the top (8). Together, two or more such changes may act in ways that we cannot yet predict (9).
Here we demonstrate that the Humboldt squid, Dosidicus gigas, has greatly extended its perennial range in the eastern North Pacific Ocean. This geographic expansion occurred during a period of ocean-scale warming, regional cooling, and the decline of tuna and billfish populations throughout the Pacific (10). In this case, environmental changes off California are concurrent with invasion by a species from an adjacent region. Examples of invasion by species at higher trophic levels are relatively rare. The subsequent ecological impact of the Humboldt squid invasion can be seen in possible top-down forcing on the local population of Pacific hake, but the ecological effects may not yet be fully expressed (11). The question of how an oceanic community will respond to climatic change must include the possibility of invading species (12), and the consequences of removing top predators may depend on whether there is an ecological understudy waiting in the wings.
Dosidicus (Fig. 1) is a large, aggressive, abundant pelagic squid that reaches mantle lengths of 1.2 m, overall lengths >2 m, and weights up to 50 kg. Its geographical distribution is centered in the eastern equatorial Pacific. From these warm waters, its historical range extends along the subtropical coasts of both North and South America, with episodic but temporary range extensions to latitudes as high as 40° (13). Only a single species is known, although genetic evidence suggests that northern and southern populations are diverging (13, 14). Dosidicus feeds opportunistically on a broad range of pelagic and demersal fishes, crustaceans, and squids (15–17), many of which undertake vertical migrations of several hundred meters during a diel cycle (18). Dosidicus has a wide range in the vertical plane (19), encompassing that of its prey (Fig. 2). In turn, Dosidicus is preyed on by tuna, billfish, sharks, pinnipeds, and toothed whales (13, 17); it is also the specific target of commercial fisheries in Mexico, Peru, and northern Chile, where it is known as the jumbo squid, the jumbo flying squid, and jibia. Like other squids in the family Ommastrephidae, Dosidicus is believed to have only a 1- to 2-year life span (20), but its reproductive cycle, seasonality, and early life stages are still largely unknown, particularly outside the tropics. Short generation times have been shown to be advantageous in cases of warming-related range shifts (2) and also in response to changes in trophic structure (8).
Fig. 1.
Video frame grab of two D. gigas, observed at 524-m depth and 5.9°C, near the mode of the vertical distribution for the species in Monterey Bay. The fish is Leuroglossus stilbius, a deep-sea smelt ≈15 cm in length.
Fig. 2.
Vertical distributions of squid, D. gigas (red bars, mode 500 m) and hake, M. productus (gray area, mode 400 m), as observed during remotely operated vehicle dives in Monterey Bay, CA. Temperatures (in blue) range from 1.6°C to 11.1°C.
Results
Our study is based on a unique data set of in situ video observations in deep water of the Monterey Submarine Canyon off central California, made during monthly dives of a remotely operated vehicle (ROV) over a period of 16 years (21). Dosidicus occurred broadly through the water column at depths between the surface and ≈1,000 m, in a temperature range from 15°C to 5°C (Fig. 2). It is apparently attracted to the lights of the ROV, and it feeds actively within their arc. In addition to hake, its local diet includes krill, Euphausia pacifica, the myctophid lanternfishes Stenobrachius leucopsarus and Tarletonbeania crenularis, the anchovy Engraulis mordax, and rockfishes Sebastes spp (17). The size range of Dosidicus specimens observed in Monterey Bay includes mantle lengths from <8 to >80 cm.
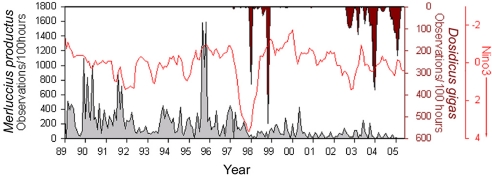
Historically, Dosidicus has been only an occasional visitor to southern and central California (22, 23), and we did not observe it in Monterey Bay until 1997, although our routine ROV surveys began in 1989. Dosidicus first appeared during the onset of the strong 1997–1998 El Niño and persisted through most of 1998. A few individuals were seen in 1999, and there was a single sighting in 2000. None were observed in 2001. In 2002 Dosidicus returned in abundance, associated with a small El Niño event, and it has been present in Monterey Bay year-round ever since (Fig. 3). Time-series cross-correlation analysis revealed that increased sea-surface temperatures in the eastern equatorial Pacific (Niño 3 index) preceded the appearance of Dosidicus off central California by 10–15 months and again by 2–3 months in both 1997 and 2002 (P < 0.05). This pattern may reflect a reproductive cycle or a secondary invasion pulse. In addition to temperature changes, El Niño events may have facilitated the range expansion of Dosidicus through increases in poleward, subsurface water flow (24) and by increasing the relative abundance of picoplankton (25), a key food source for the paralarvae of most ommastrephid squid (26).
Fig. 3.
Monthly summed observations in Monterey Bay of Pacific hake, M. productus (in gray, left y axis) and Humboldt squid, D. gigas (in dark red, inverted right y axis) normalized by hours of ROV deployment. The Niño 3 index (in bright red, inverted right y axis) is based on averages of sea-surface temperature (SST) in the equatorial Pacific (http://ingrid.ldeo.columbia.edu/SOURCES/Indices). The increased observations of D. gigas began shortly after the increased SST of the equatorial Pacific in 1997 and in 2002. Observations of hake decreased after the squid reappeared, but they rose again between 1999 and 2002, the period when D. gigas was absent.
Pacific hake (Merluccius productus) is the most abundant commercial fish species on the western coasts of the U.S. and Canada (27, 28). They are found chiefly in temperate coastal waters of the shelf and slope and historically, they overlapped with Dosidicus only at the southernmost part of the hake's geographical range, off the coast of northwestern Mexico. Hake feed chiefly on krill (29), and they undertake postspawning migrations each year that account for seasonal fluctuations in local abundance. Until 1997, they were a consistent presence, year-round, in our ROV surveys of Monterey Bay (Fig. 3). Like the squid, hake are attracted by the lights of the ROV, and their schools appear as strong targets on the vehicle's sonar even when they are beyond visual range. Coincident with the arrival of Dosidicus in 1997, the numbers of hake declined, then recovered after the squid observations dropped off, from 1999 through 2001. When Dosidicus returned in 2002, the numbers of hake dropped again and have remained very low (Fig. 3). Numbers of hake have been significantly lower during periods when Dosidicus was present (1997–1999 and 2003–2005; Mann–Whitney U test, U = 1,176, n = 196, two-tailed P = 0.000), and the seasonal pattern of their abundance has been altered [supporting information (SI) Fig. 4].
We have observed Dosidicus feeding on deep-water forage such as krill, lanternfishes, and barracudinas [see SI Movies 1–3] during ROV dives offshore, whereas their principal prey over the shelf and slope appears to be hake (17). Cross-correlation time-series analysis showed that observations of hake (Fig. 3) decreased significantly in the same month, and the month after, the number of Dosidicus observations increased (see SI Text). There was no significant relationship between our hake observations and the El Niño index.
Discussion
Climate-related changes in fish distribution have been typically characterized as range shifts or displacement away from the center of the home range, as temperatures grew warmer (2). In contrast, Dosidicus has enlarged its distribution without abandoning its historical center. The present pattern of range expansion by Dosidicus has been accompanied by a large number of mass strandings along the California coast. Sightings of Dosidicus have occurred as far north as Canada and the Gulf of Alaska (30–33). A similar pattern of strandings and range expansion has emerged off South America at the southern end of Dosidicus' range, also in conjunction with warm-water intrusions and increased poleward flow. In southern Chile, an invasion of Dosidicus may be threatening large commercial and artisinal hake fisheries (ref. 34; www.falkland-malvinas.com/Detalle.asp?NUM=5829).
The expansion of Dosidicus' range does not appear to be directly linked to a regional increase in sea-surface temperatures. Although its tropical center of distribution and the El Niño-linked episodic range expansions suggest a warm-water affinity, its vertical distribution in the water column demonstrates a physiological tolerance for temperatures far lower than it encounters near the surface (Fig. 2). Beginning in the late 1990s, a warm-to-cool regime shift occurred throughout the eastern Pacific (35). During the last half of the previous warm regime (≈1988–1998), Dosidicus was present in Monterey Bay only during warm El Niño periods. However, once it became established during the current cool regime, it has been present continuously. This demonstrates that, whereas Dosidicus may be associated with warm temperatures at the center of its distribution, it is physiologically adaptable and does not depend on the higher surface temperatures found in the tropics (19). There is no historical evidence to indicate that Dosidicus was a consistent part of the central California pelagic fauna during the previous (1950–1975) cool regime (22), which suggests that regime-shift oceanographic changes were not solely responsible for the present invasion.
In the eastern Pacific, the standing populations of tuna and billfish have recently experienced drastic depletions, effects felt not only in their abundance but also in their diversity (36, 37) and age structure (10). These large fishes are being replaced by smaller individuals, by smaller fish species, and by squid, a consequence also seen in other fisheries (8). Dosidicus, because it grows rapidly to large size and with no apparent seasonality to its reproduction (13, 38), can respond quickly to niches made accessible by the decline of large predatory fishes, which have longer life spans and delayed maturity (2, 8, 10). The removal of top predators like tuna and billfish may promote Dosidicus population growth and range expansion by reducing competition for their shared prey species. Likewise, it may reduce the mortality of juvenile Dosidicus, on which the large predatory fishes feed (13, 39).
The present situation off central California appears to be that a physiologically tolerant species (19) with a fast generation time has moved into a new area during a period of substantial climatic, oceanographic, and ecological changes. The occupation has lasted through multiple generations of the invading species (20), which indicates a sustained population rather than a relict one or multiple invasions. The geographical range of the invader now extensively overlaps that of a large commercially valuable fish stock. If this trend continues, top-down forcing could have a major impact on the most abundant commercial groundfish population off the west coast of North America. A similar pattern may also be taking place in the Southern Hemisphere.
Materials and Methods
From 1989 to 1991, ROV-based video surveys covered the upper 500 m of the water column in Monterey Bay. In 1992, the vertical coverage was extended beyond 1,000 m. Quantitative video transects in a midwater time series occurred on about a monthly basis (21). These were supplemented by additional dives in Monterey Bay and offshore that supported other types of research. The total number of dives exceeds 3,000 over 16 years. As a result, this data set has greater temporal resolution than is the case for annual fishery-based catch statistics. Quantitative video surveys are much more useful for understanding holistic effects than are catch statistics alone, because they usually include both predators and prey, whereas traditional commercial sampling seldom enumerates both. Statistical analyses were performed by using SPSS 10.1 (SPSS, Chicago, IL).
Supplementary Material
Acknowledgments
We thank the pilots of our ROVs Ventana and Tiburon and the officers and crews of the Research Vessels Point Lobos and Western Flyer. We thank Kim Reisenbichler and Rob Sherlock for assistance at sea and ashore. Thanks also to John Field for helpful discussions and comments on the manuscript and to Neil Conner for help with the data. This research was supported by the David and Lucile Packard Foundation through Monterey Bay Aquarium Research Institute, and through Hopkins Marine Station of Stanford University.
Abbreviation
- ROV
remotely operated vehicle.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702043104/DC1.
References
- 1.Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA. Science. 2005;309:1365–1369. doi: 10.1126/science.1113399. [DOI] [PubMed] [Google Scholar]
- 2.Perry AL, Low PJ, Ellis JR, Reynolds JD. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- 3.Batten SD, Welch DW. Deep-Sea Res II. 2004;51:863–873. [Google Scholar]
- 4.Hays GC, Richardson AJ, Robinson C. Trends Ecol Evol. 2005;20:337–344. doi: 10.1016/j.tree.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Richardson AJ, Schoeman DS. Science. 2004;305:1609–1612. doi: 10.1126/science.1100958. [DOI] [PubMed] [Google Scholar]
- 6.Frank KT, Petrie B, Choi JS, Leggett WC. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 7.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 8.Caddy JF, Rodhouse PG. Rev Fish Biol Fisheries. 1998;8:431–444. [Google Scholar]
- 9.Halpern BS, Cottenie K, Broitman BR. Science. 2006;312:1230–1232. doi: 10.1126/science.1128613. [DOI] [PubMed] [Google Scholar]
- 10.Sibert J, Hampton J, Klieber P, Maunder M. Science. 2006;314:1773–1776. doi: 10.1126/science.1135347. [DOI] [PubMed] [Google Scholar]
- 11.Mooney HA, Cleland EE. Proc Natl Acad Sci USA. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. Proc Natl Acad Sci USA. 2002;99:15497–15500. doi: 10.1073/pnas.242437499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigamatullin CM, Nesis KN, Arkhipkin AI. Fish Res. 2001;54:9–19. [Google Scholar]
- 14.Sandoval-Castellanos E, Uribe-Alcocer M, Diaz-Jaimes P. Fish Res. 2007;83:113–118. [Google Scholar]
- 15.Markaida U, Sosa-Nishisaki O. J Mar Biol Assoc U K. 2003;83:507–522. [Google Scholar]
- 16.Markaida U. Fish Res. 2006;79:16–27. [Google Scholar]
- 17.Field JC, Baltz K, Phillips AJ, Walker WA. Calif Coop Oceanic Fish Invest Rep. 2007;48 in press. [Google Scholar]
- 18.Barham EG. Science. 1966;151:1399–1403. doi: 10.1126/science.151.3716.1399. [DOI] [PubMed] [Google Scholar]
- 19.Gilly WF, Markaida U, Baxter CH, Block BA, Boustany A, Zeidberg L, Reisenbichler K, Robison B, Bazzino G, Salinas C. Mar Ecol Prog Ser. 2006;324:1–17. [Google Scholar]
- 20.Masuda S, Yokawa K, Yatsu A, Kawahara S. In: Large Pelagic Squids. Okutani T, editor. Tokyo: Japan Marine Fishery Resources Research Center; 1998. pp. 13–30. [Google Scholar]
- 21.Robison BH, Reisenbichler KR, Sherlock RE. Science. 2005;308:1609–1611. doi: 10.1126/science.1109104. [DOI] [PubMed] [Google Scholar]
- 22.Young RE. Smithson Contrib Zool. 1972;97:1–159. [Google Scholar]
- 23.Phillips JB. Cal Fish Game. 1933;19:129–136. [Google Scholar]
- 24.Nezlin NP, McWilliams JC. Remote Sensing Env. 2003;84:234254. [Google Scholar]
- 25.Chavez FP. Geophys Res Lett. 1996;23:265–268. [Google Scholar]
- 26.Vidal EAG, Haimovici M. Bull Mar Sci. 1998;63:305–316. [Google Scholar]
- 27.Methot RD, Dorn MW. In: Hake: Biology, Fisheries and Markets. Alheit J, Pitcher TJ, editors. London: Chapman and Hall; 1995. pp. 389–414. [Google Scholar]
- 28.Beamish RJ, Benson AJ, Sweeting RM, Neville CM. Prog Oceanogr. 2004;60:355–385. [Google Scholar]
- 29.Mackas DL, Keiser R, Saunders M, Yelland DR, Brown RM, Moore D. Can J Fish Aquat Sci. 1997;54:2080–2096. [Google Scholar]
- 30.Cosgrove JA. PICES Press. 2005;13:30–31. [Google Scholar]
- 31.Brodeur RD, Ralston S, Emmett RL, Trudel M, Auth TD, Phillips AL. Geophys Res Lett. 2006;33:L22508. [Google Scholar]
- 32.Pearcy WG. Prog Oceanogr. 2002;54:399–403. [Google Scholar]
- 33.Wing BL. PICES Press. 2006;14:26–28. [Google Scholar]
- 34.Rodriguez-Benito C, Haag C. Gayana. 2004;68:508–513. [Google Scholar]
- 35.Chavez FP, Ryan J, Lluch-Cota SE, Niquen CM. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 36.Mason JE. Calif Coop Oceanic Fish Invest Rep. 2004;45:180–190. [Google Scholar]
- 37.Myers RA, Worm B. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 38.Markaida U, Quiñonez-Velasquez C, Sosa-Nishizaki O. Fish Res. 2004;66:31–47. [Google Scholar]
- 39.Pinkas L, Oliphant MS, Iverson ILK. Calif Dept Fish Game Fish Bull. 1971;152:1–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.