Abstract
Infectious human immunodeficiency virus type 1 (HIV-1) is difficult to detect in female genital secretions by standard virus culture techniques. To improve detection of cell-free HIV-1 in female genital secretions, we adapted a short-term assay that uses the multinuclear-activation galactosidase indicator (MAGI) assay. When vaginal lavages from HIV-1-infected women were tested with the adapted MAGI assay, 25 (64%) of 39 lavages with detectable, cell-free HIV-1 RNA were shown to have infectious virus. No infectious virus was found in 10 vaginal lavages from HIV-1-infected women with undetectable vaginal viral loads. Significantly (P < 0.01) more lavages from HIV-1-infected women tested positive for infectious virus by the MAGI assay than by standard peripheral blood mononuclear cell (PBMC) coculture, which detected infectious virus in only 6 (17%) of 35 vaginal lavages. Lavages with viral loads of >10,000 copies per lavage yielded significantly (P < 0.01) more positive cultures than those with <10,000 copies by using the MAGI assay. Detection of infectious HIV-1 in vaginal lavages was not associated with the presence of genital tract infections or CD4+-T-cell counts. However, although the results were not significant (P = 0.08), the MAGI assay detected infectious virus from more vaginal lavages at a vaginal pH of ≥4.5 than at a pH of <4.5. These results indicate that the MAGI assay is more sensitive than PBMC culture methods for detecting infectious virus in female genital secretions. Accurate measurements of infectious virus in genital secretions will improve studies that evaluate sexual transmission of HIV-1.
The majority of human immunodeficiency virus type 1 (HIV-1) infections worldwide continue to occur through heterosexual transmission (7, 27). Factors that influence the amount of HIV-1 in vaginal secretions are important risk determinants for this mode of transmission. It is reported that the amount of HIV-1 RNA in vaginal secretions is correlated with that in plasma (8, 13, 17, 20) and that an increase in plasma viral load in infected persons is associated with an increase in HIV-1 transmission to their sex partners (15, 30, 35). In addition, localized cervical inflammation and ulceration, without affecting the plasma viral load, can increase HIV-1 shedding in the female genital tract and thus influence HIV-1 transmission (3, 22, 24, 47). Altogether, these studies suggest that increased HIV-1 shedding in mucosal secretions would lead to increased transmission; however, little information is available regarding the presence of infectious virus in cervicovaginal secretions.
Several studies have attempted to evaluate infectious HIV-1 in genital secretions by using virus culture methods that are highly successful for propagating virus from an infected person's peripheral blood mononuclear cells (PBMCs). However, use of these methods to culture infectious virus from female genital tract cells has had an overall low success rate (∼30%) (18, 29, 44-46). Moreover, recovery of infectious cell-free virus from genital secretions is reported to be even lower (11 to 20%) (18, 36). For example, in one study, isolation of HIV-1 from genital ulcers was reported in only 4 (11%) of 36 samples (23). In addition, extensive microbial contamination of these samples was observed, which often occurs when genital tract samples are cultured for extended periods (23). Collectively, these studies indicate that standard HIV-1 culture techniques are not adequate for detecting virus in genital secretions.
To provide a more effective method for detecting infectious virus in female genital secretions, we utilized a short-term culture method: the multinuclear-activation galactosidase indicator (MAGI) assay (21, 43). This assay uses a U373 human glioblastoma cell line (R5-MAGI) that stably expresses both the HIV-1 receptor CD4 and the coreceptor CCR5 on the cell surface. Exposure to infectious virus and subsequent infection of these cells result in the expression of a stable, integrated bacterial β-galactosidase (β-Gal) gene under transcriptional control of an HIV-1 long terminal repeat (LTR). The expressed cellular β-Gal enzyme is then detected colorimetrically.
In the present study, we compared the MAGI assay with a standard PBMC virus culture technique to detect infectious virus in female genital secretions. In addition, we investigated whether detection of infectious virus in the female genital tract was associated with peripheral blood CD4+-T-cell counts, genital tract infections (GTIs), and vaginal pH. Improved methods that better characterize infectious HIV-1 in genital secretions will allow further analysis of the parameters that may influence HIV-1 shedding and transmission, as well as the measures designed to prevent sexual transmission of HIV-1.
MATERIALS AND METHODS
Study population.
The present study includes data from examinations of women enrolled in the Emory Vaginal Ecology Study of HIV infection (17). HIV-1-infected women were eligible for enrollment if they were 18 to 49 years of age, had had a normal Pap smear within the previous 12 months, were expected to live at least 1 year, and either were not on antiretroviral therapy (ART) or had taken the same ART for at least 3 months prior to enrollment. Exclusion criteria were as previously described (17). Participants were requested to refrain from vaginal intercourse and the use of intravaginal medications for 72 h before their examinations.
Specimens.
All collected specimens were taken to the laboratory within 3 h for immediate processing. Methods for processing samples, assaying for blood contamination, and extracting RNA were as previously described (17). At each clinical exam, vaginal secretions were tested for the presence of seminal fluid (9, 17). Vaginal pH was measured by inserting pH paper into the vaginal tract. Subsequently, lavage samples were obtained by introducing 10 ml of phosphate-buffered saline (PBS) into the vagina and collecting the pooled fluid in the posterior vaginal fornix, while carefully avoiding the cervix. The total volume of recovered lavage (PBS and secretions) was recorded for each patient. Endocervical swabs were obtained to test for Neisseria gonorrhoeae and Chlamydia trachomatis. Infection with herpes simplex virus (HSV) was determined by using a standard culture technique. Wet mounts were examined to detect Trichomonas vaginalis. HIV-1 RNA was quantified in plasma and vaginal lavage samples by the use of a quantitative, competitive, reverse transcriptase PCR assay as described previously (16). HIV-1 viral load is reported as copies per milliliter in plasma and total copies per vaginal lavage (RNA copies/milliliter × total lavage volume). The limit of detection was 100 copies/ml (1,000 copies per total lavage).
MAGI cell assay.
The U373-R5-MAGI cells (43)—in DMEM medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 100 mM l-glutamine—were plated in a 24-well plate at 6 × 104 cells/well. After the cells were cultured overnight, the medium was removed, and 150 μl of filtered (0.45-μm-pore-size filter) vaginal lavage or filtered PBS was added to each well in duplicate. In some experiments, a 48-well plate seeded with 3 × 104 cells/well was used, and 75 μl of filtered vaginal lavage or filtered PBS was added to each well in duplicate. DEAE-dextran was added to each well at a final concentration of 20 μg/ml. R5-MAGI cells were incubated with lavage or PBS controls for 2 h at 37°C in 7% CO2. All plates were rocked every 45 min during the 2-h incubation. Medium was then added to each well of the plate: 1 ml of medium/well for 24-well plates or 0.5 ml of medium/well for 48-well plates.
After culture for 40 to 48 h at 37°C in 7% CO2, the medium was removed, cells were fixed with 1% formaldehyde-0.25% glutaraldehyde in PBS for 5 min and then washed twice with PBS. HIV-1-infected R5-MAGI cells containing β-Gal were stained blue after incubation with 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml containing 0.4 mM potassium ferricyanide, 0.4 mM potassium ferrocyanide, and 2 mM MgCl2 for 50 min at 37°C (21, 43). The X-Gal solution was removed, and the cells were washed twice with PBS.
Blue plaques were counted with an inverted light microscope. Single cells, groups of cells, and syncytia colored blue were counted as one infectious plaque. The number of plaques reported for an HIV-1-positive lavage culture was adjusted by subtracting the number of plaques in the negative control cultures for that experiment. The mean number of background plaques in negative control cultures was 5 ± 2.3 (mean ± the standard deviation) (see Table 3). More than four plaques above the negative controls was considered significant based on a Poisson model. As a positive control, HIV-1Ba-L (ABI, Columbia, Md.) was diluted in PBS and assayed on R5-MAGI cells to determine the amount of virus necessary to yield 30 to 50 plaques/well. In experiments with the 24-well format, 30 50% tissue culture infective dose(s) (TCID50) of HIV-1Ba-L generated 40 to 50 plaques/well. In experiments with the 48-well format, 75 TCID50 of a second HIV-1Ba-L stock generated 30 to 40 plaques/well. The negative control for each experiment was 150 μl (24-well plates) or 75 μl (48-well plates) of PBS. The R5-MAGI cells were resistant to infection with HIV-1LAI, a CXCR4-using virus.
TABLE 3.
Characteristics of the vaginal lavage samples from HIV-1-infected women
| Groupa | Patient IDb | Vaginal viral load (no. of copies/lavage) | No. of MAGI plaquesc | Cocultured | pH | GTIe |
|---|---|---|---|---|---|---|
| Low viral load | 16 | <1,000 | 3 | ND | 5.0 | |
| 21 | <1,000 | 2 | ND | 4.7 | ||
| 34a | <1,000 | 1 | ND | 5.8 | Yeast | |
| 40a | <1,000 | 4 | ND | 4.0 | Yeast | |
| 49 | <1,000 | 3 | ND | 4.0 | Yeast | |
| 77 | <1,000 | 1 | ND | 4.7 | ||
| 91 | <1,000 | 3 | ND | 4.0 | ||
| 1000 | <1,000 | 3 | ND | 4.0 | ||
| 1015a | <1,000 | 0 | ND | 5.0 | ||
| 1024 | <1,000 | 4 | ND | 5.0 | ||
| 56 | 1,800 | 5 | − | 5.0 | ||
| 40b | 2,000 | 4 | − | 4.0 | Yeast | |
| 42 | 4,300 | 0 | − | 5.0 | ||
| 34b | 4,800 | 16 | − | 6.0 | Yeast, BV | |
| 41 | 5,400 | 0 | − | 5.0 | HSV | |
| 74 | 5,400 | 6 | − | 6.0 | ||
| 1 | 9,100 | 7 | − | 5.0 | Yeast | |
| Medium viral load | 22 | 11,000 | 11 | − | 6.0 | |
| 64 | 15,000 | 5 | − | 5.0 | BV | |
| 50 | 16,000 | 21 | − | 6.0 | Yeast, BV | |
| 36 | 22,000 | 9 | − | 5.0 | ||
| 26 | 23,000 | 3 | − | 5.0 | Tric, BV | |
| 28 | 24,000 | 10 | − | 5.0 | ||
| 78 | 36,000 | 11 | − | 5.0 | Yeast | |
| 11a | 44,000 | 7 | − | 5.0 | Yeast | |
| 1021 | 47,000 | 3 | ND* | 4.1 | ||
| 1007 | 50,000 | 1 | − | 6.0 | Tric | |
| 1023 | 53,000 | 1 | − | 3.6 | Yeast | |
| 20 | 56,000 | 9 | ND* | 5.0 | Yeast, BV | |
| 13 | 57,000 | 18 | − | 6.0 | Tric | |
| 84 | 64,000 | 4 | − | 5.8 | Yeast, Tric | |
| 1008 | 71,000 | 0 | ND* | 5.0 | ||
| 14 | 88,000 | 27 | ++ | 7.0 | Yeast | |
| High viral load | 61 | 100,000 | 36 | − | 4.0 | Yeast |
| 7 | 110,000 | 9 | − | 4.0 | ||
| 11b | 110,000 | 16 | − | 4.8 | ||
| 1033 | 160,000 | 0 | − | 4.7 | ||
| 1034 | 260,000 | 19 | − | 5.6 | ||
| 71 | 260,000 | 0 | − | 5.0 | Yeast | |
| 55 | 270,000 | 54 | +++ | 5.0 | ||
| 75 | 270,000 | 2 | ND* | 4.0 | Yeast | |
| 1015b | 280,000 | 3 | ++ | 3.6 | ||
| 45 | 410,000 | 8 | ++ | 4.0 | ||
| 67 | 410,000 | 17 | +++ | 5.0 | Yeast | |
| 1013 | 430,000 | 0 | − | 4.7 | ||
| 63 | 430,000 | 9 | ++ | 6.0 | HSV | |
| 18 | 690,000 | 5 | − | 4.0 | ||
| 1030 | 4,300,000 | 6 | − | 5.0 | ||
| 3 | 12,000,000 | 18 | − | 5.0 | Yeast |
Groups were based on vaginal viral load level: low (<104 total copies, n = 17), medium (>104 to <105 total copies, n = 16), and high (>105 total copies, n = 16).
ID, identification number. Patients 11, 34, 40 and 1015 each had two lavage samples that were collected on different dates.
Number MAGI plaques above the background level. The data are expressed as the average of duplicate wells. Plaque numbers significantly above background (≥5) are indicated in boldface.
p24 detection in cultures at day 21 (in picograms/milliliter): −, <15; +, ≥15 to <100; ++, ≥100 to <1,000; and +++, ≥1,000. ND, not done; ND*, not done because of contamination.
Type of GTI documented on the day of sample acquisition, including infection with T. vaginalis (Tric), infection with HSV (HSV), bacterial vaginosis (BV), and yeast infection (yeast).
Effect of exogenous proinflammatory cytokines and cathepsin D on MAGI plaque formation.
The effect of proinflammatory cytokines on the formation of MAGI plaques was tested in the absence of virus. Tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), or IL-6 in PBS was used in MAGI cultures at or above their reported concentrations in vaginal lavage samples from HIV-1-infected women (TNF-α [6.25 to 100 pg/ml], IL-1β [0.1 to 10 ng/ml], and IL-6 [0.01 to 1.0 ng/ml]). To determine the effect of combined cytokines on the formation of plaques, IL-1β and TNF-α were diluted together in PBS at high (10 ng/ml and 100 pg/ml, respectively) and medium (1 ng/ml and 25 pg/ml, respectively) concentrations and added to MAGI cultures. Cytokines were obtained from R&D Systems (Minneapolis, Minn.). HIV-1Ba-L was pretreated with cathepsin D (1.3 to 20 μg/ml) for 30 min at 37°C before being added to the MAGI cultures. Cathepsin D was obtained from Calbiochem-Novabiochem Corp. (La Jolla, Calif.).
Standard PBMC coculture for detection of infectious HIV-1.
Normal human PBMCs were obtained by leukopheresis from HIV-1-negative blood donors. PBMCs were further purified in a Ficoll-Hypaque gradient and cryopreserved in gas-phase liquid nitrogen until use. PBMCs were enriched for CD4+ T cells by removing CD8+ T cells with anti-CD8-conjugated magnetic beads (Dynal, Oslo, Norway), according to the manufacturer's instructions. These cells were activated by culture in RPMI 1640 medium containing 0.5 μg of phytohemagglutinin-P (Difco, Detroit, Mich.)/ml, 10% FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 100 mM l-glutamine at 37°C in 7% CO2 for 2 to 3 days before use. After activation, PBMCs were cultured in the same media plus 10% IL-2 and without phytohemagglutinin-P.
For virus detection, 2 × 106 CD4-enriched PBMCs were incubated in 1 ml of filtered vaginal lavage containing 20 μg of DEAE-dextran (Amersham Pharmacia, Piscataway, N.J.)/ml and 10% FBS. Each lavage-PBMC mixture was added to one well of a 24-well plate and allowed to incubate at 37°C in 7% CO2 for 4 h. After this initial virus adsorption period, 1 ml of media was added to each well. Cultures were maintained for 3 weeks. For each culture, one-half of the medium was replenished with fresh medium every 3 to 4 days, and 106 phytohemagglutinin-stimulated cells were added every 7 days. Culture supernatants were subsequently analyzed for the presence of HIV-1 p24gag protein by enzyme-linked immunosorbent assay (Coulter Immunology, Hialeah, Fla.). Cultures producing >50 pg of p24 protein/ml were considered positive for HIV-1 isolation.
Statistical analysis.
Assessment of relationships between variables was done by using the Spearman rank correlation coefficient. The Fisher exact test was used to determine differences between and within groups.
RESULTS
Adaptation of the MAGI assay.
Previous attempts to detect infectious cell-free HIV-1 in female genital secretions by standard culture techniques have frequently been hindered by overgrowth of vaginal flora. Initial testing of vaginal lavages from HIV-1-uninfected women showed that 5 of 10 lavages had microbial flora that contaminated the cultures and inhibited MAGI cell growth. To investigate filtration as a method to remove microbial contamination while maintaining the titer of infectious virus, lavage samples from two of these HIV-1-uninfected women were spiked with HIV-1Ba-L and passed through a premoistened 0.45- or 0.22-μm-pore-size filter before use in the assay. One lavage (lavage A) was previously shown to have no signs of contamination in culture, while the other lavage (lavage B) was shown to have microbial contamination and inhibit MAGI cell growth. Both 0.22- and 0.45-μm filters removed microbial contamination from the lavages, as determined by the absence of bacterial and fungal growth in the cultures. However, lavages A and B processed with 0.22-μm filters had 20 and 35% fewer plaques, respectively, than the same samples processed with 0.45-μm filters (Table 1). The addition of a centrifugation step for concentration of the virus after filtration decreased the number of MAGI plaques by approximately twofold compared to the samples that were only filtered (Table 1). Therefore, all lavage samples were processed through a premoistened 0.45-μm filter before being used in subsequent MAGI assays.
TABLE 1.
Effect of filtering and ultracentrifugation of two HIV-1-negative lavages spiked with 30 TCID50 of HIV-1Ba-L on MAGI plaque formation
| Lavage | No. of MAGI plaquesa after:
|
||||
|---|---|---|---|---|---|
| NT | 0.22-μm filtration | 0.22-μm filtration and UC | 0.45-μm filtration | 0.45-μm filtration and UC | |
| HIV-negative lavage A + HIV-1Ba-L | 54 | 36 | 18 | 45 | 19 |
| HIV-negative lavage B + HIV-1Ba-L | 0b | 28 | 13 | 43 | 24 |
Data are expressed as the average of duplicate wells after filtration through filters with the indicated pore sizes. NT, no treatment. That is, HIV-1-negative lavages were spiked with HIV-1Ba-L (30 TCID50) and directly added to R5-MAGI cells. UC, ultracentrifugation. That is, filtered HIV-1-negative lavages spiked with HIV-1Ba-L (30 TCID50) were ultracentrifuged at 100,000 × g for 1 h at 25°C. The supernatant was then removed, and the viral pellet was resuspended in PBS and added to R5-MAGI cells.
No plaques were determined because of overt contamination.
To determine whether the number of MAGI plaques is proportional to the amount of infectious HIV-1 added to the R5-MAGI cells, duplicate cultures were infected with a dilution series of 150, 30, 15, and 3 TCID50 of HIV-1Ba-L in 150 μl of PBS or vaginal lavage from an HIV-1-uninfected woman. The highest amount of HIV-1Ba-L in PBS (150 TCID50) resulted in an average of 387 blue plaques per well. A 5-, 10-, and 50-fold decrease in the amount of virus (input of 30, 15, and 3 TCID50, respectively) added to the assay resulted in a 5.1-, 11-, and 49-fold reduction in the number of blue MAGI plaques per well, respectively. Similar results were obtained when virus was diluted into HIV-1-negative vaginal lavages (5-, 9-, and 48-fold reduction, respectively). These results indicate that the number of MAGI plaques is proportional to the amount of infectious virus added to the cultures (Spearman rank correlations of r = 0.97 [P < 0.003] and r = 0.98 [P < 0.001], respectively).
Several studies have shown that factors in genital secretions may inhibit or enhance HIV-1 replication in vitro (2, 4, 12, 39, 40). To determine whether factors in normal vaginal lavages affect the MAGI assay, aliquots from 10 HIV-1-uninfected women were filtered and used in the MAGI assay in the absence of virus. None of the 10 HIV-1-negative lavages produced more MAGI plaques than did the negative control PBS cultures. Conversely, we tested aliquots of lavages from 10 HIV-1-uninfected women that were spiked with 30 TCID50 of HIV-1Ba-L and filtered as described above. The number of MAGI plaques resulting from 9 of the 10 spiked lavages equaled the number of plaques observed when virus was spiked into PBS (not shown). The other spiked sample became contaminated during the assay and thus did not produce MAGI plaques. These results indicate that factors in these HIV-1-negative vaginal lavages do not significantly influence the MAGI assay.
It has previously been reported that the proinflammatory cytokines TNF-α, IL-1β, and IL-6 enhance HIV-1 infection in vitro (31-34) and that vaginal lavages from HIV-1-infected women have increased amounts of these cytokines (4, 39; J. M. Villanueva, K. Clancy, T. V. Ellerbrock, J. L. Lennox, T. J. Bush, T. C. Wright, T. Evans-Strickfaden, C. Schnell, and C. E. Hart, Abstr. Microbicides 2000 NIH Conf., p. 48, 2000). These cytokines may influence the integrated HIV-1 LTR-β-Gal DNA construct in MAGI cells and produce β-Gal-positive plaques above background levels. To assess this effect, duplicate serial dilutions of proinflammatory cytokines in PBS were added to R5-MAGI cells for the 2-h incubation period in the absence of virus. Concentrations at or above those found in HIV-1-positive vaginal lavage samples were used (Villanueva et al., Abstr. Microbicides 2000 NIH Conf.). The numbers of MAGI plaques for the TNF-α, IL-1β, and IL-6 dilutions did not vary from the number for the negative control (Table 2). However, R5-MAGI cell growth was inhibited at the highest IL-6 concentration (1.0 ng/ml), which was approximately threefold higher than IL-6 concentrations found in HIV-1-positive vaginal lavages. Further, when TNF-α and IL-1β were combined at high (100 pg/ml and 10 ng/ml, respectively) or medium (25 pg/ml and 1 ng/ml, respectively) levels, no synergistic effect on MAGI plaque formation was detected (not shown).
TABLE 2.
Effect of exogenous proinflammatory cytokines and cathepsin D on MAGI plaque formation
| Treatment | Concn (ng/ml) | No. of MAGI plaquesa |
|---|---|---|
| IL-1βb | 10 | 0 |
| 5 | 3 | |
| 1 | 0 | |
| 0.5 | 0 | |
| 0.1 | 0 | |
| IL-6b | 1 | NDc |
| 0.5 | 0 | |
| 0.1 | 0 | |
| 0.05 | 0 | |
| 0.01 | 0 | |
| TNF-αb | 100 pg/ml | 1 |
| 50 pg/ml | 4 | |
| 25 pg/ml | 5 | |
| 12.5 pg/ml | 0 | |
| 6.25 pg/ml | 2 | |
| Cathepsin Dd | 20 μg/ml | 43 |
| 10 μg/ml | 31 | |
| 5 μg/ml | 47 | |
| 2.5 μg/ml | 45 | |
| 1.25 μg/ml | 50 | |
| Background | 3 ± 1.3f | |
| Positive controle | 54 ± 2.9f |
That is, the number of MAGI plaques above the background level. The data are expressed as the averages of duplicate wells for single cytokine treatments and of quadruplicate wells for combined treatments.
IL-1β, IL-6, and TNF-α were separately diluted into PBS at or above reported concentrations in vaginal lavage samples from HIV-1-infected women (4, 39; Villanueva et al., Abstr. Microbicides 2000 NIH Conf.), and the effect on the formation of MAGI plaques was tested in the absence of virus.
ND, not determined. A total of 1 ng of IL-6/ml inhibited the growth of R5-MAGI cells and resulted in sloughing of the cells from the wells.
HIV-1Ba-L (30 TCID50) was pretreated for 30 min at 37°C with concentrations of cathepsin D (diluted in PBS) known to enhance HIV-1 infection before being added to the MAGI cultures.
HIV-1Ba-L (30 TCID50) was added to PBS as a positive control.
Mean number of plaques ± the standard deviation.
A similar experiment was performed with biological concentrations of cathepsin D, a known enhancer of HIV-1 expression (12). HIV-1Ba-L was preincubated with cathepsin D for 30 min at 37°C before being added to the R5-MAGI cells. Cathepsin D did not enhance the formation of MAGI plaques (Table 2). The data from both experiments show that endogenous plaque formation is not affected by proinflammatory cytokines or described HIV-1 enhancers.
Detection of infectious virus in HIV-1-positive vaginal lavages by using the MAGI assay.
Vaginal lavages from 49 HIV-1-infected women were tested for infectious virus by using MAGI cultures. The lavages were grouped according to their vaginal viral loads of cell-free HIV-1 RNA and were designated as low (below detection to <104 total copies/lavage [n = 17]), medium (104 to 105 total copies/lavage [n = 16]), and high (>105 total copies/lavage [n = 16]) (Table 3). These samples were chosen independently of the patient's corresponding plasma viral load.
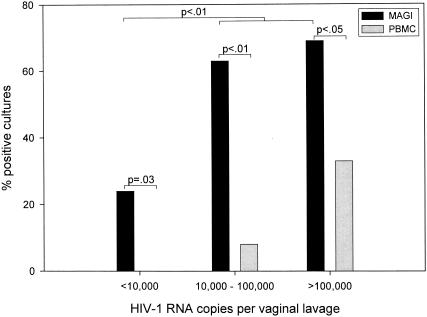
Overall, 25 (51%) of the 49 women had infectious, cell-free vaginal HIV-1 as shown by their lavages producing MAGI plaques above background levels. Of the 17 women with low vaginal viral loads, 4 (24%) had infectious virus in their lavages as determined by the MAGI assay (Fig. 1 and Table 3). No infectious virus was found in the 10 lavages from women who had undetectable vaginal viral loads (Table 3). MAGI plaques were produced by 10 (63%) of 16 and 11 (69%) of 16 vaginal lavage samples with medium and high viral loads, respectively (Fig. 1, Table 3). For women with medium and high vaginal viral loads (>104 total copies), significantly (P < 0.01) more lavages tested positive for infectious virus by the MAGI assay than for women with low vaginal viral loads (<104 total copies) (Fig. 1).
FIG. 1.
Detection of infectious HIV-1 in vaginal lavages from HIV-1-infected women by using the MAGI and traditional PBMC culture assays. Vaginal lavages from HIV-1-infected women were stratified based on HIV-1 RNA copies per lavage.
Despite this difference, there was only a weak correlation between the actual number of MAGI plaques and vaginal viral loads in the 39 lavages with detectable HIV-1 RNA (Spearman correlation coefficient r = 0.32 [P < 0.03]). To determine whether factors in vaginal secretions from HIV-1-infected women could account for this weak correlation, eight lavage samples with virus loads of >50,000 HIV-1 RNA copies were selected. Four samples originally generating ≥17 MAGI plaques (group 1) and four originally generating ≤6 MAGI plaques (group 2) were cleared of their original cell-free HIV-1 by centrifugation, spiked with cell-free HIV-1Ba-L, and applied to MAGI cultures (Table 4). Both groups of samples had a similar median level of MAGI plaques after being spiked with HIV-1Ba-L (Table 4). Compared to control MAGI cultures spiked with the same amount of HIV-1Ba-L, one of four lavage samples in each group inhibited MAGI plaque formation by >2-fold (Table 4; lavages 67 and 1030). The remaining three lavage samples in each group had MAGI plaques that were ≤2-fold more than those produced by control MAGI cultures (Table 4). These results suggest that there were no specific inhibiting factors in the vaginal lavages in group 2, compared to group 1, that affected the detection of infectious virus by the MAGI assay.
TABLE 4.
Determination of the presence of inhibitors in samples demonstrating discordance between vaginal lavage HIV-1 RNA levels and number of MAGI plaques
| Group | Patient IDf | Vaginal viral load (no. of copies/lavage) | No. of MAGI plaquesa in:
|
||
|---|---|---|---|---|---|
| Original sample | Cleared sampleb | Spiked samplec | |||
| Group 1 | 13 | 57,000 | 18 | 0 (4) | 41 |
| 14 | 88,000 | 27 | 0 (3) | 27 | |
| 1034 | 260,000 | 19 | 0 (3) | 36 | |
| 67 | 410,000 | 17 | 0 (4) | 12 | |
| Group 2 | 1033 | 160,000 | 0 | 0 (4) | 53 |
| 71 | 260,000 | 0 | 0 (3) | 40 | |
| 75 | 270,000 | 2 | 1 (6) | 31 | |
| 1030 | 4,300,000 | 6 | 0 (3) | 0 | |
| Background | 5 ± 2.9e | ||||
| Positive controld | 26 ± 11e | ||||
That is, the number of MAGI plaques above the background level. The data are expressed as the average of duplicate wells.
Filtered lavages were ultracentifuged at 100,000 × g for 1 h at 4°C to remove HIV-1. The sample supernatant was tested by the MAGI assay to determine whether the virus had been cleared. Raw values are shown in parentheses.
HIV-1Ba-L (75 TCID50) was spiked into the cleared supernatant and assayed by the MAGI assay.
HIV-1Ba-L (75 TCID50) was added to PBS as a positive control.
Mean numbers of plaques ± standard deviation.
ID, identification number.
Detection of infectious HIV-1 with standard PBMC coculture.
To compare the MAGI assay with a standard PBMC coculture assay, vaginal lavage aliquots from the 39 women with detectable vaginal viral loads were cocultured with CD8-depleted PBMCs. Although none of the 39 filtered lavages showed microbial contamination during the 48-h MAGI assay, 4 (10%) lavages with medium and high vaginal viral loads showed bacterial contamination by day 7 of coculture and were discarded. Of the remaining 35 lavage cocultures, 6 (17%) were positive for infectious virus by day 21 of culture (Table 3 and Fig. 1). None of the seven women with low but detectable vaginal viral loads and only 1 (8%) of 13 women with medium vaginal viral loads had a lavage with infectious cell-free virus as determined by PBMC coculture. However, 5 (33%) of 15 women with a high vaginal viral load (i.e., >105 total copies) had a PBMC coculture that was positive for infectious cell-free virus. These data indicate that detection of infectious, cell-free HIV-1 in vaginal lavages was significantly better with the MAGI assay (64%) than the PBMC coculture assay (17%) (P < 0.01) (Fig. 1).
Relationship between detection of infectious virus and CD4+-T-cell count, genital tract infections, and vaginal pH.
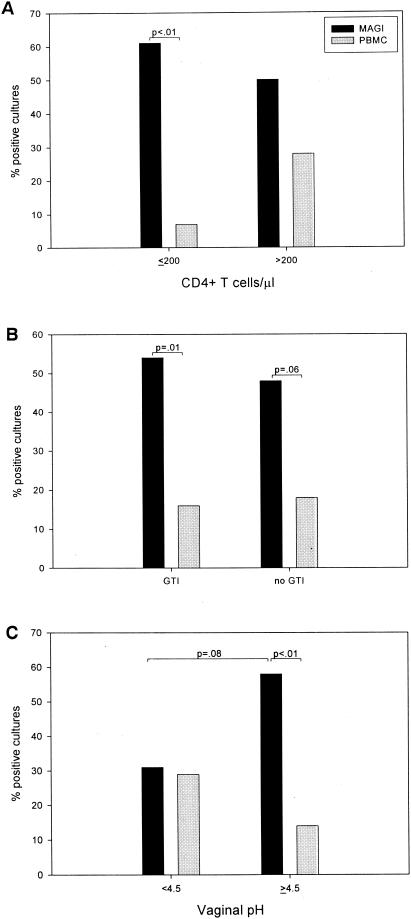
When samples were grouped according to CD4+-T-cell counts of ≤200 or >200, there was no significant difference in the number of lavages testing positive for infectious virus by the MAGI assay (P = 0.76) and by the standard PBMC culture assay (P = 0.58) (Fig. 2A). However, in comparison to the PBMC culture method, the MAGI assay detected significantly more positive cultures in samples from women with CD4+-T-cell counts of ≤200 (P < 0.01) than from women with CD4+-T-cell counts of >200 (P = 0.12) (Fig. 2A). Further, the use of ART was not a confounding factor, since no significant difference (P = 0.18) was observed in positive MAGI cultures between women who were or who were not on ART (results not shown).
FIG. 2.
Analysis of the recovery of cell-free infectious virus based on CD4+-T-cell counts (A), the presence of genital tract infections (GTIs) (B), and vaginal pH (C) by using the MAGI assay and traditional PBMC culture assay.
Since the presence of GTIs may influence the amount of infectious virus present in genital secretions, we examined lavages from women with or without GTIs. Of the 49 HIV-1-infected women, 19 had one GTI (T. vaginalis, n = 2; bacterial vaginosis, n = 1; HSV, n = 2; yeast infections, n = 14), and 5 had two concurrent GTIs (bacterial vaginosis and yeast, n = 3; T. vaginalis and yeast, n = 1; T. vaginalis and bacterial vaginosis, n = 1). The presence of one or more GTIs, compared to women without GTIs, did not significantly affect detection of infectious virus from the lavages by the MAGI assay (P = 0.76) or by the standard PBMC culture assay (P = 0.58) (Fig. 2B). The MAGI assay detected significantly more positive cultures than did the PBMC culture method in lavages from women with GTIs (P = 0.01) than from women without GTIs (P = 0.06) (Fig. 2B).
Since the presence of a GTI may influence vaginal pH and since low pH has been linked to reduced HIV-1 infectivity (28), we evaluated whether cultures positive for HIV-1 were associated with vaginal pH. No significant difference between pH groups was observed when the standard PBMC culture assay was used (P = 0.34) (Fig. 2C). However, within pH groups, the MAGI assay detected significantly more positive cultures (P < 0.01) than did standard PBMC culture in lavages from women with a vaginal pH of ≥4.5 (Fig. 2C). Although the result was not significant (P = 0.08), the MAGI assay detected more positive vaginal lavages from women with a high vaginal pH (≥4.5) than from women with a low vaginal pH (<4.5).
DISCUSSION
We show here that the MAGI assay is more effective than traditional culture methods for detecting infectious HIV-1 in vaginal secretions. A variety of factors could account for the significantly increased ability of the MAGI assay to detect infectious virus. In particular, one study reported that growth of R5-using HIV-1 in standard culture conditions is determined by the capacity of the virus to spread through the culture rather than by the infection efficiency of the initial inoculation (5). Consequently, standard coculture may have detected only infectious viruses in vaginal secretions that could have spread throughout the PBMC culture in the weeks after inoculation. These observations may very likely explain the increased sensitivity of the MAGI assay and its ability to detect infectious virus in lavage samples that did not yield positive cocultures since plaque formation depends on a single round of infection and not on a spreading infection. In addition to being more sensitive, the MAGI assay required less time and resulted in fewer contaminated cultures than did standard PBMC cultures.
The limited ability of standard techniques to detect infectious virus in genital secretions has made it difficult to determine whether an association exists between the presence of infectious HIV-1 and clinical parameters such as viral load and CD4+-T-cell counts. Although the MAGI assay detected infectious virus in more vaginal lavages with medium and high viral loads than in those with low viral loads (<104 copies/lavage), lavages from four of the seven women with low but detectable vaginal viral loads induced MAGI plaque formation. Similarly, a recent study reported that although the rates of HIV-1 isolation by standard PBMC coculture decrease as the plasma viral load decreases to <1,000 copies/ml, 38% of samples with levels of RNA of <50 copies/ml in plasma were still positive for HIV-1 by coculture (10). If transmission can be associated with the presence of infectious virus, our data support other studies that demonstrate that sexual transmission of HIV-1 can occur even when the HIV-1-infected partner has a plasma viral load of <3,500 copies/ml (14, 35). Our observation of no difference, with either method, in the ability to detect positive vaginal lavage cultures from women with a CD4+-T-cell count of <200 and those with a count of ≥200 suggests that viral shedding and possibly transmission of infectious HIV-1 are independent of a patient's clinical stage. Furthermore, the presence of infectious HIV-1, as indicated by the MAGI assay, across a wide range of vaginal viral loads suggests that viral load may not be the sole determinant in HIV-1 transmission.
Our finding indicating only a modest correlation between the numbers of MAGI plaques and the corresponding vaginal viral load suggests that there may be components in vaginal secretions from HIV-1-infected women that affect the MAGI assay. In our spiking experiments, no inhibition in the numbers of resulting MAGI plaques was observed in six of the eight tested lavage samples; however, two samples demonstrated a >2-fold reduction of infectious virus as measured by the MAGI assay. It is currently unclear what factors may account for the inhibition observed with these two samples. Recent studies suggest that HIV-1-specific neutralizing antibodies can inhibit transcytosis of virus through the mucosal epithelium (1, 6, 19) or account for the low infectivity of plasma samples with high HIV-1 viral RNA loads (11). The impact of HIV-1-specific antibodies on the infectiousness of virus in the MAGI assay remains to be investigated. Our data suggest that, in some cases, detection of infectious virus in vaginal lavages from HIV-1-infected women is multifactorial. Other potential factors in addition to HIV-1-neutralizing antibodies may include innate immune factors and genital tract flora.
Maintenance of the normal vaginal ecology is important to reduce HIV-1 transmission and to prevent acquisition of other sexually transmitted diseases. In the healthy female genital tract, a complex environment exists in which pathogenic microorganisms are exposed to local, host-derived immune factors, endogenous genital tract flora, and an acidic pH. Consequently, vaginal secretions contain factors that may affect the infectiousness of HIV-1, as well as the ability to detect infectious virus. Although multiple factors in the vaginal tract could affect the MAGI assay (4, 12, 39, 40), we were unable to demonstrate any effect on MAGI plaque formation with vaginal lavages from HIV-1-uninfected women or the addition of immune factors (proinflammatory cytokines and cathepsin D) often found in genital secretions. Other studies have indicated that GTIs such as bacterial vaginosis and infection with T. vaginalis may be associated with HIV-1 shedding and transmission. Although the MAGI assay detected more HIV-1-positive vaginal lavage cultures than did standard PBMC coculture, the presence of GTIs was not associated with the detection of infectious virus in these vaginal lavages. Moreover, plaque numbers generated by lavages from women having one or more GTI were not significantly different from those from women without GTIs.
Several cross-sectional studies have indicated an association between abnormal vaginal flora, including bacterial vaginosis, and an increased risk of acquiring HIV-1 (25, 38, 41). Since these clinical conditions are often associated with an increase in vaginal pH, it is not surprising that more recent studies have identified a basic vaginal pH (>4.5) as a risk factor for HIV-1 seropositivity (26) or cervicovaginal shedding of viral RNA (37). While not significant, our data showed that HIV-1-infected women with a vaginal pH of ≥4.5 had more detectable infectious virus in their vaginal lavage as determined by the MAGI assay than those with a low pH (<4.5). If vaginal pH is associated with shedding of infectious virus in the genital tract, this would have important implications for methods designed to prevent sexual transmission of HIV. For example, recent methods include the development of a microbicide that could be applied topically to the vagina to prevent HIV-1 infection. Although a variety of antimicrobial factors are being evaluated, one class of candidates includes products that maintain or restore the acidic environment of the vaginal tract (42). Our results with vaginal pH and recovery of infectious virus, together with the known instability of HIV-1 at low pH, suggest that this class of microbicides may prove effective in preventing sexual transmission of HIV-1.
In summary, we have demonstrated the utility of using the MAGI assay to detect infectious virus in female genital secretions. Given the small sample size, it remains to be determined whether our findings with infectious HIV-1 and other risk factors, such as vaginal pH or genital tract infections, are representative of larger study populations. Use of the MAGI assay for evaluating infectious virus could be important for studies examining the effectiveness of highly active ART, mucosal HIV-1-specific immune responses, and vaginal microbicides. Furthermore, the MAGI assay will be useful in future studies for determining whether infectious virus in genital secretions, in addition to viral RNA levels, is predictive of sexual transmission of HIV-1.
Acknowledgments
J.E.C. and J.M.V. contributed equally to this study.
J.E.C. was supported by the Oak Ridge Institute for Science and Education and by the National Research Service Award 5 F32 HD40727-02 from the National Institute of Child Health and Human Development, National Institutes of Health. S.R.A. was supported by an Emerging Infectious Diseases postdoctoral fellowship and by the amfAR postdoctoral fellowship 70553-31-RFM. S.M.S. was supported by the NCID Summer Research Fellowship Program. Financial support for this study was provided by the Centers for Disease Control and Prevention (collaborative agreements U64/CCU412279 and U64/CCU214921).
We thank Tedd Ellerbrock for support, as well as the participants in the Emory Vaginal Ecology project.
Reagent U373-MAGI-CCRSE was obtained from Michael Emerman through the AIDS Research and Reference Program, Division of AIDS, NIAID, NIH.
The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
REFERENCES
- 1.Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. [DOI] [PubMed] [Google Scholar]
- 2.Al-Harthi, L., G. T. Spear, F. B. Hashemi, A. Landay, B. E. Sha, and K. A. Roebuck. 1998. A human immunodeficiency virus (HIV)-inducing factor from the female genital tract activates HIV-1 gene expression through the κB enhancer. J. Infect. Dis. 178:1343-1351. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, D. J., J. A. Politch, L. D. Tucker, R. Fichorova, F. Haimovici, R. E. Tuomala, and K. H. Mayer. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retrovir. 14:S43-S49. [PubMed] [Google Scholar]
- 4.Belec, L., R. Gherardi, C. Payan, T. Prazuck, J. E. Malkin, C. Tevi-Benissan, and J. Pillot. 1995. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine 7:568-574. [DOI] [PubMed] [Google Scholar]
- 5.Blaak, H., L. J. Ran, R. Rientsma, and H. Schuitemaker. 2000. Susceptibility of in vitro stimulated PBMC to infection with NSI HIV-1 is associated with levels of CCR5 expression and β-chemokine production. Virology 267:237-246. [DOI] [PubMed] [Google Scholar]
- 6.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 7.Cummins, J. E., Jr., and C. S. Dezzutti. 2000. Sexual HIV-1 transmission and mucosal defense mechanisms. AIDS Rev. 2:144-154. [Google Scholar]
- 8.Cu Uvin, S., and A. M. Caliendo. 1997. Cervicovaginal human immunodeficiency virus secretion and plasma viral load in human immunodeficiency virus-seropositive women. Obstet. Gynecol. 90:739-743. [DOI] [PubMed] [Google Scholar]
- 9.Davies, A. 1978. A preliminary investigation using p-nitrophenyl phosphate to quantitate acid phosphatase on swabs examined in cases of sexual assault. Med. Sci. Law 18:174-178. [DOI] [PubMed] [Google Scholar]
- 10.Demeter, L. M., R. J. Bosch, R. W. Coombs, S. Fiscus, J. Bremer, V. A. Johnson, A. Erice, J. B. Jackson, S. A. Spector, K. M. Squires, M. A. Fischl, M. D. Hughes, and S. M. Hammer. 2002. Detection of replication-competent human immunodeficiency virus type 1 (HIV-1) in cultures from patients with levels of HIV-1 RNA in plasma suppressed to less than 500 or 50 copies per milliliter. J. Clin. Microbiol. 40:2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dianzani, F., G. Antonelli, E. Riva, O. Turriziani, L. Antonelli, S. Tyring, D. A. Carrasco, H. Lee, D. Nguyen, J. Pan, J. Poast, M. Cloyd, and S. Baron. 2002. Is human immunodeficiency virus RNA load composed of neutralized immune complexes? J. Infect. Dis. 185:1051-1054. [DOI] [PubMed] [Google Scholar]
- 12.El Messaoudi, K., L. Thiry, N. Van Tieghem, C. Liesnard, Y. Englert, N. Moguilevsky, and A. Bollen. 1999. HIV-1 infectivity and host range modification by cathepsin D present in human vaginal secretions. AIDS 13:333-339. [DOI] [PubMed] [Google Scholar]
- 13.Goulston, C., W. McFarland, and D. Katzenstein. 1996. Human immunodeficiency virus type 1 RNA in the female genital tract. J. Infect. Dis. 177:1100-1103. [DOI] [PubMed] [Google Scholar]
- 14.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 15.Gray, R. H., N. Kiwanuka, T. C. Quinn, N. K. Sewankambo, D. Serwadda, F. W. Mangen, T. Lutalo, F. Nalugoda, R. Kelly, M. Meehan, M. Z. Chen, C. Li, and M. J. Wawer. 2000. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. AIDS 14:2371-2381. [DOI] [PubMed] [Google Scholar]
- 16.Guenthner, P. C., and C. E. Hart. 1998. Quantitative, competitive, PCR assay for HIV-1 using a microplate-based detection system. BioTechniques 24:810-816. [DOI] [PubMed] [Google Scholar]
- 17.Hart, C. E., J. L. Lennox, M. Pratt-Palmore, T. C. Wright, R. F. Schinazi, T. Evans-Strickfaden, T. J. Bush, C. Schnell, L. J. Conley, K. A. Clancy, and T. V. Ellerbrock. 1999. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J. Infect. Dis. 179:871-882. [DOI] [PubMed] [Google Scholar]
- 18.Henin, Y., L. Mandelbrot, R. Henrion, R. Pradinaud, J. P. Coulaud, and L. Montagnier. 1993. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J. Acquir. Immune Defic. Syndr. 6:72-75. [PubMed] [Google Scholar]
- 19.Hocini, H., L. Belec, S. Iscaki, B. Garin, J. Pillot, P. Becquart, and M. Bomsel. 1997. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retrovir. 13:1179-1185. [DOI] [PubMed] [Google Scholar]
- 20.Iversen, A. K., A. R. Larsen, T. Jensen, L. Fugger, U. Balslev, S. Wahl, J. Gerstoft, J. I. Mullins, and P. Skinhoj. 1998. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J. Infect. Dis. 177:1214-1220. [DOI] [PubMed] [Google Scholar]
- 21.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreiss, J., D. M. Willerford, M. Hensel, W. Emonyi, F. Plummer, J. Ndinya-Achola, P. L. Roberts, J. Hoskyn, S. Hillier, and N. Kiviat. 1994. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J. Infect. Dis. 170:1597-1601. [DOI] [PubMed] [Google Scholar]
- 23.Kreiss, J. K., R. Coombs, F. Plummer, K. K. Holmes, B. Nikora, W. Cameron, E. Ngugi, J. O. Ndinya Achola, and L. Corey. 1989. Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J. Infect. Dis. 160:380-384. [DOI] [PubMed] [Google Scholar]
- 24.Lawn, S. D., S. Subbarao, T. C. Wright, Jr., T. Evans-Strickfaden, T. V. Ellerbrock, J. L. Lennox, S. T. Butera, and C. E. Hart. 2000. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix. J. Infect. Dis. 181:1950-1956. [DOI] [PubMed] [Google Scholar]
- 25.Martin, H. L., B. A. Richardson, P. M. Nyange, L. Lavreys, S. L. Hillier, B. Chohan, K. Mandaliya, J. O. Ndinya-Achola, J. Bwayo, and J. Kreiss. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J. Infect. Dis. 180:1863-1868. [DOI] [PubMed] [Google Scholar]
- 26.Mbizvo, E. M., S. E. Msuya, B. Stray-Pedersen, J. Sundby, M. Z. Chirenje, and A. Hussain. 2001. HIV seroprevalence and its associations with the other reproductive tract infections in asymptomatic women in Harare, Zimbabwe. Int. J. STD AIDS 12:524-531. [DOI] [PubMed] [Google Scholar]
- 27.Mostad, S. B., and J. K. Kreiss. 1996. Shedding of HIV-1 in the genital tract. AIDS 10:1305-1315. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor, T. J., D. Kinchington, H. O. Kangro, and D. J. Jeffries. 1995. The activity of candidate virucidal agents, low pH and genital secretions against HIV-1 in vitro. Int. J. STD AIDS 6:267-272. [DOI] [PubMed] [Google Scholar]
- 29.Panther, L. A., L. Tucker, C. Xu, R. E. Tuomala, J. I. Mullins, and D. J. Anderson. 2000. Genital tract human immunodeficiency virus type 1 (HIV-1) shedding and inflammation and HIV-1 env diversity in perinatal HIV-1 transmission. J. Infect. Dis. 181:555-563. [DOI] [PubMed] [Google Scholar]
- 30.Pedraza, M. A., J. del Romero, F. Roldan, S. Garcia, M. C. Ayerbe, A. R. Noriega, and J. Alcami. 1999. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J. Acquir. Immune Defic. Syndr. 21:120-125. [PubMed] [Google Scholar]
- 31.Poli, G., A. Kinter, J. S. Justement, J. H. Kehrl, P. Bressler, S. Stanley, and A. S. Fauci. 1990. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc. Natl. Acad. Sci. USA 87:782-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poli, G., A. L. Kinter, and A. S. Fauci. 1994. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 91:108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poli, G., and A. S. Fauci. 1992. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res. Hum. Retrovir. 8:191-197. [DOI] [PubMed] [Google Scholar]
- 34.Poli, G., P. Bressler, A. Kinter, E. Duh, W. C. Timmer, A. Rabson, J. S. Justement, S. Stanley, and A. S. Fauci. 1990. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor α by transcriptional and posttranscriptional mechanisms. J. Exp. Med. 172:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 36.Saracino, A., M. Di Stefano, J. R. Fiore, A. Lepera, D. Raimondi, G. Angarano, and G. Pastore. 2000. Frequent detection of HIV-1 RNA but low rates of HIV-1 isolation in cervicovaginal secretions from infected women. New Microbiol. 23:79-83. [PubMed] [Google Scholar]
- 37.Seck, K., N. Samb, S. Tempesta, C. Mulanga-Kabeya, D. Henzel, P. S. Sow, A. Coll-Seck, S. Mboup, I. Ndoye, and E. Delaporte. 2001. Prevalence and risk factors of cervicovaginal HIV shedding among HIV-1 and HIV-2 infected women in Dakar, Senegal. Sex. Transm. Infect. 77:190-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sewankambo, N., R. H. Gray, M. J. Wawer, L. Paxton, D. McNaim, F. Wabwire-Mangen, D. Serwadda, C. Li, N. Kiwanuka, S. L. Hillier, L. Rabe, C. A. Gaydos, T. C. Quinn, and J. Konde-Lule. 1997. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546-550. [DOI] [PubMed] [Google Scholar]
- 39.Sha, B. E., R. D. D'Amico, A. L. Landay, G. T. Spear, L. S. Massad, R. J. Rydman, N. A. Warner, J. Padnick, L. Ackatz, L. A. Charles, and C. A. Benson. 1997. Evaluation of immunologic markers in cervicovaginal fluid of HIV-infected and uninfected women: implications for the immunologic response to HIV in the female genital tract. J. Acquir. Immune Defic. Syndr. 16:161-168. [DOI] [PubMed] [Google Scholar]
- 40.Spear, G. T., L. al-Harthi, B. Sha, M. N. Saarloos, M. Hayden, L. S. Massad, C. Benson, K. A. Roebuck, N. R. Glick, and A. Landay. 1997. A potent activator of HIV-1 replication is present in the genital tract of a subset of HIV-1-infected and uninfected women. AIDS 11:1319-1326. [DOI] [PubMed] [Google Scholar]
- 41.Taha, T. E., D. R. Hoover, G. A. Dallabetta, N. I. Kumwenda, L. A. Mtimavalye, L. P. Yang, G. N. Liomba, R. L. Broadhead, J. D. Chiphangwi, and P. G. Miotti. 1998. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699-1706. [DOI] [PubMed] [Google Scholar]
- 42.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Inv. Drug 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 43.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 44.Vogt, M. W., D. J. Witt, D. E. Craven, R. Byington, D. F. Crawford, M. S. Hutchinson, R. T. Schooley, and M. S. Hirsch. 1987. Isolation patterns of the human immunodeficiency virus from cervical secretions during the menstrual cycle of women at risk for the acquired immunodeficiency syndrome. Ann. Intern. Med. 106:380-382. [DOI] [PubMed] [Google Scholar]
- 45.Vogt, M. W., D. J. Witt, D. E. Craven, R. Byington, D. F. Crawford, R. T. Schooley, and M. S. Hirsch. 1986. Isolation of HTLV-III/LAV from cervical secretions of women at high risk for AIDS. Lancet i:525-527. [DOI] [PubMed]
- 46.Wofsy, C. B., J. B. Cohen, L. B. Hauer, N. S. Padian, B. A. Michaelis, L. A. Evans, and J. A. Levy. 1986. Isolation of AIDS-associated retrovirus from genital secretions of women with antibodies to the virus. Lancet i:527-529. [DOI] [PubMed]
- 47.Wright, T. C., Jr., S. Subbarao, T. V. Ellerbrock, J. L. Lennox, T. Evans-Strickfaden, D. G. Smith, and C. E. Hart. 2001. Human immunodeficiency virus 1 expression in the female genital tract in association with cervical inflammation and ulceration. Am. J. Obstet. Gynecol. 184:279-285. [DOI] [PubMed] [Google Scholar]