Abstract
The host response to Coxsackievirus infection is complex, including T lymphocytes, B lymphocytes, natural killer cells, and macrophages. Although Coxsackievirus infection induces expression of inducible nitric oxide synthase (NOS2; EC 1.14.13.39) in macrophages, the precise role of NOS2 in the host response to Coxsackievirus myocarditis has been unclear. We show, by using mice homozygous for a disrupted NOS2 allele, that Coxsackievirus replicates to higher titers in NOS2−/− mice, that the host lacking NOS2 clears virus more slowly than the wild-type host, and that myocarditis is much more severe in infected NOS2−/− mice. These data show that NOS2 is crucial for the host response to Coxsackievirus in the mouse.
Keywords: nitric oxide
Coxsackievirus B3 (CVB3) infection is the leading cause of viral myocarditis in humans. The natural history of CVB3 myocarditis is variable; the acute myocarditis can resolve completely or develop into a chronic myocarditis, but the host resistance factors that influence the outcome of the disease are not completely understood. Prior work in a murine model of CVB3 myocarditis has demonstrated that antibodies are a specific host defense against CVB3. Nonspecific host defenses against CVB3 include natural killer (NK) cells (1, 2), macrophages (3–8), and interferon signaling (2, 3, 9–17).
Interferon-γ can induce macrophages to express inducible nitric oxide synthase (NOS2; EC 1.14.13.39) and to produce nitric oxide (NO) (18, 19). NO is a nonspecific effector molecule with antiviral properties (reviewed in ref. 20). A wide variety of viruses can induce NOS2 (21–28), and NO can inhibit the replication of many viruses in vitro and in vivo (29–34). However, experiments have shown that in some animal models, NO improves the clinical course of viral infection (31, 33); in other models NO is detrimental (21, 25), and in still other models NO has no effect (26, 35). One possible explanation for this apparent contradiction is that conclusions have been made mainly with NOS inhibitors that are nonspecific, inhibiting not only NOS2 but also NOS1 (neuronal NOS) and NOS3 (endothelial NOS), and perhaps having effects unrelated to NOS isoforms.
We and others have shown that CVB3 infection induces NOS2 in the myocardium of infected mice (10, 36, 37), and we have shown that NO can inhibit CVB3 replication in vitro (38). However, a recent report shows that inhibition of NOS in mice infected with CVB3 has no effect on viral titer and reduces mortality (39). Furthermore, the NOS inhibitors used in the experiments of us and others are not specific inhibitors of the NOS2 isoform. Thus the exact role of NOS2 and NO in the host response to CVB3 viral infection has not been precisely determined. By using mice genetically deficient in the NOS2 locus (40), we tested the hypothesis that NOS2 is a critical antiviral effector against CVB3 infection.
MATERIALS AND METHODS
Animals.
Wild-type 129/Sv mice were purchased from The Jackson Laboratory. MF1/129 NOS2-disrupted gene mice were generated as previously described (40). MF1 mice were purchased from Harlan Laboratories (Haslett, MI). All mice, MF1, 129/Sv, MF1/129 wild type, and MF1/129 NOS2, were bred and raised in our own animal facilities and maintained with their mothers until they reached 3 weeks of age. Mice were infected at age 3 weeks and then housed in isolated rooms in microisolator cages.
Cell and Viral Culture.
CVB3 (Nancy strain) (generous gift of Charles J. Gauntt, University of Texas Health Science Center at San Antonio, TX) was grown and titered with HeLa cells. In brief, HeLa cells were cultured in growing medium [Eagle’s minimal essential medium (MEM), GIBCO] supplemented with 1% l-glutamine (100 mmol), 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. After infection, HeLa cells were fed with infecting medium (MEM with 1% fetal calf serum). Viral stocks were prepared by infecting an 80–90% confluent monolayer culture of HeLa cells at a multiplicity of infection of 10. Two days after incubation at 37°C, the cells were frozen and thawed three times, and the suspension was centrifuged. Viral supernatants were titered by the plaque assay method and stored at −80°C. The CVB3 stock titer was 2 × 109 plaque-forming units/ml (pfu/ml).
Plaque assays were used to measure the amount of virus in the tissue (Charles J. Gauntt). Serial dilutions of CVB3 were added to six well plates of 90% confluent HeLa cells in a volume of 200 μl for 1 h at 37°C with gentle rocking of the plate every 15 min. Equal volumes of 2% agar (Difco) and 2× infecting medium at 42°C were mixed, and then 2 ml of the mixture was added to each well. Plates were incubated for 2 days at 37°C, the wells were fixed with Carnoy’s solution (25% acetic acid/75% ethanol), the agar plugs were removed, the cells were stained with Coomassie reagent, and the plaques were counted.
Viral Infections.
Mice that were 3 weeks old were infected by intraperitoneal injection of 0.1 ml of solution containing 105 or 107 pfu/ml CVB3 in infecting medium. Controls received 0.1 ml of solution with no virus. Animals were killed at different times after infection, according to the institutional guidelines of The Johns Hopkins University. Blood and the entire hearts, livers, kidneys, pancreas, and spleens were collected, and one-half of each specimen was frozen in liquid nitrogen for viral culture, RNA, and protein isolation. The other portion was fixed in 10% formalin buffer and embedded in plastic matrix for histopathological examination.
Western Blot Analysis.
Organs were homogenized in lysis buffer [50 mM Tris/1 mM EDTA/1 mM EGTA/0.17 mg/ml phenylmethylsulfonyl fluoride/2 μg/ml of the protein inhibitors (leupeptin, pepstatin, antipain, and antitrypsin)]. The homogenate was briefly centrifuged and supernatants were collected. Proteins were quantified by Coomassie assay, and the samples were stored at −20°C. Tissue homogenate (200 μg) was fractionated by SDS/PAGE and then transferred to a nylon membrane, which was blocked and washed. NOS2 protein expression was detected by incubating with a specific anti-NOS antibody generated in our laboratory (36) at a dilution of 1:2,000. Blots were developed with a chemiluminescent system (enhanced luminol reagent, DuPont; according to the manufacturer’s instructions).
Reverse Transcription–PCR (RT-PCR) Amplification of CVB3 RNA.
For RT-PCR, total RNA was harvested from different organs by the guanidinium thiocyanate/phenol/chloroform method (41). For the template, 0.8 μg of RNA from each of three mice was mixed and used to amplify a 200-bp cDNA fragment in a RT assay, followed by a PCR (GeneAmp, Perkin–Elmer; according to the manufacturer’s instructions). For the amplification of the corresponding cDNA fragment, the following primers based on the CVB3 genomic sequence were selected: sense primer, 5′-ACTCTGCAGCGGAACCGACTA-3′ (position 526 in the CVB3 cDNA sequence); and antisense primer, 5′-GCTGTATTCAACTTAACAATG-3′ (position 738 in the CVB3 cDNA sequence). The amplification was performed with 25 cycles consisting of denaturation for 1 min at 94°C, primer annealing for 1 min at 60°C, and primer elongation for 1 min at 72°C. The cDNA molecules were electrophoresed in 1% agarose and visualized with ethidium bromide staining. To confirm the identity of the fragments amplified, PCR products were hybridized with the 1.5-kb CVB3 probe described above in a Southern blot. The amount of RNA in a Southern blot was quantitated with NIH Image analysis software on an Apple computer.
Histology.
Hearts were harvested from mice and embedded in plastic and stained with hematoxylin and eosin as described previously (36). The severity of myocarditis was graded on a scale of 1–5 as described previously (42).
Statistics.
Data were analyzed by using an ANOVA from a Microsoft Excel application run on a Power Macintosh 8500 from Apple Computer.
RESULTS
CVB3 Infection Induces NOS2 Expression.
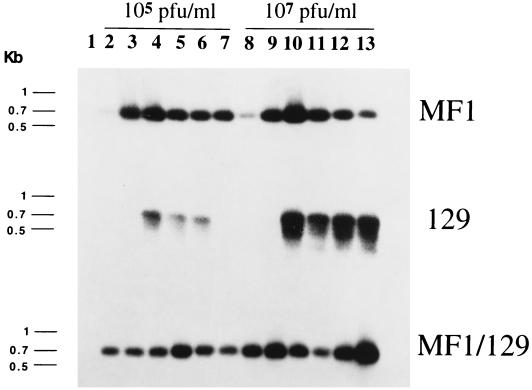
CVB3 infection induces NOS2 mRNA in wild-type mice. [The wild-type control mice included MF1 mice, 129 mice, and hybrid (MF1 × 129)F2 wild-type mice; the NOS2 null mice are (MF1,129) chimeras (40).] NOS2 RNA is absent from noninfected mice. Wild-type mice infected with 105 or 107 pfu of CVB3 express NOS2 mRNA in the myocardium (Fig. 1). NOS2 RNA is detected day 1 after infection and is then expressed at greater levels, peaking at day 5. Wild-type mice infected with a larger dose of CVB3 express more NOS2 mRNA than mice infected with lower doses. In contrast, NOS2 null mice did not express NOS2 mRNA (data not shown).
Figure 1.
CVB3 infection induces NOS2 mRNA in hearts. Wild-type mice (MF1, 129, and MF1/129 hybrid F2) were infected with 105 or 107 pfu of CVB3. Hearts were harvested from noninfected animals (lane 1) or from infected mice at times 1, 3, 5, 7, 10, and 15 days after infection (lanes 2–7 and lanes 8–13). NOS2 expression was analyzed by Southern analysis of RT-PCR from total RNA (n = 3 mice per RT-PCR).
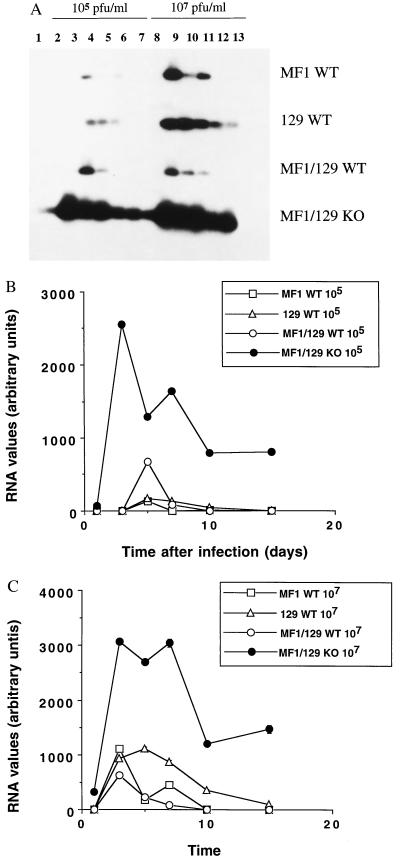
CVB3 Genome Replicates to Higher Levels in NOS2 Null Mice.
To explore the effect of NOS2 on the replication of CVB3, we performed RT-PCR of CVB3 RNA from hearts of infected mice. RNA was harvested from hearts of infected NOS2 null and wild-type mice and analyzed by RT-PCR with primers designed to amplify a 200-bp fragment from the CVB3 genome (see Materials and Methods). The CVB3 genome is present for longer periods of time at higher levels in the NOS2 null mice, as compared with the wild-type controls (Fig. 2). When a lower dose of 105 pfu is injected into the three wild-type control mice, CVB3 RNA appears at day 5 and disappears by day 10 (Fig. 2A). In contrast, CVB3 is detected on day 1 in infected NOS2 null mice infected with 105 pfu and persists through day 15. When a higher dose of 107 pfu is injected into the three strains of wild-type mice, CVB3 RNA appears at day 3 and disappears at day 10 (except in the 129 strain, in which RNA persists through day 15). CVB3 RNA in the NOS2 null mice is expressed at much higher levels than in controls and appears earlier, and at day 15 is still present at high levels.
Figure 2.
CVB3 RNA is present in hearts of infected mice. Wild-type mice (MF1, 129, and MF1/129 hybrid F2) and NOS2 null mice (MF1/129 KO) were infected with 105 or 107 pfu of CVB3. Hearts were harvested from noninfected animals (lane 1) or from infected mice at times 1, 3, 5, 7, 10, and 15 days after infection (lanes 2–7 and lanes 8–13). The CVB3 genome was analyzed by Southern analysis of RT-PCR from total RNA (A). The Southern blot was quantitated by densitometry for mice infected with (B) 105 pfu CVB3 or (C) 107 pfu CVB3. For B and C the differences between the NOS2 null mice and the MF1/129 wild-type mice at each time point have a P < 0.001 (n = 3 mice per RT-PCR ± SD although the error bars are not visible).
Much higher levels of CVB3 RNA are found in infected mice lacking NOS2 than in wild-type mice (Fig. 2 B and C). Quantitation of the CVB3 RNA by densitometry reveals that on day 7, the peak of viral RNA steady state levels in NOS2 null mice is 20-fold greater than in wild-type mice infected with 105 pfu. In mice infected with 107 pfu, the amount of CVB3 RNA is 40-fold greater than in controls.
Increased CVB3 Replication in Mice Lacking NOS2.
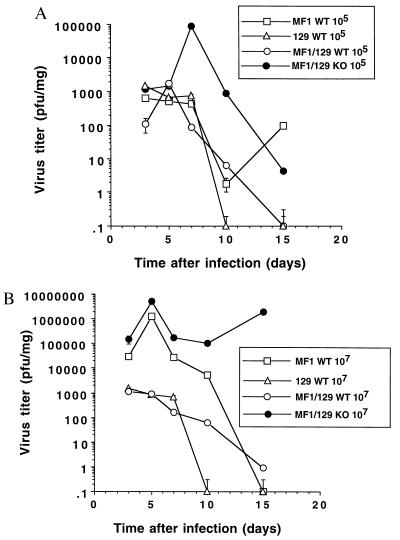
We then compared the kinetics of viral growth in wild-type mice and in mice lacking the NOS2 gene. Mice were infected with CVB3 at two different doses, 105 and 107 pfu i.p. The amount of virus in organs from infected mice was measured 0, 3, 5, 7, 10, and 15 days after infection (see Materials and Methods).
The greatest amount of CVB3 is found in the heart 5–7 days after infection (Fig. 3). However, CVB3 titers in the hearts of mice lacking the NOS2 gene are approximately 40-fold higher than titers in (MF1 × 129)F2 wild-type mice at an initial dose of 105 CVB3 and are approximately 500-fold higher at an initial dose of 107 (Fig. 3). Compared with the MF1 wild type, titers in the NOS2 null mice are still greater, although not as much as the (MF1 × 129)F2 wild-type mice (Fig. 3).
Figure 3.
CVB3 infectious particles are present in hearts of infected mice. Wild-type mice (MF1, 129, and MF1/129 hybrid F2) and NOS2 null mice (MF1/129 KO) were infected with (A) 105 or (B) 107 pfu CVB3. Hearts were harvested from noninfected animals or from infected mice at times 1, 3, 5, 7, 10, and 15 days after infection. The amount of CVB3 pfu/mg of tissue was determined by the plaque assay. For A the differences between the NOS2 null mice and the MF1/129 wild-type mice at days 7, 10, and 15 have a P < 0.01. For B the differences between the NOS2 null mice and all other mice at all time points have a P < 0.001 (n = triplicate measurements from each of 3 mice ± SD).
Initially, hearts of the NOS2 null and the wild-type mice start out with similar titers of CVB3 on day 3. However, mice with the NOS2 gene clear the viral load from the hearts more rapidly than those lacking the NOS2 gene. These differences are most pronounced when mice are infected with a higher titer of CVB3 at 107 pfu. Virus is eliminated from the wild-type mice but persists at high titers in the NOS2 null mice (Fig. 3B).
Titers of CVB3 are higher in other organs of NOS2 null mice as well, approximately 10–100-fold greater than wild-type (MF1 × 129)F2 mice (Table 1). The most significant differences between NOS2 null and wild-type mice are in the titers found in the heart and blood. (For both wild-type and NOS2 null mice, CVB3 titers peak earlier in the other organs and blood compared with the heart; maximal titers in most organs occur at day 3, but in the heart at day 5.)
Table 1.
Viral titers in infected wild-type (WT) and NOS2 null (KO) mice
| Organ | 105 pfu
|
107 pfu
|
Peak day | ||
|---|---|---|---|---|---|
| WT | KO | WT | KO | ||
| Blood | 4 × 103 | 8 × 105 | 4 × 104 | 1 × 106 | 3 |
| Heart | 2 × 103 | 9 × 104 | 9 × 102 | 5 × 106 | 5, 7 |
| Liver | 2 × 102 | 4 × 103 | 2 × 102 | 3 × 103 | 3 |
| Kidney | 2 × 102 | 7 × 102 | 4 × 102 | 2 × 101 | 3 |
| Pancreas | 5 × 105 | 2 × 106 | 6 × 105 | 7 × 105 | 3 |
| Spleen | 2 × 102 | 4 × 103 | 8 × 102 | 2 × 103 | 3 |
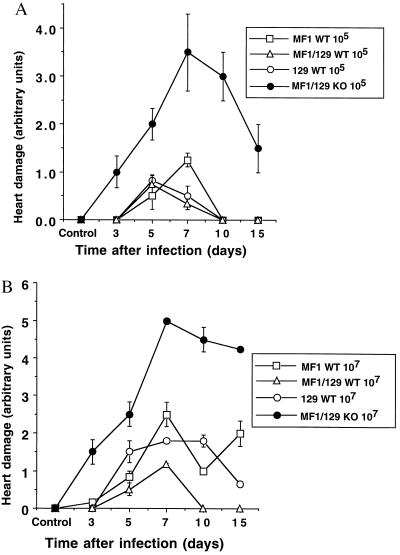
Increased Myocarditis in NOS2 Null Mice.
The severity of heart damage was quantified by histological grading of sections with a scale previously described (42). The hearts of infected mice developed myocarditis in a time- and dose-dependent manner (Fig. 4). Heart damage in all strains of mice peaks 5–7 days after infection with 105 pfu CVB3. However, myocarditis begins earlier in infected NOS2 null mice, is more severe, and resolves more slowly (Fig. 4A). Similar but more pronounced results are observed in mice infected with 107 pfu of CVB3 (Fig. 4B).
Figure 4.
Severity of myocarditis in infected mice. Wild-type mice (MF1, 129, and MF1/129 hybrid F2) and NOS2 null mice (MF1/129 KO) were infected with (A) 105 or (B) 107 pfu of CVB3. Hearts were harvested from noninfected animals or from infected mice at times 1, 3, 5, 7, 10, and 15 days after infection. Tissue sections were stained with hematoxylin and eosin, and the severity of the myocarditis was graded. For A the differences between the NOS2 null mice and all other wild-type controls for day 5 are P < 0.05 and for days 7–15 are P < 0.001. For B the differences between the NOS2 null mice and all other wild-type controls are P < 0.005. (n = 3 sections from each of 3 mice ± SD.)
As has been reported by others, the histological appearance of CVB3 myocarditis follows a typical pattern in wild-type mice. From days 0 to 5, scattered, small foci of inflammation arise with cells histologically consistent with macrophages and lymphocytes, along with myocyte necrosis in all mice. By days 6–10, more abundant and larger foci are present, with myocyte necrosis; areas of calcification are observed only in MF1 wild-type mice. After day 10, the myocarditis resolved in wild-type mice infected with low doses of CVB3, with some minor regions of fibrosis.
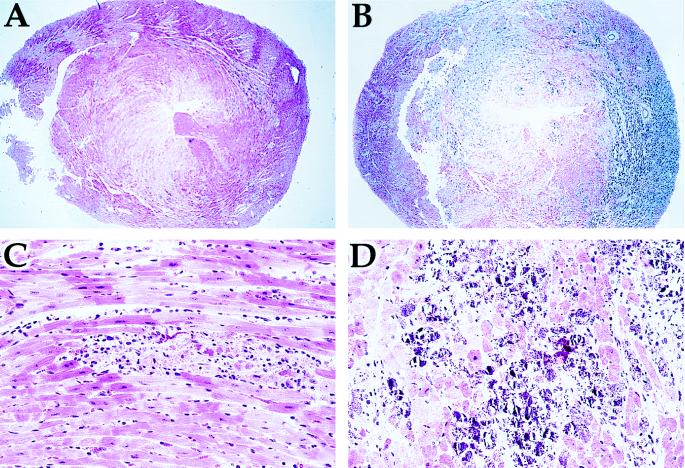
In contrast, the histological appearance of CVB3 myocarditis in NOS2 null mice is much more severe after day 10 than in wild-type mice. There is more degeneration of myocytes and more extensive dystrophic calcification with extracellular deposits of calcium (Fig. 5). This pattern is also seen in CVB3 mice lacking NK cells (2). The extensive damage does not resolve 14 days after infection in the NOS2 null mice, as it does in the wild-type controls.
Figure 5.
Myocarditis in wild-type and NOS2 null mice. Wild-type mice (A and C) and NOS2 null mice (B and D) were infected with 107 pfu of CVB3. Hearts were harvested and tissue sections were stained with hematoxylin and eosin. (A and B, ×40; C and D, ×240.)
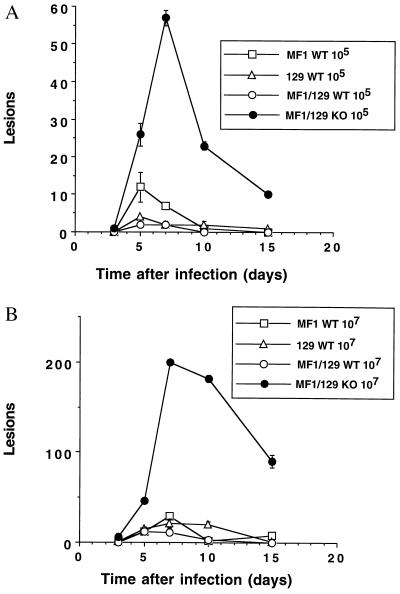
Increased Inflammatory Lesions in NOS2 Null Mice.
Lack of NOS2 is associated with an increase in the number of focal lesions in hearts of infected mice (Fig. 6). Mice infected with 105 or 107 pfu i.p. were sacrificed on days 3, 5, 7, 10, and 15, and the number of inflammatory lesions was counted. Wild-type mice infected with 105 pfu of CVB3 have less than 15 foci, whereas NOS2 null mice have 57 foci on day 7. Wild-type mice infected with 107 pfu of CVB3 have less than 30 foci, whereas NOS2 mice had greater than 200 foci on day 7.
Figure 6.
Lesions in hearts of infected mice. Wild-type mice (MF1, 129, and MF1/129 hybrid F2) and NOS2 null mice (MF1/129 KO) were infected with (A) 105 or (B) 107 pfu of CVB3. Hearts were harvested from noninfected animals or from infected mice. Tissue sections were stained with hematoxylin and eosin, and the number of lesions was counted. The difference between the NOS2 null mice and all controls is P < 0.001 (n = 3 sections from each of 3 mice ± SD).
DISCUSSION
The data presented here demonstrate that NO (or a reactive oxygen species derived from NO) plays an important role in the immunologic response to Coxsackievirus infection. In NOS2 null mice, CVB3 replicates to higher titers in all organs, particularly the blood and the heart, and causes more damage than in wild-type mice. Prior work by us and others showed that CVB3 induces NOS2 and that nonspecific inhibition of all NOS isoforms increased the mortality of infected mice (10, 36–38). Use of mice lacking the NOS2 gene permits a more specific analysis of the role of NOS2 in isolation from the other NOS isoforms and eliminates the possibility that effects of arginine analogs may influence viral replication by mechanisms other than NO production.
Our study focused on the acute phase of viral myocarditis, which takes place within the first 10–15 days after infection. CVB3 was virtually eliminated from wild-type mice 10 days after infection. In contrast, CVB3 appears earlier and persists later in hearts of animals lacking NOS2, compared with CVB3 in wild-type mice. CVB3 RNA was detected at day 1 in NOS2 null mice as compared with day 5 in wild-type mice (Fig. 2). These data support the idea that NOS2 is a component of an early host response to infection.
The CVB3 content in blood is 100-fold greater in mice lacking NOS2 than in mice with NOS2. Perhaps this inability to reduce the circulating viral load permits more virus to enter other organs of the infected host, increasing the total viral burden. For example, the NOS2 null mice had between 40- and 1000-fold more virus in their hearts than the wild-type controls. These data suggest that NO (or a reactive oxygen species derived from NO) inhibits viral replication in vivo, confirming previous work that demonstrates NO inhibition of CVB3 replication in vitro (10, 36–38). A reduction in CVB3 RNA in mice with the NOS2 gene also confirms in vitro studies showing that NO reduces replication of the Coxsackieviral RNA genome (38). However, this work contradicts the results of others that show that nitroarginine has no effect on viral titer in vivo and actually increases survival of infected mice (39). Perhaps nitroarginine has effects on other NOS isoforms or other enzymatic pathways.
It has been suggested that NO can be either beneficial or harmful to the host, depending on the amount of NO produced. In our study, mice lacking NOS2 had a much more severe myocarditis than mice expressing NOS2. Mice lacking NOS2 had extensive damage to the myocardium, including dystrophic calcification that was not seen in wild-type mice. Extensive calcification is also found in mice lacking NK cells in which CVB3 also replicates to high titers, suggesting that calcification is the end result of severe viral damage (2). [However, in contrast to mice lacking NK cells, mice lacking NOS2 have greater numbers of heart lesions compared with normal mice (2).] Thus NO (or a reactive oxygen species derived from NO) protects the myocardium against damage from CVB3 infection by inhibiting viral replication.
The replication of CVB3 depends in part on the genetics of the host (43, 44). Substantial variation in viral titer is observed in infections of the same virus in different strains of mice in our experiments (Fig. 3). Because the NOS2 null (NOS2−/−) mouse is a hybrid of the MF1 and the 129 strain, we used the following mice as controls: wild-type MF1, wild-type 129, and also wild-type (MF1,129)F2 hybrids (45). Other possible strains that could be used as controls for the homozygous NOS2−/− (MF1,129) mice include progeny of matings between heterozygote NOS2wt/− (MF1,129) mice. In spite of the variation in viral growth in different strains, the increase in viral titer in mice lacking NOS2 is significant compared with the titer in all strains of wild-type mice.
The host’s cellular response to CVB3 infection is complex, depending on lymphocytes, NK cells, and macrophages. One potential role of macrophages is to migrate to foci of CVB3 replication and produce NO. Previously we demonstrated the expression of NOS2 in macrophages infiltrating into the hearts of CVB3-infected mice (36). Although macrophages might be the major source of NO in the infected heart, cardiac myocytes and lymphocytes can also produce NO from NOS2 in different experimental systems (46–49). Thus, many cells in the inflamed heart could conceivably produce NO from NOS2, thereby reducing the replication of CVB3.
The host response to CVB3 infection also depends on interferon-γ, which can induce intracellular signals via interferon regulatory factor 1 (IRF-1) (50). Mice lacking IRF-1 are highly susceptible to CVB3 infection (51). NOS2 is not induced in IRF-1 null mice, because IRF-1 is a critical component in the transcriptional activation of NOS2 (52, 53). However, IRF-1 may activate other antiviral responses in addition to NOS2. For example, IRF-1 regulates NK function (54), regulates expression of major histocompatibility class I genes and (2′-5′)oligoadenylate syntheses (55), and affects T cell development resulting in a decrease in CD8+ T cells (56, 57). Thus the diminished resistance of IRF-1 null mice to viral infection may be because of lack of induction of a variety of genes, including NOS2.
Although CVB3 replicated to much higher titers in mice lacking NOS2 than in wild-type mice, eventually the virus was cleared from the knock-out mice. These data support the hypothesis that NOS2 is part of a rapidly activated but nonspecific component of the immune system, which inhibits the growth of a wide variety of pathogens until slower but more specific host resistance pathways are activated (58). Data presented here thus suggest that NOS2 protects the host in murine Coxsackievirus myocarditis.
Acknowledgments
This work was supported by a grant from the Spanish Ministry of Education and Culture (to M.S.), by Grants R01 HL53615 and P50 HL52315 from the National Institutes of Health (to C.Z. and C.J.L.), by a grant from the Cora and John H. Davis Foundation (to C.J.L.), and by a grant from the Bernard Bernard Foundation (to C.J.L.).
ABBREVIATIONS
- CVB3
Coxsackievirus strain B type 3
- NK
natural killer
- NOS2
inducible nitric oxide synthase
- NOS
nitric-oxide synthase
- pfu
plaque-forming units
- RT
reverse transcription
- IRF-1
interferon regulatory factor 1
References
- 1.Godeny E K, Gauntt C J. J Immunol. 1987;139:913–918. [PubMed] [Google Scholar]
- 2.Godeny E K, Gauntt C J. J Immunol. 1986;137:1695–1702. [PubMed] [Google Scholar]
- 3.Huber S A, Mortensen A, Moulton G. J Virol. 1996;70:3039–3044. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook D N. J Leukocyte Biol. 1996;59:61–66. doi: 10.1002/jlb.59.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Bendinelli M, Matteucci D, Toniolo A, Patane A M, Pistillo M P. J Infect Dis. 1982;146:797–805. doi: 10.1093/infdis/146.6.797. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff J F. J Immunol. 1979;123:31–35. [PubMed] [Google Scholar]
- 7.Godeny E K, Gauntt C J. Am J Pathol. 1987;129:267–276. [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff J F, Woodruff J J. J Immunol. 1974;113:1726–1730. [PubMed] [Google Scholar]
- 9.Heim A, Stille-Seigener M, Pring-Akerblom P, Grumbach I, Brehm C, Kreuzer H, Figulla H R. J Interferon Cytokine Res. 1996;16:283–287. doi: 10.1089/jir.1996.16.283. [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka Y, Kishimoto C, Takada H, Nakamura M, Kurokawa M, Ochiai H, Shiraki K. J Am Coll Cardiol. 1996;28:1610–1615. doi: 10.1016/s0735-1097(96)00372-5. [DOI] [PubMed] [Google Scholar]
- 11.Huber S A, Pfaeffle B. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada I, Matsumori A, Matoba Y, Tominaga M, Yamada T, Kawai C. J Lab Clin Med. 1992;120:569–573. [PubMed] [Google Scholar]
- 13.Heim A, Canu A, Kirschner P, Simon T, Mall G, Hofschneider P H, Kandolf R. J Infect Dis. 1992;166:958–965. doi: 10.1093/infdis/166.5.985. [DOI] [PubMed] [Google Scholar]
- 14.Capobianchi M R, Matteucci D, Giovannetti A, Soldaini E, Bendinelli M, Stanton J G, Dianzani F. Viral Immunol. 1991;4:103–110. doi: 10.1089/vim.1991.4.103. [DOI] [PubMed] [Google Scholar]
- 15.Langford M P, Kadi R M, Ganley J P, Yin-Murphy M. Intervirology. 1988;29:320–327. doi: 10.1159/000150062. [DOI] [PubMed] [Google Scholar]
- 16.Matsumori A, Tomioka N, Kawai C. Am Heart J. 1988;115:1229–1232. doi: 10.1016/0002-8703(88)90013-0. [DOI] [PubMed] [Google Scholar]
- 17.Kandolf R, Canu A, Hofschneider P H. J Mol Cell Cardiol. 1985;17:167–181. doi: 10.1016/s0022-2828(85)80019-5. [DOI] [PubMed] [Google Scholar]
- 18.Ding A H, Nathan C F, Stuehr D J. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 19.Stuehr D J, Marletta M A. J Immunol. 1987;139:518–525. [PubMed] [Google Scholar]
- 20.Mannick J B. Res Immunol. 1995;146:693–697. doi: 10.1016/0923-2494(96)84920-0. [DOI] [PubMed] [Google Scholar]
- 21.Kreil T R, Eibl M M. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 22.Melkova Z, Esteban M. J Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 23.Bukrinsky M I, Nottet H S, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R H, Jacob J R, Tennant B C, Hotchkiss J H. Cancer Res. 1992;52:4139–4143. [PubMed] [Google Scholar]
- 25.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolph M S, Ramshaw I A, Rockett K A, Ruby J, Cowden W B. Virology. 1996;217:470–477. doi: 10.1006/viro.1996.0141. [DOI] [PubMed] [Google Scholar]
- 27.Hooper D C, Ohnishi S T, Kean R, Numagami Y, Dietzschold B, Koprowski H. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Dam A M, Bauer J, Man-A-Hing W K, Marquette C, Tilders F J, Berkenbosch F. J Neurosci Res. 1995;40:251–260. doi: 10.1002/jnr.490400214. [DOI] [PubMed] [Google Scholar]
- 29.Croen K D. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 31.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi Z, Reiss C S. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo M A. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu T, Bi Z, Reiss C S. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 35.Butz E A, Hostager B S, Southern P J. Microb Pathog. 1994;16:283–295. doi: 10.1006/mpat.1994.1029. [DOI] [PubMed] [Google Scholar]
- 36.Lowenstein C J, Hill S L, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose N R, Herskowitz A. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikami S, Kawashima S, Kanazawa K, Hirata K, Katayama Y, Hotta H, Hayashi Y, Ito H, Yokoyama M. Biochem Biophys Res Commun. 1996;220:983–989. doi: 10.1006/bbrc.1996.0519. [DOI] [PubMed] [Google Scholar]
- 38.Zaragoza C, Ocampo C J, Saura M, McMillan A, Lowenstein C J. J Clin Invest. 1997;100:1760–1767. doi: 10.1172/JCI119702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikami S, Kawahima S, Kanazawa K, Hirata K I, Hotta H, Hayashi Y, Itoh H, Yokoyama M. Circ Res. 1997;81:504–511. doi: 10.1161/01.res.81.4.504. [DOI] [PubMed] [Google Scholar]
- 40.Wei X Q, Charles I G, Smith A, Ure J, Feng C J, Huang F P, Xu D M, Muller W, Moncada S, Liew F Y. Nature (London) 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 41.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 42.Khatib R, Chason J L, Silberberg B K, Lerner A M. J Infect Dis. 1980;141:394–403. doi: 10.1093/infdis/141.3.394. [DOI] [PubMed] [Google Scholar]
- 43.Wolfgram L J, Beisel K W, Herskowitz A, Rose N R. J Immunol. 1986;136:1846–1852. [PubMed] [Google Scholar]
- 44.Herskowitz A, Beisel K W, Wolfgram L J, Rose N R. Hum Pathol. 1985;16:671–673. doi: 10.1016/s0046-8177(85)80149-0. [DOI] [PubMed] [Google Scholar]
- 45.Davisson M T. JAX Notes. 1996;464:1–4. [Google Scholar]
- 46.Yang X, Chowdhury N, Cai B, Brett J, Marboe C, Sciacca R R, Michler R E, Cannon P J. J Clin Invest. 1994;94:714–721. doi: 10.1172/JCI117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirk S J, Regan M C, Barbul A. Biochem Biophys Res Commun. 1990;173:660–665. doi: 10.1016/s0006-291x(05)80086-5. [DOI] [PubMed] [Google Scholar]
- 48.Keller R, Keist R, Erb P, Aebischer T, De Libero G, Balzer M, Groscurth P, Keller H U. Cell Immunol. 1990;131:398–403. doi: 10.1016/0008-8749(90)90264-r. [DOI] [PubMed] [Google Scholar]
- 49.Mannick J B, Asano K, Izumi K, Kieff E, Stamler J S. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 51.Liu, P., Penninger, J., Aitken, K., Sole, M. & Mak, T. (1995) Eur. Heart J. 16, Suppl., 25–27. [DOI] [PubMed]
- 52.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh S I, Kimura T, Green S J. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 53.Martin E, Nathan C, Xie Q W. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan G S, Mittrucker H W, Kagi D, Matsuyama T, Mak T W. J Exp Med. 1996;184:2043–2048. doi: 10.1084/jem.184.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruffner H, Reis L F, Naf D, Weissmann C. Proc Natl Acad Sci USA. 1993;90:11503–11507. doi: 10.1073/pnas.90.24.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundig T M, Amakawa R, Kishihara K, Wakeham A. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 57.Reis L F, Ruffner H, Stark G, Aguet M, Weissmann C. EMBO J. 1994;13:4798–4806. doi: 10.1002/j.1460-2075.1994.tb06805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathan C. Cell. 1995;82:873s–876s. [Google Scholar]