Abstract
The National Toxicology Program (NTP) hosted a workshop, “Animal Models for the NTP Rodent Cancer Bioassay: Strains & Stocks -- Should We Switch?” on June 16-17, 2005, at the National Institute of Environmental Health Sciences (NIEHS) in Research Triangle Park, NC. The workshop’s objectives were to determine (1) whether the currently used models, the F344/N rat and B6C3F1/N mouse, continue to be appropriate to identify substances that may pose a carcinogenic hazard for humans and (2) whether the NTP should consider conducting cancer bioassays using multiple strains of rats and/or mice to better capture the range of genetic variability. Workshop participants advised the NTP to discontinue using the current F344/N strain due to the recent issues with fertility, seizure activity, and chylothorax and provided several options on how the program should approach identifying and selecting a new rat model. Participants believed that the B6C3F1/N mouse is still appropriate for use by the NTP, but suggested the NTP take steps to better understand and address increases in background rates of liver tumors in this strain. Finally, the participants supported the NTP exploring the use of the multiple strain approach, although they raised many questions concerning data interpretation and feasibility. This article also outlines the NTP’s next steps in pursuing the workshop recommendations.
INTRODUCTION
The National Toxicology Program (NTP) hosted a workshop on June 16-17, 2005, at the National Institute of Environmental Health Sciences (NIEHS) in Research Triangle Park, NC to discuss rodent strains used in the NTP two-year chronic toxicology and carcinogenicity bioassay. This workshop is part of a series of activities associated with the NTP Roadmap to critically evaluate the NTP testing program and determine whether any refinements or new strategies are needed to maximize its positive impact on public health (National Toxicology Program (NTP) 2005). The last review of the NTP rodent cancer bioassay occurred in 1984 (National Toxicology Program (NTP) Board of Scientific Counselors 1984).
The workshop’s objectives were to determine (1) whether the currently used models, the F344/N rat and B6C3F1/N mouse, continue to be appropriate to identify substances that may pose a carcinogenic hazard for humans and (2) whether the NTP should consider conducting cancer bioassays using multiple strains of rats and/or mice to better capture the range of genetic variability as suggested by Festing (Festing 1995). Approximately 100 persons from academia, industry, government, and the private sector attended the workshop including an invited panel of 18 scientists1 with expertise in rodent genetics, cancer biology, statistics, and other related fields. The workshop opened with a series of presentations on characteristics of existing models, perspectives on selecting rodent models for carcinogenicity testing, and the use and theoretical statistical power of the multiple strain approach. In order to address the workshop objectives, the invited panel also met in three breakout groups: (1) rat models, (2) mouse models, and (3) the multiple strain approach. Each breakout group addressed a series of questions determined by the organizers. While the multiple strain breakout group was devoted to discussing the multiple strain approach, the rat and mouse breakout groups also addressed this issue to a lesser degree. The meeting reconvened in plenary session on the second day, and the attendees heard and discussed summaries of the deliberations of the breakout groups. Public participation occurred during both the plenary sessions and the breakout group sessions. The workshop agenda, presentations, background materials, roster of the invited panel and other attendees, and public comments can be found on the NTP website (http://ntp.niehs.nih.gov see “Meetings & Workshop”).
BREAKOUT GROUP CONCLUSIONS AND RECOMMENDATIONS
Rat Models
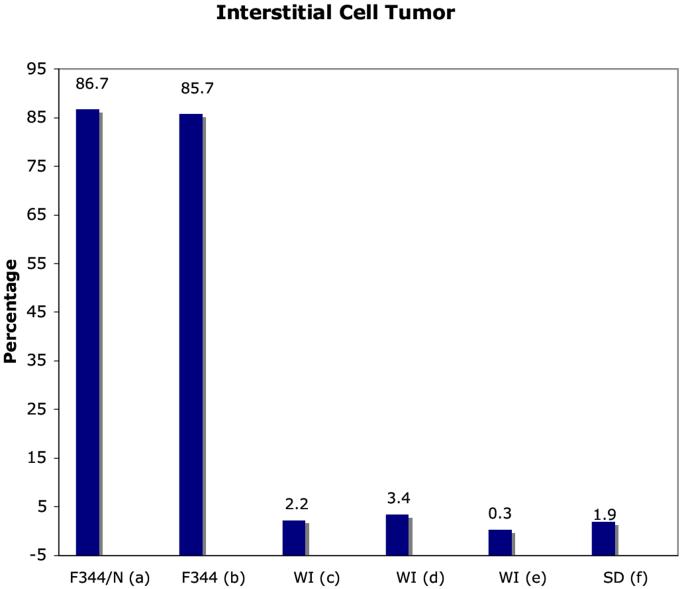
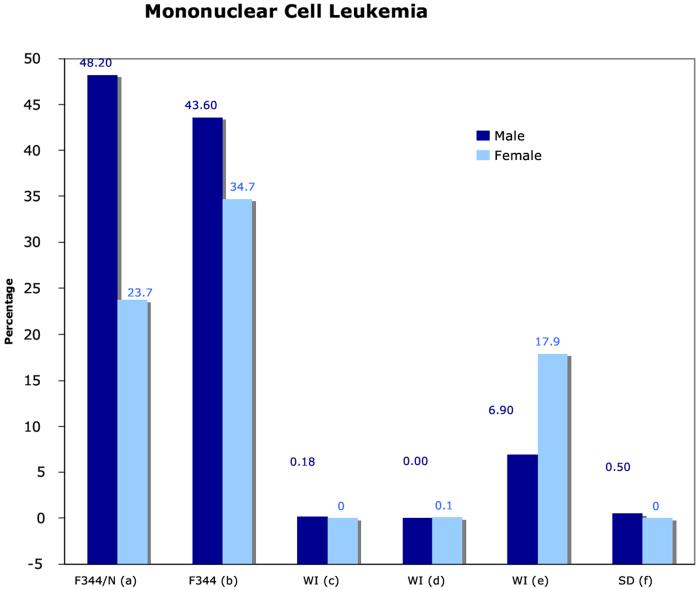
The F344/N rat has been used in the NTP two-year chronic toxicity and carcinogenicity bioassays for almost 30 years. The F344/N rat is known to have high background incidences of certain types of tumors including testicular interstitial cell tumors (Figure 1a) and mononuclear cell leukemia (Figure 1b). From a statistical perspective, high background rates of such tumors in control animals will generally decrease the ability to detect an exposure-related effect. In addition, when a statistically significant tumor effect is found in test animals relative to concurrent controls, the effect may not be considered exposure-related if it falls within the range observed in historical controls (Haseman, Arnold et al. 1990).
Figure 1.
Incidence of interstitial cell tumor in the F344/N rat and comparisons with other rats.
Incidence of mononuclear cell leukemia in the F344/N rat and comparisons with other rats.
Data presented at the NTP workshop (http://ntp.niehs.nih.gov see “Meetings & Workshop). (a): NTP F344/N, (b): NCTR F344, (c): Wistar Han CRL Data, (d): Wistar Han RCC Data, (e): Wistar proprietary data, and (f): Sprague Dawley proprietary data
Besides these inherent issues with the F344/N strain, declining fertility, sporadic seizure activity, and chylothorax have occurred within the past five years in the NTP F344/N rat colony. These issues are unique to our F344/N colony maintained at Taconic Farms, Inc. and to the best of our knowledge do not appear in other colonies maintained for commercial purposes at Taconic or other suppliers. The reasons for the development of these conditions in this specific colony have not been identified.
The rat breakout group strongly advised that the NTP discontinue use of its current F344/N strain and proposed three options: (1) re-establish the F344/N from another source, although such an approach would not address the general issues confronting the strain; (2) create an F1 hybrid such as the F344/Brown Norway cross (FBNF1); and (3) consider using an alternative strain or stock such as the outbred Wistar-Han. The group proposed a FBNF1 hybrid because it may have a lower incidence of testicular interstitial cell tumors than the F344 (Thurman, Moeller et al. 1995) and may also have lower rates of mononuclear cell leukemia. However, the group indicated that the spontaneous tumor rates in the FBNF1 for other types of tumors have not been rigorously established. The group suggested that the NTP consider the Wistar-Han because it is an outbred strain used by several pharmaceutical companies, and has a high survival rate and a low incidence of background tumors.
The rat breakout group could not agree on a specific isogenic strain (inbred or F1 hybrid) or outbred stock to recommend for future NTP studies. Instead they noted advantages of several commonly used inbred and outbred strains. The group proposed that studies already initiated with the F344/N be completed; however, for future bioassays, they recommended that the NTP select a new strain to use as the “default” unless other factors, such as metabolism data for the agent being tested suggested otherwise. The group also suggested that the NTP place greater emphasis on the use of “highly predictive” strains rather than “highly sensitive” strains.
Mouse Models
The B6C3F1 mouse has a long history of use in cancer bioassays (Rao and Boorman 1999). The National Cancer Institute selected the B6C3F1 as the model for its cancer bioassays based on the results of a study of multiple strains, using B6C3F1 and B6AKF1 hybrids2. Briefly, the B6C3F1 correctly identified significantly more known carcinogens when compared to the B6AKF1. The B6C3F1 also was considered hardy, had good reproductive capacity, was resistant to disease, and had low spontaneous tumor rates compared to other strains and hybrids (Innes, Ulland et al. 1969). The NTP continued to use this strain when it became responsible for conducting the two-year rodent bioassay in 1978, and this choice was reconfirmed at an NTP-sponsored workshop in 1988 (Rao, Birnbaum et al. 1988)
The B6C3F1 mouse is known to have higher background rates of liver tumors compared to other mouse strains (Figure 2). Liver tumors and body weight are highly correlated in the NTP B6C3F1/N mouse (Haseman, Young et al. 1997). Body weights in the NTP B6C3F1/N mouse have increased during the same time frame in which increased liver tumors have been observed suggesting that these two events are related.
Figure 2.
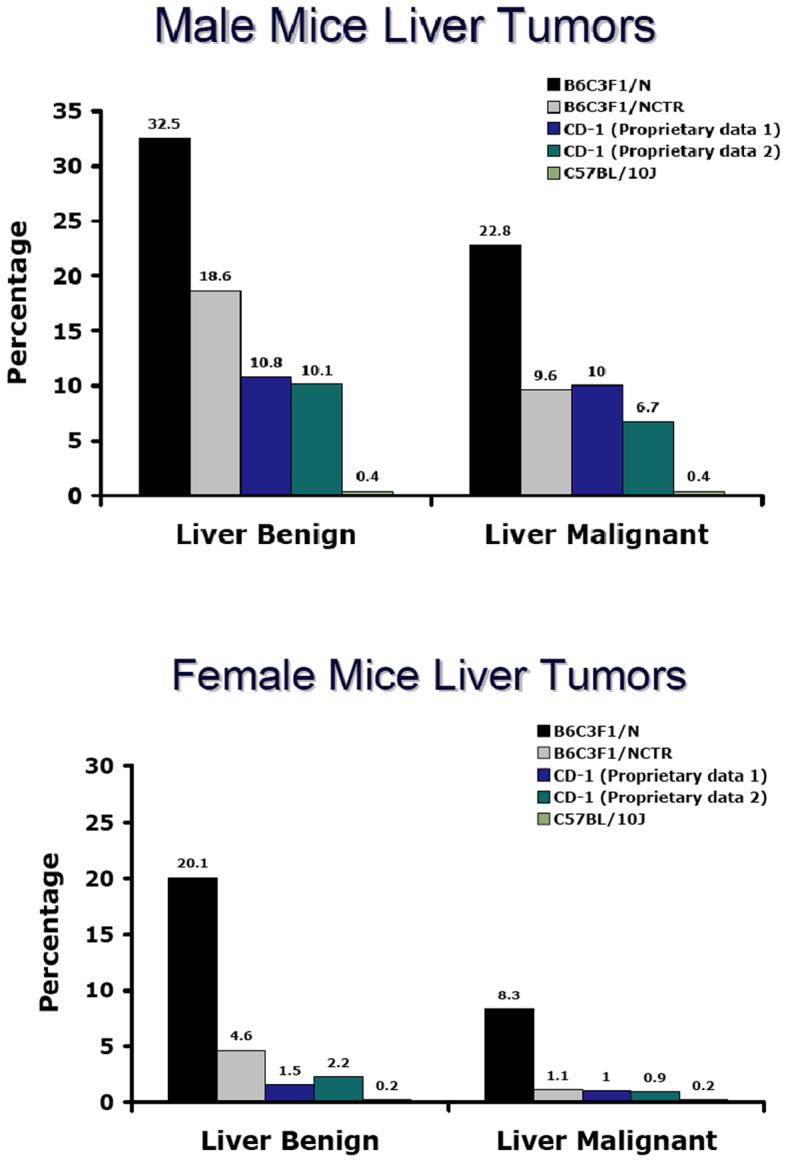
Incidence of benign and malignant hepatocellular liver tumors in male and female B6C3F1/N mice and comparisons with other mice
The mouse breakout group questioned whether the high incidence of background liver tumors in the B6C3F1 mouse was sufficient to warrant the NTP switching to another mouse model. In addition, the group supported the NTP’s ongoing effort to have the DNA of the parent strains of the B6C3F1 sequenced to better understand the genetic makeup of the model.
The mouse breakout group recommended that the NTP use isogenic strains to ensure reproducibility over time and facilitate genetic monitoring and mechanistic studies. Furthermore, the group suggested the use of a F1 hybrid rather than an inbred strain, because many of the identified cancer modifiers are semidominant and F1 mice often display sensitivity intermediate between that of resistant and sensitive parents.
Although, the group did not recommend changes in the current mouse model, they did offer suggestions should the NTP decide to explore the use of multiple mouse strains. These suggestions included (1) concurrently testing new strains and the NTP B6C3F1 strain, (2) using a fixed panel of strains and F1 hybrids in the bioassay, and (3) when choosing which strains to use, start with the inbred strains being re-sequenced by NIEHS, and choose parental pairs for F1 hybrids that are genomically distant from each other.
Although the breakout groups were not asked to discuss the need for both rat and mouse models in the bioassay, the mouse breakout group emphasized the importance of the continued use of the mouse. They thought a tumor response in multiple species translates to greater concern with implications for eventual risk assessment. In addition, having data from both species would be helpful to better interpret instances where the study’s tumorigenic outcome yielded equivocal responses. Finally, the group thought that the availability of genomic sequences for multiple strains would enhance the NTP’s understanding of mechanisms and genetic modifiers for cancer and other diseases.
Multiple Strain Approach
The issue of the use of multiple strains of rodents to better capture the range of genetic variability was a topic of considerable discussion and debate. In brief, a multiple strain approach would involve the use of more than one strain of rat or mouse in each dose group. The total group size per dose would be comparable to the current single strain approach (n = 50 per sex per group). For example, if 5 strains of mice were used, each dose group could be comprised of 10 animals per strain for a total 50 animals per dose per sex.
From a research perspective, the use of multiple rodent strains would potentially increase our understanding of the influence of certain genetic polymorphisms on the biological response to environmental exposures and improve our ability to extrapolate findings to sensitive subpopulations of humans. For these reasons, the NIEHS strategic plan for 2006 highlights the use of a variety of rodent strains to improve the availability of relevant in vivo models for human disease (National Institute of Environmental Health Sciences (NIEHS) 2006). To help achieve this objective, in 2004 the NIEHS and the NTP initiated a research plan for whole genomic DNA sequencing of 15 inbred mouse strains (http://ntp.niehs.nih.gov see “Mouse Genome Resequencing Project”).
While there is considerable support for the multiple strain approach as a research tool, its utility for hazard identification is uncertain. Based on simulations using 1, 2, 3, or 4 strains, the statistical power to detect a carcinogenic response is generally similar between the multiple and single strain approaches except in situations where there is a considerable amount of heterogeneity in tumor response among the strains and where the most sensitive strains display a “very strong” response. In the latter case, the multiple strain approach may increase power by 45 to 70 percent, depending upon the number of strains used. However, any theoretical advantage in increasing statistical power with a multiple strain approach is lost if the data from each strain is analyzed separately instead of pooling data across strains. This issue is potentially a major hurdle for routine use of the multiple strain approach as it is unclear whether regulatory and scientific communities would accept the results of an analysis of data pooled across strains.
Overall, workshop participants thought the use of multiple strains is a viable strategy for cancer hazard identification3 even though the approach has several disadvantages. For example, the multiple strain approach would be costly and logistically complicated, creating more opportunity for operational error. In addition, there are study design issues to address, such as dose selection when each strain may have a different maximum tolerated dose. The multiple strain group did not attempt to weigh the advantages and disadvantages of the multiple strain approach and provide an overall recommendation on whether the NTP should adopt it routinely.
In terms of which strains to select, the multiple strain group suggested the NTP develop a pool of strains for which two-year background data are known (body weight, two-year survival, natural life span, and histopathology) from which several strains could be selected for a given bioassay. The choice of what specific strains to select could then be based on known sensitivities. The group recommended testing substances in sensitive strains when possible. In addition, the relevance of mechanisms in human should be considered. The multiple strain group was unanimous in preferring isogenic to outbred strains. More specifically, they recommended using commercially viable and sequenced strains as much as possible. As a strategy for switching, the group recommended that the NTP conduct pilot strains studies to collect adequate background information for proposed strains. If feasible, these strains should then be characterized with respect to response to known human carcinogens or compounds generally recognized as safe. Additional strains should be added incrementally to the two-year bioassay while also routinely including the B6CF1/N mouse and 344/N rat.
NEXT STEPS FOR THE NTP
The NTP carefully considered the advice provided by workshop participants. Shortly after the workshop the NTP discontinued use of the NTP F344/N rat in all new studies and began using a commercial source of the F344 (F344/NTac). The NTP intends to continue to use an isogenic rat strain to maximize reproducibility in tumorigenic response over time and facilitate genetic monitoring and interpretation of subsequent mechanistic studies. The NTP will consider the FBNF1 rat as an alternative strain. While the FBNF1 lacks a historical control database to assess spontaneous neoplastic and non-neoplastic lesions, the NTP could establish a control database by using the FBNF1 as a second concurrent control in chronic studies. If needed, the NTP could also test the FBNF1 with a known carcinogen(s) and compare the results to those observed in the initial animal strain to validate the sensitivity of the FBNF1 to carcinogens.
At this time the NTP has no plans to replace the B6C3F1/N mouse in cancer bioassays. Alternative mouse strains may be included in selected future NTP studies to examine the genetic basis for adverse responses to environmental agents. The strains would be selected following a thorough evaluation of the genomic data from the mouse sequencing project noted above.
The NTP is deferring the final decision on whether/how to pursue the multiple strain approach until additional workshops related to the NTP Roadmap are completed. At that time, the program will reevaluate priorities related to the testing program and consider broader issues related to an increased institutional emphasis on addressing host susceptibility. For example, the next workshop will focus on identifying changes to the protocols for prechronic studies that might enhance the NTP’s ability to detect early biomarkers of toxicity to the lung and cardiovascular system, as well as changes to lipid and carbohydrate metabolism. This ongoing series of workshops is intended to strengthen the NTP’s experimental approaches to identifying and understanding environmental influences on human disease and dysfunction. We welcome comments and suggestions on these and other topics from the scientific community.
Footnotes
Drs. Molly Bogue, Norman Drinkwater, John DiGiovanni, Jeffery Everitt, Michael Festing, Thomas Hamm, Jerry Hardisty, Joseph Haseman, William Hooks, Howard Jacob, Ralph Kodell, Daniel Morton, James Popp, Julian Preston, Carlos Sonnenschein, David Threadgill, Hiroyoshi Toyshiba, and Vernon Walker.
The B6C3F1 hybrid is produced by crossing the inbred C57BL/6 female with the inbred C3H male. The B6AKF1 is produced by crossing the inbred C57BL/6 female with the inbred AKR male.
The rat breakout group was not supportive of a multiple strain approach that involves keeping group sizes the same as the current single strain study design (n = 50 per sex per group), but composed of animals from different strains (e.g., 10 animals per sex from 5 strains). Primarily, they were concerned about interpreting a study based on pooling data from small groups of animals from different strains. For this reason, they felt the multiple strain approach would not be practical, as it would have to be scaled up appropriately to mimic the design of a single strain study.
References
- Festing MF. “Use of a multistrain assay could improve the NTP carcinogenesis bioassay.”. Environ Health Perspect. 1995;103(1):44–52. doi: 10.1289/ehp.9510344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman JK, Arnold J, Eustis SL. Pathology of the Fischer Rat. G. A. Boorman. San Diego, Academic Press; 1990. Tumor incidences in Fischer 344 rats: NTP historical data; pp. 557–564. [Google Scholar]
- Haseman JK, Young E, Eustis SL, Hailey JR. “Body weight-tumor incidence correlations in long-term rodent carcinogenicity studies.”. Toxicol Pathol. 1997;25(3):256–63. doi: 10.1177/019262339702500302. [DOI] [PubMed] [Google Scholar]
- Innes JR, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER, Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, Peters J. “Bioassay of pesticides and industrial chemicals for tumorigenicity in mice: a preliminary note.”. J Natl Cancer Inst. 1969;42(6):1101–14. [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences (NIEHS) 2006. New Frontiers in Environmental Sciences and Human Health: The 2006-2011 NIEHS Strategic Plan.
- National Toxicology Program (NTP) “Roadmap to achieve the NTP Vision (released March 10, 2005).”. 2005 Available at http://ntp.niehs.nih.gov see “NTP Vision & Roadmap” [Accessed August 2005].
- National Toxicology Program (NTP) Board of Scientific Counselors 1984. “Report of the Ad Hoc Panel on Chemical Carcinogenesis Testing and Evaluation of the NTP Board of Scientific Counselors (August 17, 1984).”.
- Rao GN, Birnbaum LS, Collins JJ, Tennant RW, Skow LC. “Mouse strains for chemical carcinogenicity studies: overview of a workshop.”. Fundamental & Applied Toxicology. 1988;10(3):385–94. doi: 10.1016/0272-0590(88)90285-0. [DOI] [PubMed] [Google Scholar]
- Rao GN, Boorman G. Pathology of the Mouse. Cache River Press; R. Maronpot. Vienna, IL: 1999. History of the B6C3F1 mouse; pp. 1–6. [Google Scholar]
- Thurman JD, Moeller RB, Jr., Turturro A. “Proliferative lesions of the testis in ad libitum-fed and food-restricted Fischer-344 and FBNF1 rats.”. Lab Anim Sci. 1995;45(6):635–40. [PubMed] [Google Scholar]