Abstract
The enzymatic properties and the physiological function of the type IV apurinic/apyrimidinic (AP)-endonuclease homolog of Bacillus subtilis, encoded by yqfS, a gene specifically expressed in spores, were studied here. To this end, a recombinant YqfS protein containing an N-terminal His6 tag was synthesized in Escherichia coli and purified to homogeneity. An anti-His6-YqfS polyclonal antibody exclusively localized YqfS in cell extracts prepared from B. subtilis spores. The His6-YqfS protein demonstrated enzymatic properties characteristic of the type IV family of DNA repair enzymes, such as AP-endonucleases and 3′-phosphatases. However, the purified protein lacked both 5′-phosphatase and exonuclease III activities. YqfS showed not only a high level of amino acid identity with E. coli Nfo but also a high resistance to inactivation by EDTA, in the presence of DNA containing AP sites (AP-DNA). These results suggest that YqfS possesses a trinuclear Zn center in which the three metal atoms are intimately coordinated by nine conserved basic residues and two water molecules. Electrophoretic mobility shift assays demonstrated that YqfS possesses structural properties that permit it to bind and scan undamaged DNA as well as to strongly interact with AP-DNA. The ability of yqfS to genetically complement the DNA repair deficiency of an E. coli mutant lacking the major AP-endonucleases Nfo and exonuclease III strongly suggests that its product confers protection to cells against the deleterious effects of oxidative promoters and alkylating agents. Thus, we conclude that YqfS of B. subtilis is a spore-specific protein that has structural and enzymatic properties required to participate in the repair of AP sites and 3′ blocking groups of DNA generated during both spore dormancy and germination.
During unpredicted periods of dormancy Bacillus subtilis spores are constantly exposed to environmental conditions that have the potential to cause several types of DNA damage. Therefore, the existence of spore-specific protecting mechanisms would seem to be fundamental for spore survival. One of the factors intricately involved in protecting spore DNA from several types of damage, such as oxidative stress, UV-C irradiation, and desiccation, is the presence of α/β type small acid-soluble proteins (reviewed in references 16, 28, and 27). Although α/β type small acid-soluble proteins protect spore DNA from several stresses, they confer protection neither to base alkylation (29) nor to UV-induced DNA strand break formation (30). Thus, while the physiological state of the B. subtilis spores prevents or dramatically slows DNA damage during the long periods of dormancy, it is clear that spores do accumulate potentially lethal and mutagenic DNA lesions such as the spore photoproduct, strand breaks, cyclobutane pyrimidine dimers, chemically altered bases and apurinic/apyridiminic (AP) sites which could affect transcription and replication processes during germination (16, 26, 29). To remove these potentially deleterious DNA damages and alterations, B. subtilis spores utilize spore-specific and general DNA repair systems such as the spore photoproduct lyase (SplB), the nucleotide excision repair system (UVR) and Rec proteins (reviewed in reference 16).
AP sites can be potentially generated during spore germination not only by the action of DNA glycosylases but also by the spontaneous depurination and depyrimidination of DNA. AP sites are inherently toxic and highly mutagenic; therefore, they should be rapidly processed and eliminated during spore germination. Moreover, 3′-blocking groups such as phosphates, phosphoglycolates, and 3′α,β-unsaturated aldehydes existing in DNA as products of reactive oxygen species attack or generated by the combined action of glycosylase/lyase activities must be also eliminated by AP-endonucleases as they inhibit DNA replication (4).
The first catalytic event during repair of AP sites is carried out by AP-endonucleases which cleave the DNA backbone immediately 5′ of an AP site, generating a 5′ deoxyribose-phosphate group and a 3′ deoxyribose-hydroxyl group (6). On the other hand, 3′ blocking groups on DNA strand breaks are also processed by AP-endonucleases to generate a 3′-OH group (4).
Analysis of the genome of B. subtilis (10) revealed the existence of two open reading frames (ORFs), named exoA and yqfS, whose predicted products share amino acid sequence homology with Escherichia coli exonuclease III (ExoIII) and Nfo, respectively. Except for a lower 3′-5′exonuclease activity the biochemical properties of a B. subtilis ExoA purified protein were very similar to those reported for E. coli ExoIII (30). Interestingly a B. subtilis mutant lacking the ExoA function was as tolerant to hydrogen peroxide and alkylating agents as was the repair proficient isogenic parental strain (30), suggesting that YqfS or other noncharacterized AP-endonucleases might compensate the functions of ExoA in B. subtilis.
In E. coli the expression of nfo is linked to the oxidative stress generated by superoxide radicals (2). However, in B. subtilis the regulation of yqfS expression occurs in a temporal manner and the mRNA for this gene is apparently localized within the forespore (32). Furthermore, the promoter responsible for the regulation of yqfS expression appears to be part of the σG regulon (32). In addition the lack of induction of a yqfS-lacZ fusion inserted at the yqfS locus of the B. subtilis chromosome following treatment by either hydrogen peroxide or the DNA damaging agent mitomycin C revealed that this gene is not under the control of the PerR or SOS regulons (32).
The research reported in this manuscript demonstrates that the AP-endonuclease YqfS exists in mature spores and that its DNA coding sequence possesses the ability to genetically complement the DNA repair deficiency of an E. coli mutant lacking the major AP-endonucleases Nfo and ExoIII. Furthermore, a His6-YqfS protein synthesized in E. coli and purified to homogeneity has biochemical properties similar to those exhibited by the type IV family of endonucleases. Therefore, we conclude that the spore protein YqfS of B. subtilis is a new functional member of the type IV family of AP-endonucleases.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this work are shown in Table 1. The medium used was Luria-Bertani (LB) medium (15). When appropriate, ampicillin (AMP) (100 μg/ml) or kanamycin (25 μg/ml) was added to the medium. Liquid cultures were incubated with aeration (shaking at 250 rpm) at 37°C. Cultures on solid media were grown at 37°C. The optical density (OD) of liquid cultures was monitored with a Pharmacia Ultrospec 2000 spectrophotometer set at 600 nm.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| E. coli strains | ||
| SURE | e14− McrA− (mcrCB-hsd SMR-mrr)171 endA1 supE44 thl-1 gyrA96 relA1 lac recB recJ sbcC umuC::Tn5(Kmr) uvrC [F′ proAB lacqZ M15 Tn10(Tcr)] | Stratagene |
| XL10-Gold Kan | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lac1qZΔM15 Tn10(Tetr) Tn5(Kanr) Amy] | Stratagene |
| PERM282 | Strain E. coli SURE carrying plasmid pPERM 282 | This study |
| PERM337 | Strain E. coli XL10-Gold Kanr carrying plasmid pPERM 337 | This study |
| PERM348 | Strain E. coli XL10-Gold Kanr carrying plasmid pPERM348 | This study |
| RPC501 | nfo-1::Kan Δ(xth-pncA) | R. P. Cunningham |
| PERM398 | RPC 501 carrying pPERM 348 | This study |
| PERM399 | RPC 501 carrying pQE30 | This study |
| Plasmids | ||
| pUC18 | Multisite E. coli cloning vector; Apr | Laboratory stock |
| pQE30 | Vector that contains a T5 promoter that enables six-His-tagged protein expression; Apr | Qiagen |
| pPERM282 | pUC18 with 1.07-kb XbaI-BamHI PCR product containing yqfS; Apr | This study |
| pPERM348 | pQE30 with 1.07-kb BamHI-BamHI yqfS fragment from pPERM282; Apr | This study |
Design of a plasmid to overexpress yqfS and purify a His6-YqfS protein.
The ORF of yqfS lacking the first codon and extending through 157 bp downstream of the yqfS stop codon was amplified by PCR, using 0.1 μg of chromosomal DNA from B. subtilis 168 and the oligonucleotide primers 5′-GCGGATCCCTG AGA ATA GGC TCA CAC G-3′ (forward) and 5′-CGGGATCCGGC CGT TGA AGT AGC GAA CC-3′ (reverse). The primers were designed to insert BamHI restriction sites into the cloned DNA (underlined). Amplification was performed on a MJ Research (Watertown, Mass.) Minicycler using Vent DNA polymerase (New England Biolabs, Beverly, Mass.). The PCR fragment (1,070 bp) was first ligated into HincII-treated pUC18 to generate pPERM282 which was replicated into E. coli XL-1 Blue (Stratagene, La Jolla, Calif.). pPERM282 was digested with BamHI, and the 1,070-bp yqfS fragment was inserted in-frame into the BamHI site of pQE30 (QIAGEN Inc., Valencia, Calif.); the resulting construction pPERM348 was replicated in E. coli XL10-Gold Kan (Stratagene). The proper in-frame insertion of the yqfS fragment was assessed by both restriction analysis and DNA sequencing as previously described (22, 23).
Purification of His6-YqfS.
E. coli PERM348 was grown in 50 ml of LB medium supplemented with AMP to an optical density of 0.5. Expression of the yqfS gene was induced during 4 h at 37°C by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 0.5 mM. Cells were collected by centrifugation and washed two times with 10 ml of 50 mM Tris-HCl (pH 7.5)-300 mM NaCl (buffer A). The cells were disrupted in 10 ml of buffer A containing lysozyme (10 mg/ml) for 30 min at 37°C. The cell homogenate was subjected to centrifugation (29,200 × g) to eliminate undisrupted cells and cell debris and the supernatant was applied to a 5 ml Ni-nitrilotriacetic acid (NTA)-agarose (QIAGEN Inc.) column, previously equilibrated with buffer A. The column was washed with 50 ml of buffer A containing 10 mM imidazole plus 50 ml of buffer A containing 20 mM imidazole, and the protein bound to the resin was eluted with 15 ml of buffer A containing 100 mM imidazole, 2-ml fractions were collected during this last step. Aliquots (15 μl) of the cell homogenate and the flowthrough as well as the bound fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (11).
Complementation of the DNA repair-deficient strain E. coli RPC501 [nfo-1::Kan Δ(xth-pncA)].
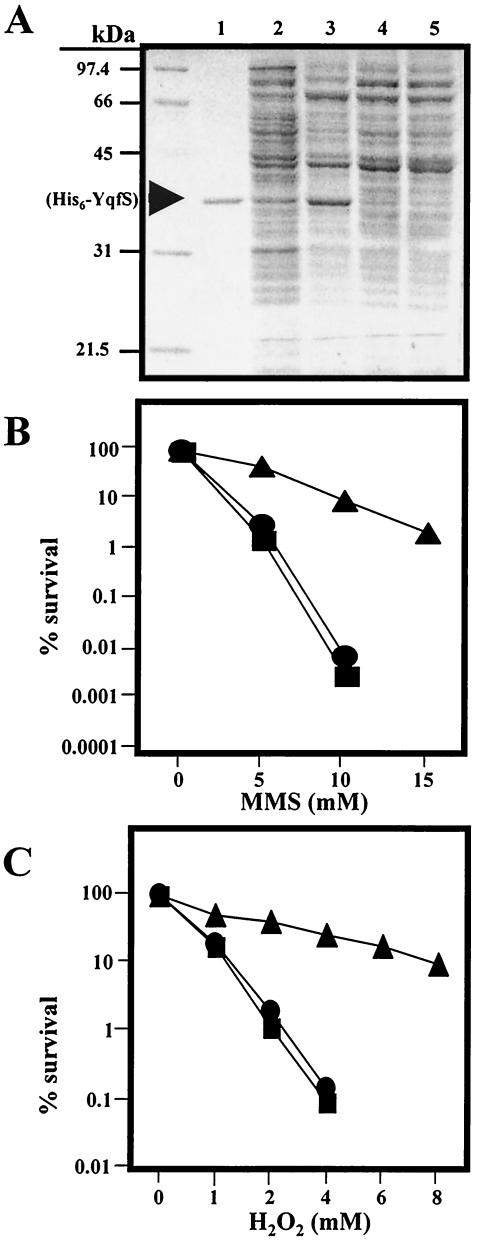
The plasmid pPERM348 was introduced by transformation into competent cells of the strain E. coli RPC501 (kindly provided by Richard P. Cunningham), following the calcium chloride protocol (22). Transformant colonies were selected in LB medium supplemented with AMP. The presence of the plasmid pPERM348 in the transformant strain (E. coli PERM398) was determined by restriction analysis of plasmid DNA mini-preparations (22). The overproduction of His6-YqfS in the strain E. coli RPC501 harboring pPERM348 was corroborated by taking a culture to an OD at 600 nm (OD600) of 0.5 and inducing with 0.1 mM IPTG for 1 h. After disrupting the cells with lysozyme, the cell extracts were analyzed by SDS-PAGE using as a marker the His6-YqfS protein purified as described above. The sensitivity of E. coli RPC501 and its derivative E. coli PERM398 to the DNA damaging agents H2O2 and methyl methanosulfonate (MMS) was determined. Essentially, strains were grown overnight in LB containing the appropriate antibiotics and then diluted (1:50) into fresh medium. The cultures were shaken at 37°C to an OD600 of 0.5 then IPTG (0.1 mM) was exclusively added to the strain RPC501 (a subculture of this strain was left with no IPTG) and incubation was continued for 15 min. The cells were collected by centrifugation, washed once and suspended in 10 mM sodium phosphate (pH 7.5)-150 mM NaCl (buffer B). The cultures of each strain were treated with different concentrations of either MMS or H2O2 and incubation was continued for 1 h at 37°C with shaking. The cell suspensions were diluted serially 10-fold in buffer B and plated on solid LB containing the appropriate selective antibiotics. The viable colonies were counted after 1 to 2 days of incubation at 37°C to estimate survival.
Substrates and enzyme assays for AP-endonuclease activity.
AP-endonuclease activity of His6-YqfS was assayed against pBluescript (pBS) (Stratagene) which was partially depurinated following a previously described protocol (7). A typical mixture reaction in a volume of 25 μl contained 600 ng of purified His6-YqfS, 100 ng of substrate in 50 mM Tris-HCl (pH 7.5) containing 1 mM dithiothreitol (DTT). The reactions were incubated at 37°C during 30 min, and analyzed by electrophoresis on a 1% agarose gel which was stained with ethidium bromide. AP-endonuclease activity of His6-YqfS was also determined utilizing a radioactive double-stranded 19-mer nucleotide containing a single AP site which was synthesized as previously described (5). Essentially, the nucleotide 5′-GCAGCGCAGUCAGCCGACG-3′ was treated with uracil-DNA glycosylase following the instructions of the provider (Roche, Mannheim Germany). The AP site containing 19-mer nucleotide was labeled on its 5′end with [γ-32P]ATP and T4 polynucleotide kinase (Promega, Madison, Wis.) as previously reported (5). Finally, the AP radioactive oligonucleotide was annealed to a threefold excess of the complementary oligonucleotide 5′-CGTCGGCTGACTGCGCTGC-3′ on ice for 3 h (5).
The reactions were performed in a total volume of 15 μl containing 50 mM Tris-HCl (pH 7.5), 1 mM DTT, and 500 nM unlabeled and 10 nM double-stranded radioactive 19-mer containing a single AP-site. His6-YqfS (300 ng) was added to the mixture reaction and incubated for 30 min at 37°C. The reactions were separated on a 20% denaturing polyacrylamide gel which was dried and then exposed to X-Omat films (Kodak) during 12 h.
To assay 5′-phosphatase activity the 19-mer nucleotide 5′-GCAGCGCAGUCAGCCGACG-3′ was labeled on its 5′end with [γ-32P]ATP and T4 polynucleotide kinase. 5′-Phosphatase reactions were performed in 25-μl reactions which contained 500 ng of His6-YqfS, 20 ng of the 5′-end radiolabeled 19-mer (22,000 cpm/ng of DNA) in 50 mM Tris-HCl (pH 7.5)-1 mM DTT. As a positive control 1 U of alkaline phosphatase (New England Bio Labs) was added to the mixture reaction instead of His6-YqfS. Mixture reactions were incubated at 37°C for up to 30 min. The amount of radioactive phosphate released was determined from the norit-nonadsorbed fraction by liquid scintillation, as previously described (12).
The 3′-exonuclease activity of His6-YqfS was determined against a substrate containing 3′-terminal [α-32P]dCMP, which was synthesized by treating pUC19 with EcoRI followed by end-filling with [α-32P]dCTP and the Klenow fragment of DNA polymerase (Promega) according to the manufacturer's procedures. To this end, mixture reactions of 25 μl were mounted, which contained, 500 ng of His6-YqfS, 13 ng of the [α-32P]dCTP radioactively labeled pUC19 substrate (11,500 cpm/ng of DNA) in 50 mM Tris-HCl (pH 7.5) containing 1 mM DTT. A mixture reaction containing 100 U of ExoIII (New England Bio Labs) instead of His6-YqfS was used as a positive control. The 3′-exonuclease activity was determined by acid precipitation as previously published (12). The 3′-phosphatase activity of His6-YqfS was determined by measuring the ability of His6-YqfS to stimulate nick translation of DNA containing 3′-phosphate termini, according to a previously described protocol (7). Briefly, micrococcal nuclease-treated pBS (50 nmol) was incubated with either 1 U of E. coli Nfo, 500 ng of His6-YqfS, or 1 U of alkaline phosphatase for 30 min at 37°C. Each sample of enzyme-treated plasmid was incubated with the Klenow fragment of DNA polymerase at 37°C in the presence of deoxynucleoside triphosphates (10 μM) and [α-32P]dCTP in 50 mM Tris-HCl (pH 7.5) containing 10 mM MgCl2 and 1 mM DTT. Reactions were terminated by adding bovine serum albumin (2 mg/ml) and ice-cold 5% trichloroacetic acid. The radioactivity incorporated into the material precipitated by trichloroacetic acid was quantified by liquid scintillation.
Electrophoretic mobility shift assays (EMSA).
Protein-DNA interactions were carried out in 20-μl reaction mixtures that typically contained Tris-HCl 50 mM (pH 7.5), 1 mM DTT, 300 mM NaCl, ∼20 pmol of the [32P] labeled 19-mer containing a single AP site, and the indicated amounts of purified His6-YqfS protein. Reactions were incubated at 4°C for 10 min and then loaded onto 3% agarose gels. Gels were first subjected to electrophoresis at 70 V in 1× Tris-acetate buffer and after drying subjected to autoradiography.
RESULTS
Expression and purification of YqfS from E. coli.
A gene designated yqfS was retrieved from the genome of B. subtilis (10); whose primary structure revealed an ORF of 891 bp with enough information for the synthesis of a predicted protein of 32, 915 Da. Amino acid alignments (1) showed that YqfS posses homologies of 53, 52, and 32% with E. coli Nfo (24), Saccharomyces cerevisiae Apn1 (18), and Thermotoga maritima endonuclease IV (5), respectively. As shown in Fig. 1, YqfS conserves important amino acid residues present in several members of the AP-endonuclease family, including the nine residues, His-69, His-110, His145, Asp-179, His-182, His-214, Asp-227, His-229, and Glu-25, putatively involved in the coordination of three Zn atoms in the active site of E. coli Nfo (6). Moreover, equivalent residues, His-7, Phe-31, Tyr-72, Trp-266, and the Zn2+-ligand Glu-259, involved in forming a deep AP site pocket for cleaving the phosphodiester bond of the AP-site in E. coli Nfo (6), are also conserved in YqfS (Fig. 1).
FIG. 1.
Amino acid sequence alignment of B. subtilis YqfS with homologs from E. coli (12), S. cerevisiae (18), and T. maritima (5). Symbols: *, residues involved in the coordination of three Zn atoms in the active site of E. coli Nfo; ♦, residues involved in forming a deep AP site pocket for cleaving the phosphodiester bond of the AP-site in E. coli Nfo.
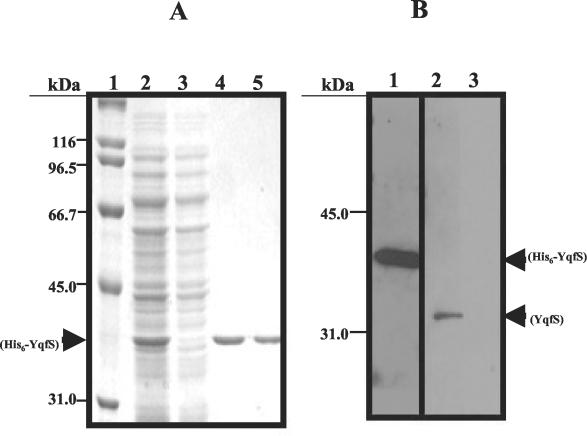
Among the type IV AP-endonuclease homologs described in the literature, only the functions of those from E. coli (12), S. cerevisiae (9, 20), and T. maritima (5) have been assessed. As shown in Fig. 1, the predicted product of B. subtilis yqfS conserves a high level of sequence homology with E. coli Nfo, therefore we investigated whether yqfS encodes a functional type IV AP-endonuclease homolog of B. subtilis. As a necessary step to accomplish this goal a protocol was devised to purify a recombinant YqfS protein. Thus, the yqfS gene was amplified by PCR and expressed from the IPTG inducible T5 promoter of the plasmid pQE-30 to generate a protein tagged with 6 histidines on its N terminus. Initially, induction of yqfS was performed for 2 h with 5 mM IPTG; however, these conditions caused a very high level of His6-YqfS synthesis, resulting in the formation of inclusion bodies in the E. coli host cells (results not shown). To improve the amount of soluble His6-YqfS protein, the strain E. coli PERM 348 was grown at 37°C to an OD600 of 0.5 and then induction was carried out at 37°C with 0.5 mM IPTG, for 2 h. SDS-PAGE analysis confirmed the existence of a highly abundant soluble 36-kDa protein in cell homogenates obtained from E. coli cells subjected to these induction conditions (Fig. 2A, lane 2). When the His6-YqfS protein present in the cell extracts was subjected to purification by metal chelate affinity chromatography, on a Ni-NTA-agarose column, a major protein band with a molecular mass of around 36 kDa was eluded from the column with 100 mM imidazole, as revealed by SDS-PAGE (Fig. 2A, lanes 4 and 5).
FIG. 2.
(A) SDS-PAGE analysis of His6-YqfS purification through a Ni-NTA-agarose column. Aliquots (15 μl) of each sample were electrophoresed on a 10% polyacrylamide gel which was stained with Coomassie blue. Lane 1, molecular weight standards; lane 2, E. coli PERM348 lysate; lane 3, flowthrough; lanes 4 and 5, fractions eluted from the column with 100 mM imidazole. (B) Immunoblot analysis of protein samples prepared with 5 μg of pure His6-YqfS (lane 1) or 100 μg of cell extracts prepared from either mature spores (17) (lane 2) or vegetative cells (lane 3) of B. subtilis 168, which were separated on an SDS-12% polyacrylamide gel and transferred to a nitrocellulose membrane. The blot was probed with a polyclonal anti-His6-YqfS chicken antibody which was diluted 5,000-fold and then processed with an ECL Western blotting analysis system (Amersham Pharmacia, Buckinghamshire, United Kingdom).
YqfS is located in mature spores.
Our recent results demonstrated that in B. subtilis the expression of yqfS is neither constitutive nor induced by oxidative or DNA damaging agents but regulated in a spatial and temporal manner (32). The expression of yqfS was shown to occur during the last steps of sporulation in the developing spores from a sigG type promoter (32). Therefore, we wanted to determine if the site and timing of yqfS expression was consistent with the location of the YqfS protein. Specifically, is YqfS found in the spore and/or in growing cells? To this end, cellular extracts prepared from vegetatively growing cells and mature spores of B. subtilis 168 were separated by SDS-PAGE, transferred to a nitrocellulose filter and then probed with a polyclonal chicken antiserum against His6-YqfS. As expected, the antibodies recognized the His6-YqfS protein purified from E. coli cells (Fig. 2B, lane 1); on the other hand, the anti-His6-YqfS antibodies exclusively recognized a band of about 33 kDa in cell extracts prepared from B. subtilis spores (Fig. 2B, lane 2) but not in those prepared from vegetative cells (Fig. 2B, lane 3). These results, revealed for the first time that the type IV AP-endonuclease enzyme encoded by yqfS exists in B. subtilis dormant spores.
Enzyme activities of His6-YqfS.
To investigate whether the purified His6-YqfS protein possessed AP-endonuclease activity the recombinant protein was first incubated with partially depurinated plasmid DNA (AP-pB). The results analyzed by electrophoresis in agarose gels, revealed that YqfS was able to convert the AP-pB plasmid from the closed covalently circular form to the open circular form (Fig. 3A; lane 4). The nuclease reaction performed by His6-YqfS was specific for the AP substrate as the enzyme was unable to attack the DNA of the nondepurinated plasmid (U-pB) (Fig. 3A, lane 3). The AP-endonuclease activity encoded by the His6-YqfS protein was also tested against a 5′-end radioactively labeled double-stranded 19-mer nucleotide containing a single AP site. The products of the reaction analyzed on a denaturing acrylamide gel revealed that the endonucleolytic activity of YqfS specifically processes the cleavage of the substrate containing an apurinic site (Fig. 3B, lane 6) since it showed no endonuclease activity against the 32P-labeled 19-mer substrate lacking an AP site (Fig. 3B, lane 5). As shown in Fig. 3B (lane 4), the AP 32P-labeled 19-mer was also cleaved by E. coli Nfo, which on the other hand was not able to process the cleavage of the intact 19-mer substrate (Fig. 3B, lane 3). Utilizing the 32P-labeled 19-mer substrate containing a single AP site as a substrate the apparent Km of YqfS for AP cleavage site was 86 nM. Furthermore, we investigated whether, in addition to possessing AP-endonuclease activity, the purified His6-YqfS enzyme also possessed 3′-phosphatase activity. Therefore, plasmid pBluescript was first treated with micrococcal nuclease to generate 3′-phosphates and then used as a substrate to determine whether His6-YqfS stimulates the nick translation activity of the Klenow fragment of DNA polymerase (7). The results shown in Fig. 4A revealed that YqfS possessed 3′-phosphatase activity since it was able to stimulate the incorporation of radioactivity into DNA during the nick translation assay. In this experiment, a positive control showed that as expected, Nfo from E. coli also was able to stimulate the nick translation activity of the polymerase. Although His6-YqfS was able to function as an AP-endonuclease and possessed 3′-phosphatase activity it showed no activities of either 5′-phosphatase (Fig. 4B) or 3′-exonuclease (Fig. 4C). These results demonstrated that the product encoded by the yqfS gene shares enzyme properties similar to those reported for members of the type IV AP-endonuclease family (5, 8, 12).
FIG. 3.
Endonuclease activity of His6-YqfS against a plasmid containing AP sites (A) and against a double-stranded 19-mer containing a single AP site (B). (A) Aliquots (0.6 μg) of His6-YqfS were incubated with 0.5 μg of either nontreated (pBS) (lane 3) or AP-site-containing (AP-pB) (lane 4) pBluescript. Lane 1, untreated plasmid incubated with 50 mM Tris-HCl, (pH 7.5), 300 mM NaCl; lane 2, AP sites-containing plasmid incubated with 50 mM Tris-HCl (pH 7.5)-300 mM NaCl. The reactions were incubated at 37°C during 30 min and analyzed by electrophoresis on a 1% agarose gel which was stained with ethidium bromide. Abbreviations: CCC, covalent closed circular plasmid; OC, open circular plasmid. (B) A 510 nM concentration of 32P-, 5′-end-labeled double-stranded 19-mer nucleotide containing a single AP site was incubated in the absence (lane 2) or presence of 300 ng of His6-YqfS (lane 6) and 1 U of E. coli Nfo (lane 4). A 32P-labeled 19-mer substrate lacking an AP site (510 nM) was incubated under the same conditions in the absence (lane 1) or presence of 300 ng of His6YqfS (lane 5) and 1 U of E. coli Nfo (lane 3). The reactions were separated on a 20% denaturing acrylamide gel and then subjected to autoradiography. Abbreviations: U, uncleaved substrate; C, cleaved substrate.
FIG. 4.
Determination of 3′-phosphatase (A), 5′-phosphatase (B), and 3′-exonuclease (C) activities for YqfS. (A) The ability of either E. coli Nfo (1 U), His6-YqfS (YqfS) (500 ng), or alkaline phosphatase (A.P.) (1 U) to stimulate the nick translation activity of DNA containing 3′-phosphate termini was determined as described in Materials and Methods. Error bars show standard deviation. (B) 5′-Phosphatase activity was determined as described in Materials and Methods to either E. coli Nfo (1 U), His6-YqfS (500 ng) or alkaline phosphatase (1 U). (C) 3′-Exonuclease activity was determined as described in Materials and Methods to either, E. coli Nfo (1 U), His6-YqfS (500 ng), or ExoIII (100 U). C, no added enzyme. For all three panels, the y axis shows counts per minute incorporated (A, 105; B, 105; C, 104). The data are expressed as averages of two independent duplicate determinations.
Biochemical properties of recombinant His6-YqfS.
Elucidation of the crystal structure of E. coli Nfo revealed that its active site consists of a trinuclear Zn center which is putatively involved in catalyzing the phosphodiester cleavage in the AP sites of DNA (6). Alignments between the primary structures of YqfS and functional homologs of E. coli Nfo, revealed that the former contains nine invariably conserved residues putatively involved in coordinating three Zn atoms (Fig. 1). Therefore, we investigated whether YqfS is also a metal dependent enzyme. Increasing concentrations of EDTA were added to reactions containing His6-YqfS and the substrate AP-pB. Results analyzed on agarose gels, revealed that concentrations as high as 100 mM of EDTA were unable to inhibit the AP-endonuclease activity of YqfS (Fig. 5A, lanes 2 to 5). In fact, a concentration of 250 mM of EDTA was required to inactivate the AP-endonuclease activity of this protein (results not shown). However, as shown in Fig. 5B, when His6-YqfS was preincubated at room temperature with different concentrations of EDTA and then assayed for endonuclease activity against the AP-pB substrate, a concentration of as low as 10 mM of EDTA was enough to inactivate the enzyme. Taken together these results we conclude that B. subtilis YqfS is a metal dependent enzyme which shows similar properties of inactivation by EDTA as those observed for E. coli Nfo (12) and S. cerevisiae ApnI (8).
FIG. 5.
Effect of EDTA on the AP-endonuclease activity of His6-YqfS. (A). Aliquots of His6-YqfS (0.1 μg) were incubated with 0.5 μg of AP containing sites pBluescript (APpB), either in the absence (lane 1) or presence (lanes 2 to 5) of different concentrations of EDTA. The reactions were incubated at 37°C during 30 min and analyzed by electrophoresis on a 1% agarose gel which was stained with ethidium bromide. (B). Aliquots of His6-YqfS (0.5 μg) were incubated either in the absence (lane 1) or presence (lanes 2 to 6) of different amounts of EDTA for 15 min at 37°C, and then 0.5 μg of APpB was added to each reaction mixture and the mixtures were incubated at 37°C during 30 min and analyzed by electrophoresis on a 1% agarose gel which was stained with ethidium bromide.
DNA binding properties of YqfS.
Analysis of the crystal structure of Nfo revealed for the first time that an α8β8 TIM barrel possesses structural properties that enable it to bind DNA (6). Due to the evident structural similarities between YqfS and Nfo (Fig. 1) we analyzed the DNA binding properties of YqfS by utilizing DNA shift mobility analysis by probing with a 32P 5′-end labeled double-stranded 19-mer nucleotide. As shown in Fig. 6, the His6-YqfS enzyme was able to recognize and bind to the AP site containing 32P-labeled 19-mer substrate causing a shift on its electrophoretic mobility. Formation of the His6-YqfS:AP-19-mer complex was dependent on the concentration of the enzyme used in the reaction (Fig. 6, lanes 2 to 4). Moreover, addition of antibody for the His6-YqfS to the binding reaction between His6-YqfS and the radioactive AP double-stranded 19-mer caused the formation of a highly retarded His6-YqfS:AP-DNA:Ab complex (Fig. 6, lane 5). A control experiment showed that the His6-YqfS antibody was not able to interact with the AP radioactive 19-mer substrate by itself (Fig. 6, lane 6).
FIG. 6.
EMSA of His6-YqfS binding to an AP site containing 32P-labeled double-stranded 19-mer nucleotide at different protein concentrations. Reaction mixtures (20 μl) containing ∼20 pmol of 32P-AP-19 bp, Tris-HCl (50 mM; pH 7.5), 1 mM DTT, and 300 mM NaCl were incubated for 10 min at 4°C, either in the absence (lanes 1 and 6) or presence (lanes 2 to 5) of the indicated amounts of His6-YqfS. A polyclonal anti-His6-YqfS antibody was added to the mixture reactions shown in lane 5. The reaction mixtures were loaded onto an agarose gel which was developed at 70 V in 1× Tris-acetate buffer and after drying subjected to autoradiography.
Crystallographic analysis of Nfo as well as of a complex between Nfo and a 15-bp DNA containing tetrahydrofuran as a synthetic abasic site suggested that the TIM barrel fold structure has molecular properties not only for binding and scanning normal DNA but also to specifically recognize AP sites on DNA (6). To test this hypothesis, the purified His6-YqfS enzyme was incubated with a radioactive double-stranded 19-mer substrate containing (Fig. 7A) or not containing (Fig. 7B) a single AP site. In this protocol different amounts of sodium chloride were added to the reactions, and the results were then analyzed by EMSA. The data revealed that in the absence or in the presence of low concentrations of salt (50 and 100 mM) the enzyme was able to recognize both types of DNA independent of the presence or absence of an AP site (Fig. 7, lanes 2 to 4). However, when the concentrations of the salt were increased to 100 to 250 mM, the enzyme was exclusively detached from the nondamaged DNA substrate (Fig. 7B, lanes 3 to 4). The bonding complex formed between YqfS and the double-stranded 19-mer substrate containing an AP site was strong since the protein remained attached to the damaged substrate even at salt concentrations as high as 500 mM (Fig. 7A, lanes 4 to 6). In fact concentrations of 1 M of NaCl were required to disrupt the His6-YqfS:AP-DNAcomplex (Fig. 7A, lanes 7).
FIG. 7.
EMSA of His6-YqfS binding to a 32P-labeled double-stranded 19-mer nucleotide containing (A) or not containing (B) a single AP site under different ionic strengths. Reaction mixtures (20 μl) containing ∼20 pmol of either 32P-AP-19bp (A) or 32P-19bp (B), Tris-HCl 50 mM (pH 7.5), and 1 mM DTT were incubated for 10 min at 4°C, either in the absence (lane 1) or presence (lanes 2 to 8) of 8 μg of His6-YqfS; containing (lanes 3 to 7) or not containing (lanes 1 to 2) different concentrations of NaCl. The reaction mixtures were loaded onto an agarose gel which was developed at 70 V in 1× Tris-acetate buffer and after drying subjected to autoradiography.
yqfS genetically complements an E. coli mutant deficient in ExoIII and Nfo activities.
To investigate the physiological role played by YqfS, it was determined whether its encoding DNA sequence complemented the DNA repair-deficient phenotype of the mutant E. coli strain RPC501 which lacks both the exoIII and nfo genes (33). Accordingly, the plasmid pPERM348, which contained the yqfS ORF cloned in frame into the multiple cloning site of the expression plasmid pQE30, was introduced into the mutant strain E. coli RPC501. The production of a His6-YqfS protein in the resulting strain E. coli PERM398 was investigated by growing the cells to an OD600 of 0.5 and then inducing with IPTG to a final concentration of 0.1 mM. The results of Fig. 8A show that even in the absence of IPTG the cells produce a soluble protein (Fig. 8A, lanes 2 and 3) with a molecular mass identical to the pure His6-YqfS protein (Fig. 8, lane 1). On the other hand, isogenic strains lacking the yqfS ORF were unable to induce the synthesis of a protein band with the molecular mass of His6-YqfS, in the absence (Fig. 8, lane 4) or presence (Fig. 8, lane 5) of IPTG. Once it was determined that YqfS was being produced in the appropriate genetic background, E. coli RP501, the ability of this strain to survive treatment with different concentrations of either H2O2 or MMS was determined. The results revealed that the high sensitivity of E. coli RPC501 to both MMS (Fig. 8B) and H2O2 (Fig. 8C) can be complemented by the expression of yqfS in the strain E. coli RPC501. However, this complementation was not observed for the strain E. coli PERM399, which harbors only the plasmid pQE30, which lacks the ORF for yqfS. Interestingly, we observed that the resistance of E. coli PERM398 to H2O2 and MMS treatment was better in the absence of IPTG compared to when the yqfS gene was induced to overproduce the recombinant protein (results not shown). These data suggest that high levels of expression of His6-YqfS from the T5 promoter might have a negative effect on the growth and survival of E. coli PERM398.
FIG. 8.
Survival curves generated in response to MMS (B) and H2O2 (C) for strains E. coli RPC501 (xth/nfo) (▪), PERM398 (RPC501 + pQE30-yqfS) (▴), and PERM399 (RPC501 + pQE30) (•). The E. coli strains were grown in LB to an OD600 of 0.5. The cells were collected by centrifugation, washed once and suspended in buffer B. The cultures of each strain were treated with either H2O2 or MMS atthe concentrations indicated, and incubation was continued for 1 h at 37°C with shaking. The cell suspensions were diluted serially 10-fold and plated on solid LB containing the appropriate selective antibiotics. The viable colonies were counted after 1 to 2 days of incubation at 37°C to estimate survival. (A) SDS-PAGE analysis of His6-YqfS synthesis in cell extracts of strains E. coli PERM398, in the absence (lane 2) or presence (lane 3) of 0.1 mM IPTG. Lane 1, 5 μg of purified His6-YqfS; lanes 4 and 5, cell extracts of E. coli RPC501 and E. coli PERM399, respectively.
DISCUSSION
Previous results revealed that in B. subtilis the regulation of yqfS expression occurs in a temporal and forespore-specific manner (32), suggesting that its product is involved in protecting spores from the environmental conditions which result in the generation of AP sites and strand breaks. Therefore, it was of relevance to investigate whether YqfS posses enzymatic and biochemical properties characteristic of the type IV AP-endonuclease family (18). Accordingly, the yqfS gene was expressed in E. coli with a N-terminal hexahistidine tag and purified to apparent homogeneity by Ni-NTA-agarose chromatography. As has been described for other His-tagged proteins (21, 25), His6-YqfS exhibited a slow migration on SDS-PAGE, showing a molecular mass of ∼36 kDa, 3 kDa above its predicted mass.
The His6-YqfS protein was utilized to produce polyclonal antibodies which when utilized in Western blot experiments recognized in cell extracts a 33-kDa protein from B. subtilis spores. This protein had the expected molecular mass for the predicted product of yqfS. No similar protein was recognized from extracts of growing cells. These results are in agreement with data that demonstrated that in B. subtilis the expression of yqfS occurs in the developing spores during the last steps of sporulation from a sigG type promoter (32). It has been reported that other sigG-dependent genes such as ssp genes and splAB are transcribed during sporulation and their products are packed into the spores to confer protection to DNA against the mutagenic and deleterious effects of chemical agents and environmental stresses (16, 29). The specific location of YqfS in B. subtilis mature spores strongly suggests that a base excision repair pathway is involved in protecting spores from the environmental damage, which results in the generation of AP sites and strand breaks during either dormancy or germination. It remains to be investigated whether other components of the BER pathway follow a spore-specific expression pattern.
The functionality of the pure His6-YqfS protein was demonstrated by its ability to catalyze the cleavage of a plasmid containing AP sites as well as of a 32P-labeled double-stranded 19-mer nucleotide containing a single AP site. The apparent Km of YqfS for AP cleavage site of the last substrate was 86 nM. A Km value around three times larger, i.e., 270 nM, was found for T. maritima endonuclease IV, during the degradation of the same AP site-containing 19-mer nucleotide (5). Evaluation of other enzymatic properties revealed that YqfS was capable of stimulating the nick translation activity of DNA polymerase by catalyzing the cleavage of 3′ blocking phosphates of DNA treated with micrococcal nuclease. This result suggests that YqfS might play an important role in processing not only AP sites but also single strand breaks on DNA, particularly those which block the 3′-OH of a single DNA strand as a result of free radical attack (4). In addition, our results revealed that YqfS lacks both 5′-phosphatase and 3′-exonuclease activities, a common characteristic shared by the other members of the type IV AP-endonuclease family (5, 12, 20). The type IV family of AP-endonucleases currently includes three characterized members, namely, E. coli Nfo (12), S. cerevisiae Apn (9, 20), and T. maritima endonuclease IV (5). Eukaryotes such as Schizosaccharomyces pombe and Caenorhabditis elegans possess genes which potentially encode endonuclease IV homologs. However, in the former case the SpApn1 gene is apparently not expressed, and no AP-endonuclease activity has been detected in S. pombe extracts (19). On the other hand, a CeApn recombinant gene did not express a functional protein in E. coli; thus, the activities of this homolog are currently unknown (13). Therefore, YqfS of B. subtilis not only represents the second homolog of bacterial origin but also the fourth member of the family IV of AP-endonucleases with a demonstrated biochemical function.
Upon determination of other biochemical properties of YqfS, our experiments revealed that in the presence of AP-DNA, concentrations of EDTA as high as 500 mM were required to inactivate this endonuclease. On the other hand, in the absence of DNA a concentration of EDTA 50 times lower inhibited the AP-endonuclease activity of YqfS. The resistance of YqfS to EDTA inactivation is not unprecedented and is actually a reported distinctive characteristic of type IV AP-endonucleases (12, 20) which is not shared by Mg2+-dependent endonucleases such as ExoIII and APE-1 (4, 6). Based on these results as well as on the existence in YqfS of the nine amino acids involved in forming the trinuclear Zn center in Nfo (Fig. 1), we suggest that YqfS is a Zn-dependent enzyme.
It has been predicted that E. coli Nfo must bind and scan normal DNA via electrostatic complementarity and hydrogen bonding to the DNA phosphate backbone from β-barrel bonds and α-helical dipoles ideally positioned by an α8β8 framework (6). Results of amino acid alignments revealed that YqfS shares a high degree of amino acid identity with E. coli Nfo (Fig. 1). Thus, we suspected that the structural properties required to bind DNA by Nfo should be also conserved in YqfS. This suggestion was analyzed by EMSA. Results revealed that YqfS was able to recognize and retard the electrophoretic mobility of a 32P-labeled 19-mer nucleotide containing an AP site, in a reaction which was dependent on the concentration of the YqfS used in the assay. These results combined with the high degree of sequence similarity between YqfS and Nfo tend to support the notion that YqfS most probably adopts an α8β8 TIM barrel which shares structural properties with E. coli Nfo to bind DNA. Results of crystallographic analysis predicted that the α8β8 TIM barrel adopted by Nfo should be capable of binding to DNA and discriminate between nondamaged DNA from DNA containing AP sites (6). Thus, in a first level of interaction Nfo should be capable of binding nondamaged DNA to scan it in search of AP sites. It is also expected that a complex of this nature should be very unstable. Our results confirmed this prediction for YqfS. Essentially, the enzyme was able to form a complex with nondamaged DNA which was disrupted at a low ionic strength. On the other hand, in a second level of interaction Nfo should be able the recognize an AP site on DNA and establish a highly stable complex. Such a complex was indeed formed between YqfS and substrate DNA containing a single AP site. Disruption of this complex required concentrations of salt as high as 1 M. Taking these results together, we conclude that YqfS is an enzyme which possess structural properties not only to bind undamaged DNA but also to strongly interact with DNA containing AP sites.
Although E. coli mutants deficient in either exoIII or nfo show few biological abnormalities (3, 14), it has been reported that the combination of both mutations generates cells which exhibit not only a high sensitivity to MMS, H2O2, and tert-butyl hydroperoxide but also an enhanced mutation rate by MMS (33). Therefore, we used the exoIII nfo double mutant of E. coli, RPC501, to investigate whether yqfS can genetically complement the high sensitivity of this strain to H2O2 and MMS. Our results demonstrated that expression of the His-tagged yqfS complements the DNA repair-deficient phenotype of E. coli RPC501. Although yqfS was shown to be more efficient in complementing the sensitivity to H2O2, it was evident that yqfS was also proficient in reverting the DNA damaging effects produced by the alkylating agent MMS. Taking into account that H2O2 treatment of cells induces the formation of single strand breaks whereas MMS indirectly generates AP site on DNA, it is appropriate to conclude that YqfS possesses the ability to correct, in vivo, both types of DNA lesions. Based on these results, we conclude that YqfS not only possesses amino acid sequence similarity to functional members of the type IV AP-endonuclease family but also fulfills similar physiological functions by conferring protection to cells against the deleterious effects of oxidative promoters and alkylating agents. Furthermore, the enzymatic activity of YqfS is in agreement for a role for this protein in the repair of the damage done to the DNA of spores and promoting the successful process of germination (26, 27, 31).
Acknowledgments
This work was supported by grant 31767-N from the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México to Mario Pedraza-Reyes. José M. Salas-Pacheco and Norma Urtiz-Estrada were supported by a doctoral fellowship from CONACYT. R.E.Y. was supported by MCB-9975140 from the National Science Foundation.
We thank Edmundo Chavez-Cosio for facilities provided during the obtaining of the anti His6-YqfS antibodies and thank Juan A. Rojas for technical assistance.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped blast and PSI-blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, E., and B. Weiss. 1987. Endonuclease IV of Escherichia coli is induced by paraquat, DNA damage by oxygen-derived species. Proc. Natl. Acad. Sci. USA 84:3189-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham, R. P., S. M. Saporito, S. G. Spitzer, and B. Weiss. 1986. Endonuclease IV (nfo) mutant of Escherichia coli. J. Bacteriol. 168:1120-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 5.Haas, B. J., M. Sandigursky, J. A. Tainer, W. A. Franklin, and R. P. Cunningham. 1999. Purification and characterization of Thermotoga maritima endonuclease IV, a thermostable apurinic/apyrimidinic endonuclease and 3′-repair diesterase. J. Bacteriol. 181:2834-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosfield, D. J., Y. Guan, B. J. Haas, R. P. Cunningham, and J. A. Tainer. 1999. Structure of the DNA repair enzyme endonuclease IV and its DNA complex: double-nucleotide flipping at abasic sites and three-metal-ion catalysis. Cell 98:397-408. [DOI] [PubMed] [Google Scholar]
- 7.Izumi, T., K. Ishizaki, M. Ikenaga, and S. Yonei. 1992. A mutant endonuclease IV of Escherichia coli loses the ability to repair lethal DNA damage induced by hydrogen peroxide but not that induced by methyl methanesulfonate. J. Bacteriol. 174:7711-7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, A. W., and B. Demple. 1988. Yeast DNA 3′-repair diesterase is the major cellular apurinic/apyrimidinic endonuclease: substrate specificity and kinetics. J. Biol. Chem. 263:18017-18022. [PubMed] [Google Scholar]
- 9.Kunz, B. A., E. S. Henson, H. Roche, D. Ramotar, T. Nunoshiba, and B. Demple. 1994. Specificity of the mutator caused by deletion of yeast structural gene (APN1) for the major apurininc endonuclease. Proc. Natl. Acad. Sci. USA 91:8165-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V, Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Conerton, A. Danchin, et al. 1997. The complete genome sequence of the Gram positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Levin, J. D., A. W. Johnson, and B. Demple. 1988. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J. Biol. Chem. 263:8066-8071. [PubMed] [Google Scholar]
- 13.Mason, J. Y., S. Tremblay, and D. Ramotar. 1996. The Caenorhabditis elegans gene CEAPN1 encodes a homolog of Escherichia coli and yeast apurinic/apyrimidinic endonuclease. Gene 179:291-293. [DOI] [PubMed] [Google Scholar]
- 14.Milcarek, C., and B. Weiss. 1972. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease III. J. Mol. Biol. 68:303-318. [DOI] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y.
- 16.Nicholson, W. L., N. Munakata, G. Horneck, H. G. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.). Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
- 18.Ramotar, D. 1997. The apurinic-apyrimidinic endonuclease IV family of DNA repair enzymes. Biochem. Cell. Biol. 75:327-336. [PubMed] [Google Scholar]
- 19.Ramotar, D., J. Vadnais, J. Mason, and S. Tremblay. 1998. Schizosaccharomyces pombe apn1 encodes a homologue of the Escherichia coli endonuclease IV family of DNA repair proteins. Biochim. Biophys. Acta 1396:15-20. [DOI] [PubMed] [Google Scholar]
- 20.Ramotar, D., S. C. Poppoff, E. B. Gralla, and B. Demple. 1991. Cellular role of yeast Apn1 apurinic endonuclease /3′-diesterase:repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol. Cell. Biol. 11:4537-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebeil, R. Y., Y. Sun, L. Chooback, M. Pedraza-Reyes, and W. L. Nicholson. 1998. Spore photoproduct (SP) lyase from Bacillus subtilis spores is a novel iron-sulfur enzyme which shares features with class III anaerobic enzymes such as ribonucleotide reductase and pyruvate-formate lyases. J. Bacteriol. 18:4879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saporito, S. M., and R. P. Cunningham. 1988. Nucleotide sequence of the nfo gene of Escherichia coli K-12. J. Bacteriol. 170:5141-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schon, U., and W. Schumann. 1994. Construction of His6-tagging vectors allowing single-step purification of GroES and other polypeptides produced in B. subtilis. Gene 141:91-94. [DOI] [PubMed] [Google Scholar]
- 26.Setlow, B., and P. Setlow. 1994. Heat inactivation of Bacillus subtilis spores lacking small, acid-soluble spore proteins is accompanied by generation of abasic sites in spore DNA. J. Bacteriol. 176:2111-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow, P. 1988. Small acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu. Rev. Microbiol. 42:319-338. [DOI] [PubMed] [Google Scholar]
- 28.Setlow, P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29-54. [DOI] [PubMed] [Google Scholar]
- 29.Setlow, B., K. J. Tautvydas, and P. Setlow. 1998. Small acid-soluble spore proteins of the α/β-type do not protect the DNA in Bacillus subtilis spores against base alkylation. Appl. Environ. Microbiol. 64:1958-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shida, T., T. Ogawa, N. Ogasawara, and J. Sekiguchi. 1999. Characterization of Bacillus subtilis exoA protein: a multifunctional DNA-repair enzyme similar to Escherichia coli exonuclease III. Biosci. Biotechnol. Biochem. 9:1528-1534. [DOI] [PubMed] [Google Scholar]
- 31.Slieman, T. A., and W. L. Nicholson. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl. Environ. Microbiol. 66:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uriz-Estrada, N., J. M. Salas-Pacheco, R. E. Yasbin, and M. Pedraza-Reyes. 2003. Forespore-specific expression of Bacillus subtilis yqfS, which encodes type IV apurinic/apyrimidinic endonuclease, a component of the base excision repair pathway. J. Bacteriol. 185:340-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yajko, D. M., and B. Weiss. 1975. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc. Natl. Acad. Sci. USA 72:688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]