Abstract
ATP-dependent glucokinase is suggested to have evolved from a hypothetical polyphosphate (polyP)-dependent glucokinase (polyP-GK) via a bifunctional polyP/ATP glucokinase (polyP/ATP-GK). Here we showed that polyP-GK is present in a polyP-accumulating bacterium, Microlunatus phosphovorus. The polyP-GK produced glucose-6-Pi from glucose and polyP, but it could not phosphorylate glucose with ATP. The polyP-GK was most closely related to the polyP/ATP-GK of Mycobacterium tuberculosis.
Inorganic polyphosphate (polyP) is a linear polymer of tens or hundreds of orthophosphate (Pi) residues linked in the same manner as the two high-energy phosphoanhydride bonds in ATP (4, 5). PolyP is readily formed by dehydration of Pi and found in abundance in volcanic condensates and deep-oceanic steam vents (14). Hence, ancient organisms may have utilized polyP instead of ATP in their metabolic reactions (4, 9, 14).
Glucokinases that use ATP as the sole phosphoryl donor to catalyze the phosphorylation of glucose (ATP-GKs) have been present in all contemporary organisms examined (2). Bifunctional glucokinase (polyP/ATP-GK), which utilizes polyP or ATP as the phosphoryl donor to phosphorylate glucose, was found first in Mycobacterium phlei (10) and then in many other bacteria, including Corynebacterium diphtheriae (11), Mycobacterium tuberculosis (12), and Propionibacterium shermanii (13). The polyP/ATP-GK of M. tuberculosis also utilizes GTP, UTP, and CTP as phosphoryl donors (12). PolyP is recognized as one of the earliest biopolymers and is most likely a prominent precursor in prebiotic evolution (4). Thus, it has been hypothesized that glucose phosphorylation was originally mediated by polyP and that when ATP became available in the environment, a transition was made by the GKs to utilize the latter phosphoryl donor (9). However, nobody has discovered the strictly polyP-dependent glucokinase that utilizes polyP as the sole phosphoryl donor (polyP-GK).
Microlunatus phosphovorus strain NM-1 is a gram-positive, coccus-shaped, non-spore-forming bacterium (7). Strain NM-1, which was originally isolated with an enhanced biological phosphorus removal process, accumulates large amounts of polyP (a maximum of approximately 48% of its dry weight as phosphate [Pi]) in a glucose medium (7). We discovered the existence of polyP-GK in M. phosphovorus strain NM-1.
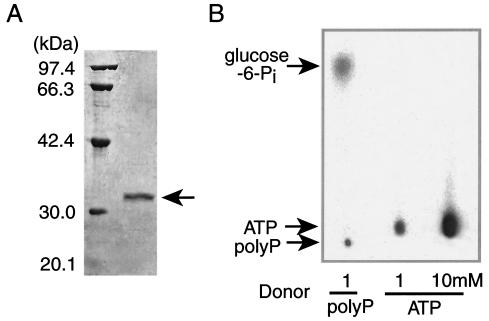
M. phosphovorus strain NM-1 was grown for 2 days under aerobic conditions at 27°C in the glucose medium (7). The cells harvested at the mid-logarithmic phase of growth were disrupted by using Bead-beater (Biospec), and the polyP-GK was precipitated by adding 70% ammonium sulfate. The polyP-GK was further purified by using a DEAE-cellulose (Whatman), a phenyl-Sepharose (Amersham Biosciences), a PE hydrophobic (Poros), and two anion exchange (HQ [Poros] and miniQ [Amersham Biosciences]) columns. The polyP-GK was purified 253-fold (Fig. 1A), and the recovery of polyP-GK activity was 6.7%. The polyP-GK migrated as a 32-kDa protein on sodium dodecyl sulfate (SDS)-polyacrylamide gels, while it fractionated as a 64-kDa protein by gel filtration, suggesting that it was a dimer. The purified enzyme produced glucose-6-Pi from glucose and [32P]polyP (Fig. 1B). The polyP-GK does not catalyze the reverse reaction (data not shown).
FIG. 1.
SDS-polyacrylamide gel electrophoresis (PAGE) of polyP-GK and analysis of polyP-GK activity. (A) The purified polyP-GK was analyzed by SDS-10% PAGE. (B) [32P]polyP (6) and [γ-32P]ATP were used for the phosphorylation of 1 mM glucose by 0.1 μg of the purified polyP-GK. The reaction was performed in the presence of TMB buffer (0.1 M Tris, 0.1 M boric acid, 0.1 M maleic acid [pH 5.5])-50 mM KCl-4 mM MgCl2 at 30°C. After 1 h of incubation, the reaction mixture was plotted onto a polyethyleneimine-cellulose plate and the plate was developed with 1 M formic acid and 0.4 M LiCl. The 32P-labeled products were visualized using BAS1000 (Fujifilm).
The production of glucose-6-Pi was confirmed by oxidizing it to glucose-6-phosphonate with glucose-6-Pi dehydrogenase and NADP. The polyP-GK could not phosphorylate glucose with [γ-32P]ATP even at concentrations as high as 5 to 10 mM (the usual intracellular levels of ATP) (Fig. 1B). The polyP-GK utilizes neither pyrophosphate nor ADP as a phosphoryl donor. The polyP-GK does not degrade polyP in the absence of glucose (data not shown). The Km values for a long-chain polyP with 700 Pi residues and two short-chain polyPs with 30 and 8 Pi residues were 0.06, 3.8, and 12 μM, respectively. The kcat values for these polyPs were 183, 270, and 87 s−1, respectively. Thus, the polyP-GK prefers a long-chain polyP as a phosphoryl donor. The polyP-GK was able to phosphorylate glucosamine at a rate similar to that of glucose and was able to slowly phosphorylate mannose. The polyP-GK activity required 1 to 10 mM Mg2+. Mn2+, Co2+, and Zn2+ (in that order) were also effective. The optimal temperature and pH were 30°C and 5.5, respectively.
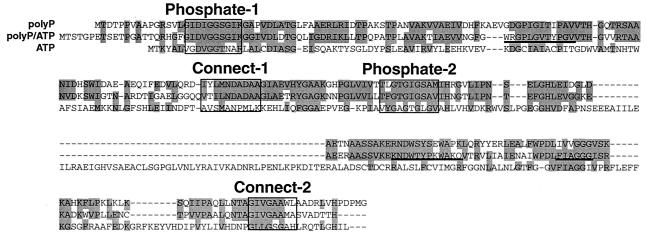
The N-terminal amino acid sequence of the purified polyP-GK was determined by using a peptide sequencer (Applied Biosystems). After the enzyme was digested with trypsin, the internal amino acid sequences were also determined by using a mass spectrometer (Micromass). On the basis of the amino acid sequences, the DNA primers T(ACGT)TT(CT)GCIGC(ACGT)GA(AG)(AC)G (containing an inosine [I]) and GC(AG)TC(ACGT)GC(AG)TC(AG)TTCAT were designed for amplification of a DNA fragment containing the gene (ppgk) that encodes polyP-GK. Using the amplified DNA as a probe, a 1.8-kb SphI fragment which contains the entire ppgk gene was cloned from its chromosomal DNA. The M. phosphovorus ppgk gene encoded a putative polypeptide of 266 amino acids. The deduced amino acid sequence of the polyP-GK showed 50% identity (64% similarity) to that of the M. tuberculosis polyP/ATP-GK (Fig. 2). The polyP-GK contained regions that are homologous to common motifs interacting with the ATP molecule that are conserved in the ATP- and polyP/ATP-GKs from different sources (Fig. 2). These regions are the Phosphate-1 and Phosphate-2 motifs, which contact the β- and γ-Pi of ATP (1). The highly conserved residues in these motifs are Asp and Gly (in Phosphate-1) and Gly and Thr (in Phosphate-2) (1). Two hinge regions at the interface between the two Pi-binding domains, the Connect-1 and Connect-2 motifs, were also identified in the polyP-GK.
FIG. 2.
Comparison of the deduced amino acid sequences of the M. phosphovorus polyP-GK, the M. tuberculosis polyP/ATP-GK, and the E. coli ATP-GK. The shaded amino acids were conserved among the GKs (CLUSTALW analysis; DDBJ). The Phosphate-1, Phosphate-2, Connect-1, and Connect-2 motifs are boxed. The underlined sequences (WRGPLGVTYPGV, KNDWTYPKWAKQ, and FIAGGG) are the proposed regions of glucose, polyP, and adenosine binding in the polyP/ATP-GK (9), respectively.
In addition, the polyP-GK was found to possess a putative polyP-binding region which was homologous to that proposed for the M. tuberculosis polyP/ATP-GK (3, 9). The internal region of the M. phosphovorus polyP-GK between Phosphate-1 and Connect-1 was longer by two amino acids than that of the polyP/ATP-GK. A putative glucose-binding region proposed in the polyP/ATP-GK (9) was conserved in polyP-GK but not well conserved in ATP-GK. An adenosine-binding region (9) was slightly changed in the polyP-GK (Fig. 2). However, the location of the adenosine-binding region is highly speculative in the polyP-GK. The amino acid sequences which may specifically enable the polyP-GK to utilize polyP as the sole phosphoryl donor remain to be identified. Interestingly, ATP-GK contains a larger insertion between Phosphate-2 and Connect-2 motifs than those seen with polyP and polyP/ATP-GKs (Fig. 2).
A long-standing question was whether a strictly polyP-dependent glucokinase exists in contemporary organisms. The present results convincingly showed the existence of polyP-GK, which has been considered a hypothetical evolutionary origin of glucokinases. Very interestingly, M. phosphovorus strain NM-1 was found to release Pi, concomitantly with the degradation of intracellular polyP, when it took up glucose and accumulated glycogen under anaerobic conditions (8). This evidence, together with the existence of polyP-GK, strongly suggests that polyP can be directly used to phosphorylate glucose in M. phosphovorus. After the glucose-Pi is converted to glycogen, the resultant Pi might be released. The polyP-GK may be responsible for the release of Pi of this bacterium. We could not detect a polyP-dependent phosphofructokinase activity in M. phosphovorus strain NM-1. Only ATP phosphofructokinase was present in this organism (data not shown). These results suggest that polyP is not a sole phosphoryl donor for driving the whole glycolytic pathway in M. phosphovorus strain NM-1. When the M. phosphovorus cells are grown aerobically in the glucose medium with excess Pi, the cellular levels of polyP (mainly 100 to 200 Pi residues) reach over 5 mmol/g of cells in Pi equivalents (8), which far exceeds those seen with ATP (typically 7 μmol/g of Escherichia coli cells). However, it is unclear whether ATP is directly used for the biosynthesis of polyP, because significant polyphosphate kinase activity was not detected with M. phosphovorus strain NM-1. The cellular environment of this unique bacterium may represent a hypothetical ancient world in which polyP could be preferentially utilized in metabolic roles.
Nucleotide sequence accession number.
The nucleotide sequence of the ppgk gene has been deposited in DDBJ with the accession number AB075018.
Acknowledgments
We thank A. Yamagata and K. Yoshizato for determination of the internal amino acid sequences.
REFERENCES
- 1.Bork, P., C. Sander, and A. Valencia. 1992. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc. Natl. Acad. Sci. USA 89:7290-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bork, P., C. Sander, and A. Valencia. 1993. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci. 2:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh, P. C., B. C. Shenoy, D. Samols, and N. F. B. Phillips. 1996. Cloning, expression, and characterization of polyphosphate glucokinase from Mycobacterium tuberculosis. J. Biol. Chem. 271:4909-4915. [DOI] [PubMed] [Google Scholar]
- 4.Kornberg, A. 1995. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J. Bacteriol. 177:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulaev, I. S. 1979. The biochemistry of inorganic polyphosphate. Wiley Interscience, New York, N.Y.
- 6.Kuroda, A., H. Murphy, M. Cashel, and A. Kornberg. 1997. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 272:21240-21243. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura, K., A. Hiraishi, Y. Yoshimi, M. Kawaharasaki, K. Masuda, and Y. Kamagata. 1995. Microlunatus phosphovorus gen. nov., sp. nov., a new gram-positive polyphosphate-accumulating bacterium isolated from activated sludge. Int. J. Syst. Bacteriol. 45:17-22. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura, K., S. Ishikawa, and M. Kawarasaki. 1995. Phosphate uptake and release activity in immobilized polyphosphate-accumulating bacterium Microlunatus phosphovorus strain NM-1. J. Ferment. Bioeng. 80:377-382. [Google Scholar]
- 9.Phillips, N. F. B., P. C. Hsieh, and T. H. Kowalczyk. 1999. Polyphosphate glucokinase. Prog. Mol. Subcell. Biol. 23:101-125. [DOI] [PubMed] [Google Scholar]
- 10.Szymona, M. 1957. Utilization of inorganic polyphosphates for phosphorylation of glucose in Mycobacterium phlei. Bull. Acad. Pol. Sci. Ser. Sci. Biol. 5:379-381. [Google Scholar]
- 11.Szymona, M., and O. Szymona. 1961. Participation of volutin in the hexokinase reaction of Corynebacterium diphtheriae. Bull. Acad. Pol. Sci. Ser. Sci. Biol. 9:371. [Google Scholar]
- 12.Szymona, M., and J. Widomski. 1974. A kinetic study on inorganic polyphosphate glucokinase from Mycobacterium tuberculosis H37Ra. Physiol. Chem. Phys. 6:393-404. [PubMed] [Google Scholar]
- 13.Wood, H. G., and N. H. Goss. 1985. Phosphorylating enzymes of the propionic acid bacteria and the roles of ATP, inorganic pyrophosphate, and polyphosphates. Proc. Natl. Acad. Sci. USA 82:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamagata, Y., H. Watanabe, M. Saitoh, and T. Namba. 1991. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature. 352:516-520. [DOI] [PubMed] [Google Scholar]