Abstract
A major goal of tumor immunotherapy is the effective eradication of established metastases associated with the induction of a T cell-mediated protective immunity. We achieved this in a poorly immunogenic murine neuroblastoma model by gene therapy with a single chain interleukin 12 (scIL-12) fusion protein that assures equal expression of its p35 and p40 subunits. Thus, NXS2 hybrid neuroblastoma cells (C1300 × dorsal root ganglion cells), which form experimental bone marrow and liver metastases in syngeneic A/J mice, were transduced with a gene encoding murine interleukin 12, monomerized by introduction of a protein linker between the p35 and p40 protein chains of this heterodimeric cytokine. We demonstrate for the first time that subcutaneous vaccination with these transduced cells induces a protective immunity, as indicated by the complete absence of liver and bone marrow metastasis after challenge with NXS2 wild-type tumor cells. Furthermore, vaccination of animals with established liver and bone marrow metastases completely eradicated liver metastases and suppressed bone marrow metastases. The local and systemic immune response against scIL-12-transduced NXS2 cells is largely dependent on CD8+ T cells. This was demonstrated in vivo by depletion of immunocompetent A/J mice with monoclonal anti-CD4 and anti-CD8 antibodies and in vitro by specific major histocompatibility complex, class I-restricted CD8+ T cell-mediated killing of NXS2 and their parental C1300 neuroblastoma cells. In conclusion, we demonstrate successful anti-tumor immunotherapy with an scIL-12 fusion protein that could facilitate clinical application of interleukin 12 gene therapy.
The induction or redirection of T cell-mediated immune responses followed by a protective immunity in malignancies characterized by poor immunogenicity is a most challenging goal for adjuvant immunotherapy. A new approach in this regard is the tumor-specific delivery of immunomodulators, which achieve effective concentrations in the tumor microenvironment and effectively stimulate cellular immune responses against syngeneic malignancies. Interleukin 12 (IL-12), a disulfide-linked heterodimeric cytokine consisting of a 35- (p35) and a 40 (p40)-kDa subunit, proved to be very effective in this regard. After its discovery as a strong stimulatory factor for natural killer cells (1) and cytotoxic T cells (2), the anti-tumor effect of IL-12 was established in various in vivo systems following local and systemic administration. However, the latter has been reported to cause fatal toxicity in a clinical trial (3), which led investigators to focus mainly on local administration of this cytokine by gene therapy or gene gun applications, that proved to be very effective and nontoxic at the same time (4–6). Both particle-mediated gene delivery by gene gun or transduction of autologous tumor cells with genes encoding for IL-12 require the delivery of two genes, due to the heterodimeric structure of this cytokine, encoding a p35 and p40 subunit, respectively. This has previously been achieved in various ways, including cotransfection of two plasmids separately expressing each subunit gene (7), by a more complicated vector design, such as single plasmids with p35 and p40 expression cassettes in tandem array (4), or by retroviruses containing internal ribosome entry site sequences for the expression of both subunits from a single mRNA transcript (8). Most of these constructs share the independent expression of both cytokine subunits, which can subsequently lead to a difference in molar production of p35 and p40. Excess production of p40 is associated with the formation of inhibitory p40 dimers that were reported to have specific IL-12 inhibitory effects (9, 10). To circumvent uneven expression of the two IL-12 subunits, the genetic linkage of p35 followed by the p40 subunit was proposed by means of a DNA sequence encoding a flexible protein linker commonly used in antibody engineering (11). Such a construct was shown to have IL-12 bioactivity; however, for the p35–p40 fusion protein, the specific IL-12 bioactivity was reported to be 100-fold less than that of the IL-12 standard (12).
Here, we demonstrate that subcutaneous vaccination with a single chain IL-12 fusion protein induces a T cell-mediated immunity that completely protects mice from challenge with wild-type tumor cells, as indicated by the complete absence of liver and bone marrow metastases in a novel syngeneic model of neuroblastoma. The poor immunogenicity of this model clearly demonstrates the feasibility of efficient gene therapy with a single chain IL-12 fusion protein.
MATERIALS AND METHODS
Construction of a Bioactive Single Chain Mouse IL-12 Fusion Protein.
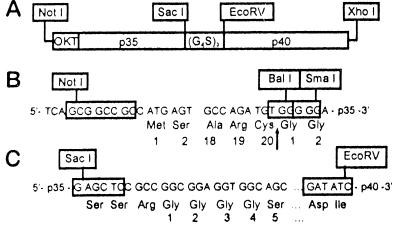
The cDNAs encoding the p35 and p40 chains of mouse IL-12 (mIL-12) were generated from Con A stimulated mouse splenocytes by reverse transcriptase PCR. Primers selected from the 5′- and 3′-end of the coding sequences of each chain were designed to introduce SmaI, EcoRV, and XhoI restriction sites (Fig. 1). The cDNA for the single chain IL-12 fusion protein was constructed by linkage of the p35 and p40 cDNAs with a synthetic linker encoding for the naturally occurring SacI restriction site at the 3′-end of p35 and its last two amino acids followed by (Gly4 Ser)3 and ending with an EcoRV restriction site. The p40 chain was cloned downstream of the linker with the EcoRV restriction site, which encodes for aspartic acid and isoleucine (Fig. 1 A and C). To assure secretion in eukaryotic cells, this construct was joined at its 5′ SmaI site with the 3′ BalI site of an OKT3 leader sequence, thereby replacing the amino-terminal arginines of p35 with two glycines downstream from the cleavage site (Fig. 1B). After introduction into the pBK-CMV vector (Stratagene), using the NotI and XhoI restriction sites, NXS2 cells were transfected and stable clones selected in the presence of 500 μg/ml G418 (Sigma).
Figure 1.
Construction of a vector insert for expression of a single chain IL-12 fusion protein. (A) Schematic of the DNA construct encoding for a single chain IL-12 fusion protein. The p35 and p40 subunits were genetically fused with a DNA linker encoding for the 15 amino acids (glycine4 serine)3 using the SacI and EcoRV restriction sites. (B) To assure secretion in eukaryotic cells, an OKT3 leader sequence was introduced upstream from the p35 gene. For this purpose, the BalI and SmaI restriction sites were used, replacing the N-terminal arginine of p35 with two glycine residues downstream from the cleavage site, as indicated by the black arrow. (C) Fusion of p35 and p40 with a synthetic DNA linker was established by use of a naturally occurring SacI site at the 3′-end of the p35 gene and an engineered EcoRV site upstream from the 5′-end of the p40 gene, which introduced additional aspartic acid and isoleucine residues.
The specific mIL-12 bioactivity of the scIL-12 construct was one-sixth that of the mIL-12 standard (Hoffmann–La Roche), as determined in supernatants of such clones by measuring the ability to induce mouse interferon-γ after incubation with mouse splenocytes, as previously described (13). The mIL-12 protein content was measured by an ELISA for mIL-12-p70 (Genzyme). Three NXS2 clones secreting either low (1.2 ng/106 cells/24 h), intermediate (4.0 ng/106 cells/24 h), or high levels (6.5 ng/106 cells/24 h) of scIL-12 were chosen for vaccination experiments. One NXS2 clone transduced with the empty vector was used for control experiments. All clones revealed the same proliferation characteristics in vitro as NXS2 wild-type cells.
Experimental Bone Marrow and Liver Metastases Model of Neuroblastoma.
Syngeneic female A/J and C.B-17 SCID mice were obtained at 8 weeks of age from The Jackson Laboratory and Taconic Farms, respectively. They were housed in the pathogen-free mouse colony at our institution, and all animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals. The murine NXS2 hybrid neuroblastoma cell line was cultured and used for induction of metastases, as described previously (14, 15). Experimental bone marrow and liver metastases were induced by tail vein injection of either 1 × 105 or 5 × 104 NXS2 cells, respectively, and mice were sacrificed for evaluation after 26 days. The number of metastatic liver foci were counted after injection of 1 × 105 cells. The percentage of liver surface covered by fused metastases was determined after inoculation of 5 × 104 cells, and liver weights were always measured with fresh tissue specimen. For evaluation of bone marrow metastases, the bone cavities of both femurs and tibiae of each animal were flushed with 3 ml of PBS (pH 7.4). The cell pellet was used for total RNA isolation.
RNA Isolation, Reverse Transcription, and PCR Amplification.
Total cellular RNA isolation, synthesis of cDNA, and amplification of mouse tyrosine hydroxylase was done, as described previously (15). For the detection of tyrosine hydroxylase, the PCR was adjusted at low and high sensitivity. According to results of both high and low sensitivity PCR assays, bone marrow metastases were designated as stage 0 with no PCR signal, stage 1 with an exclusive signal for high sensitivity PCR, and stage 2 in the presence of both high and low sensitivity PCR signals.
Cytotoxicity Assays.
Effector cells were prepared from mouse spleen cells by hypotonic lysis of red blood cells with ACK lysis buffer (GIBCO/BRL). These cells were cultured in the presence of irradiated NXS2 cells in complete DMEM with 10% fetal calf serum and 5% T-STIM culture supplement (Becton Dickinson) for 3 days and either used for cytotoxicity assays or for subsequent separation into T cell subpopulations. I-Ak anti-major histocompatibility complex (MHC) class II antibodies (clone 10–3.6) (PharMingen) were used at a concentration of 25 μg/ml, 24 h after initiation of the stimulation phase of the assay. Pure CD8+ and CD4+ effector cells were prepared by magnetic activated cell sorting (Mini MACS, Miltenyi Biotec, Auburn, CA). The purity of cell fractions was determined by fluorescence-activated cell sorter (FACS) analysis. Only preparations exceeding a purity of 95% were used for the cytotoxicity assays. These 51Cr release cytotoxicity assays were performed, as described previously (16).
Statistics.
The statistical significance of differential findings between experimental groups of animals was determined by the nonparametric Mann–Whitney rank sum test. Findings were regarded as significant if two-tailed P values were ≤0.01.
RESULTS
Effect of scIL-12 Production on Tumorigenicity of NXS2 Cells.
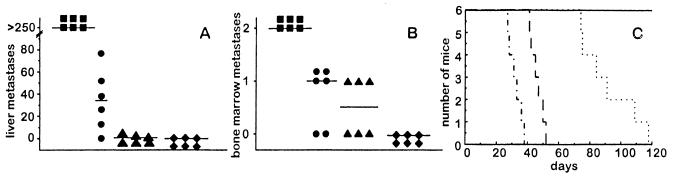
The induction of experimental liver and bone marrow metastasis by scIL-12-producing NXS2 clones of low, intermediate and high expression levels was assessed 26 days after i.v. injection of 1 × 105 cells each into syngeneic A/J mice. NXS2 cells producing the highest amount of scIL-12 formed neither liver nor bone marrow metastases (Fig. 2 A and B) and resulted in a tripling of live span (Fig. 2C), as compared with mice injected with NXS2 cells transduced only with the empty vector. Injection of NXS2 clones with low and intermediate scIL-12 production resulted in a significant reduction in liver and bone marrow metastases followed by an increase in life span (Fig. 2). This anti-tumor effect correlated with the amount of scIL-12 produced by the various clones. In contrast to systemic application, subcutaneous injection of up to 10 × 106 NXS2 cells of the low, intermediate, and high scIL-12 producers lead to a complete tumor rejection 14 days after inoculation in all animals, irrespective of the scIL-12 level produced. No differences in s.c. tumor growth were observed between NXS2 wild-type cells and NXS2 cells transduced with the empty vector (data not shown). These data indicate a strong immunogenicity of genetically modified NXS2 cells that were used as live cellular vaccines for further immunization studies.
Figure 2.
Tumorigenicity of NXS2 cells after scIL-12 transduction. Syngeneic A/J mice (n = 6) were injected intravenously with 1 × 105 NXS2 cells from the low, intermediate, or high scIL-12 secreting clones and analyzed for liver (A) or bone marrow metastasis (B) 26 days after inoculation or kept for survival studies (C). (A) Tumor foci to the liver were counted after injection of low (•), intermediate (▴), and high (⧫) scIL-12-producing clones of NXS2 and compared with NXS2 cells carrying the empty vector (▪). Horizontal lines represent the mean value per experimental group. (B) Bone marrow metastases were analyzed by tyrosine hydroxylase RT-PCR and staged, as described in Materials and Methods after intravenous injection of low (•), intermediate (▴), and high (⧫) scIL-12-producing clones of NXS2 and compared with NXS2 cells carrying the empty vector (▪). The horizontal lines represent the mean value per experimental group. (C) The survival of mice was studied after intravenous injection of low (— · —), intermediate (⋯ ·), and high (———) scIL-12-producing clones of NXS2 and compared with that of mice injected with NXS2 cells carrying the empty vector (· — · —). Differences between all experimental groups and the control group were statistically significant (P < 0.005).
Effect of Local scIL-12 NXS2 Cell Application on Systemic Anti-Tumor Immunity.
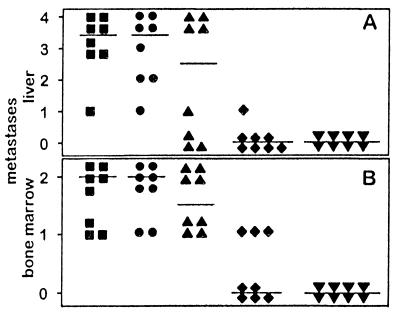
The induction of a protective immunity after subcutaneous injection of scIL-12-producing NXS2 cells was established in two separate experimental settings. First, syngeneic A/J mice were vaccinated with scIL-12-producing NXS2 clones, followed after 7 days with an i.v. challenge of 5 × 104 NXS2 wild-type cells. Mice receiving 4 × 106 cells of the intermediate scIL-12-producing clone revealed a complete protective immunity against subsequent NXS2 challenge. This was indicated by the absence of bone marrow metastases, as determined by tyrosine hydroxylase reverse transcription (RT)-PCR (Fig. 3B), detecting one NXS2 cell in 100,000 naïve bone marrow cells (15). Complete tumor protection was also demonstrated by the absence of liver metastases in such vaccinated animals, which revealed an average liver weight of 967 ± 54 mg standard deviation, which is the same as that of naïve animals (Fig. 3A). This finding contrasts with the numerous bone marrow and liver metastases observed in mice receiving either no vaccination or only NXS2 cells transduced with the empty vector. Mice receiving NXS2 cells transduced with the empty vector all developed continuously growing s.c. tumors. Consequently, they were surgically removed on day 14, simultaneously with the occurrence of s.c. tumor rejection in A/J mice receiving scIL-12-secreting NXS2 cells (Fig. 5A). Both control groups developed bone marrow and liver metastases with increased liver weights of 2,138 ± 619 mg standard deviation and 2,028 ± 483 standard deviation, respectively. A reduction in bone marrow and liver metastasis was observed with lower cell numbers of the intermediate scIL-12 producer used for the initial vaccination or with the low scIL-12-producing clone, respectively, indicating a dose-dependent protection. Second, A/J mice with established experimental micrometastases to liver and bone marrow, 5 days after intravenous injection of 5 × 104 NXS2 cells (16), were subsequently vaccinated by s.c. injection of scIL-12-producing NXS2 clones. Mice receiving 10 × 106 of the high scIL-12-producing NXS2 clone showed a complete eradication of established liver metastases (Fig. 4A) and a significant reduction in established bone marrow metastases (Fig. 4B) as compared with control groups, which received either no vaccine or 10 × 106 NXS2 cells expressing only the empty vector. Again, a reduction in tumor cell number and use of the intermediate scIL-12 producer partially protected mice from developing metastatic bone marrow and liver disease.
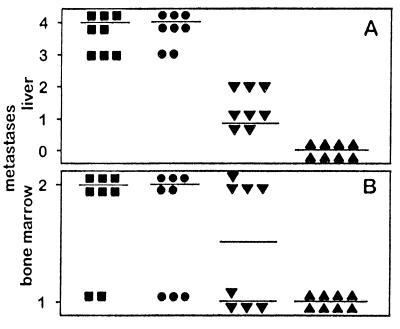
Figure 3.
Effect of single chain IL-12-producing NXS2 cells on subsequent challenge with NXS2 parental cells. Syngeneic A/J mice (n = 8) were injected s.c. with low and intermediate scIL-12-producing NXS2 cell clones and subsequently challenged 7 days thereafter by i.v. injection of 5 × 104 NXS2 parental cells. Tumors were surgically removed 14 days after s.c. inoculation. After 26 days, mice were sacrificed and analyzed for liver (A) and bone marrow metastases (B). Horizontal lines represent the median of each experimental group. (A) Liver metastases were staged after s.c. injection with 2 × 106 low (▴) and either 2 × 106 (⧫) or 4 × 106 intermediate (▾) scIL-12-producing NXS2 cells according to the percent liver surface covered with metastatic lesions. 4 = >75%; 3 = 50–75%; 2 = 25–50%; 1= >0–25%; 0 = 0%. Results were compared with mice receiving no s.c. injection (▪) or injection with 4 × 106 NXS2 cells carrying only the empty vector (•). Differences between results with intermediate scIL-12-producing NXS2 cells and all control groups were statistically significant (P < 0.001). (B) Bone marrow metastases were analyzed by tyrosine hydroxylase RT-PCR and scored, as described in Materials and Methods, after s.c. injection of 2 × 106 low (▴) and either 2 × 106 (⧫) or 4 × 106 intermediate (▾) scIL-12-producing NXS2 cells and compared with control animals receiving no s.c. injection (▪) or injection with 4 × 106 NXS2 cells carrying only the empty vector (•). Differences between results with intermediate scIL-12-producing NXS2 cells and all control groups were statistically significant (P < 0.005).
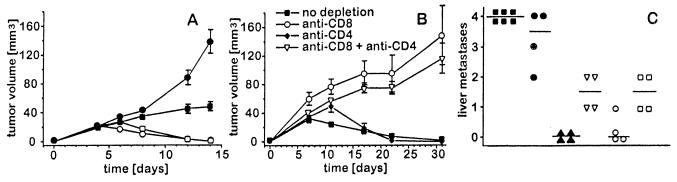
Figure 5.
Characterization of local (A and B) and systemic immune responses (C) induced by s.c. injection of NXS2 cells genetically engineered to produce scIL-12. T cell-deficient C.B-17 SCID mice (A) and immunocompetent A/J mice depleted of CD4+ and/or CD8+ T cell subsets in vivo (B) received s.c. injections with various scIL-12-producing NXS2 clones. Subcutaneous tumor volumes were calculated by measuring width/2 × width × length and expressed as cubic millimeters. T cell-depleted A/J mice were challenged 7 days after s.c. injection of 5 × 106 intermediate scIL-12-producing NXS2 cells with 5 × 104 NXS2 parental cells and sacrificed 26 days after challenge for evaluation of liver metastases. (A) C.B-17 SCID mice (filled symbols) and immunocompetent A/J mice (open symbols) in experimental groups of six mice each were injected with either 2 × 106 of low scIL-12-producing (circles) or 2 × 106 of intermediate scIL-12-producing NXS2 cells (squares). (B) Syngeneic A/J mice in vivo depleted of T cell subsets were injected with 5 × 106 intermediate scIL-12-producing NXS2 cells. T cell subsets were depleted by i.p. injection of 350 μg anti-CD4 and/or anti-CD8 monoclonal antibodies, as previously described (16). (C) Comparison of experimental liver metastases observed in T cell subset depleted (open symbols) versus T cell nondepleted (closed symbols) A/J mice previously vaccinated with 5 × 106 intermediate scIL-12-producing NXS2 cells and subsequently challenged with 5 × 104 NXS2 parental cells 7 days after vaccination. ▪, no depletion and no vaccination; •, no depletion, 5 × 106 NXS2 cells s.c. carrying the empty vector. Tumors were surgically removed 14 days after s.c. inoculation. In the following experimental groups, all mice received 5 × 106 intermediate scIL-12-producing NXS2 cells s.c.: ▴, no depletion; ▿, CD8+ T cell depletion; ○, CD4+ T cell depletion; □, CD8+ and CD4+ T cell depletion. Liver metastases were scored according to the liver surface coverage with metastatic lesions. 4 = >75%; 3 = 50–75%; 2 = 25–50%; 1 = >0–25%; 0 = 0%. Horizontal lines represent the median for each experimental group. Differences between results obtained with CD8+ T cell depletion (▿) or CD8+ and CD4+ T cell depletion (○) and no depletion (▴) were statistically significant (P < 0.05).
Figure 4.
Effect of single chain IL-12-producing NXS2 cells on established neuroblastoma metastases. Syngeneic A/J mice (n = 8) injected i.v. with 5 × 104 NXS2 cells received s.c. injections with intermediate and high scIL-12-producing NXS2 cell clones 5 days later. Developing s.c. tumors were surgically removed 14 days after s.c. inoculation. Twenty-six days after i.v. inoculation, mice were sacrificed and analyzed for liver (A) and bone marrow metastases (B). Horizontal lines represent the median for each experimental group. (A) Liver metastases were scored after s.c. injection with 4 × 106 intermediate (▾) and 10 × 106 high (▴) scIL-12-producing NXS2 cells according to the percent liver surface covered with metastatic lesions. 4 = >75%; 3 = 50–75%; 2 = 25–50%; 1= >0–25%; 0 = 0%. Results were compared with those of mice receiving either no s.c. injection (▪) or injection with 10 × 106 NXS2 cells carrying only the empty vector (•). Differences between all experimental and control groups were statistically significant (P < 0.001). (B) Bone marrow metastases were analyzed by tyrosine hydroxylase RT-PCR and scored, as described in Materials and Methods, after s.c. injection of 4 × 106 intermediate (▾) or 10 × 106 high (▴) scIL-12-producing NXS2 cells and compared with control animals receiving either no s.c. injection (▪) or injection with 10 × 106 NXS2 cells carrying only the empty vector (•). Differences between results with 10 × 106 high scIL-12-producing NXS2 cells and all control groups were statistically significant (P < 0.05).
Effector Mechanisms of the Immune Response Induced by Subcutaneous Vaccination with scIL-12-Producing NXS2 Cells.
In vivo. The local anti-tumor response induced after injection of NXS2 cells, genetically engineered to produce scIL-12, was investigated either in scid/scid mice deficient in mature T cells or in immunocompetent A/J mice depleted of CD4+ and/or CD8+ T cell subpopulations. Subcutaneous injection of 2 × 106 NXS2 cells, producing either intermediate or low amounts of scIL-12, into T cell-deficient mice lead to continuous s.c. tumor growth. The growth delay observed with intermediate versus low scIL-12 producing NXS2 cells is most likely due to a response of natural killer cells, which are fully functional in this mouse strain. This is in contrast to immunocompetent syngeneic A/J mice, which completely rejected NXS2 cells 14 days after inoculation (Fig. 5A), indicating the involvement of T cells in the initial response to the vaccine. This contention is further supported by results of experiments with in vivo-depleted immunocompetent A/J mice. Thus, only mice depleted of CD8+ T cells or CD8+ and CD4+ T cells revealed a continuous s.c. tumor growth after injection of 5 × 106 NXS2 cells producing intermediate amounts of scIL-12. In contrast, mice not depleted of T cells or depleted only of CD4+ T cells showed tumor rejection (Fig. 5B). After challenging such mice with NXS2 wild-type cells 7 days after subcutaneous vaccination, only those mice depleted of either CD8+ T cells or both CD8+ and CD4+ T cells developed significant macroscopic liver metastases, as compared with nondepleted controls. These findings indicate a major role for CD8+ T cells (Fig. 5C) in the protective systemic immunity induced by scIL-12-producing NXS2 cells.
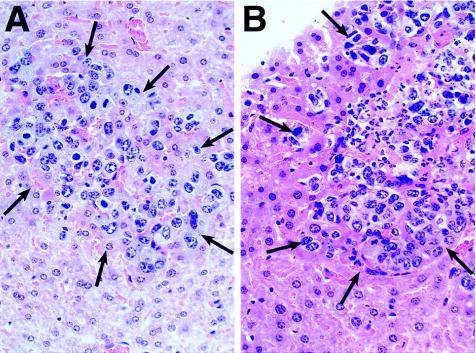
This conclusion is further supported by histological analyses of livers from mice with established liver metastases 7 days after s.c. vaccination. Only mice that were injected s.c. with 10 × 106 scIL-12-producing NXS2 cells developed an inflammatory polymorphonuclear infiltrate in established metastatic liver foci (Fig. 6B). In contrast, control mice receiving 10 × 106 NXS2 cells carrying only the empty vector showed the complete absence of such a response (Fig. 6A). This finding was confirmed by immunohistochemical analysis using a common leukocyte marker, e.g., mouse anti-CD45 mAb. In this case, only established liver metastases from mice previously vaccinated with scIL-12-producing NXS2 cells stained positively, as compared with control mice which were negative in this regard (data not shown).
Figure 6.
Morphology of tumor infiltrating lymphocytes in established liver metastasis after vaccination with NXS2 cells genetically engineered to produce scIL-12. Mice, with established liver metastases 5 days after injection of 5 × 104 NXS2 cells, were injected s.c. with 10 × 106 cells of either the high scIL-12-producing NXS2 clone (B) or NXS2 cells carrying the empty vector (A). Seven days after s.c. injection, livers were analyzed morphologically following embedding with paraffin and staining with hematoxylin/eosin. Slides were photographed at ×400. Arrows delineate metastases from surrounding liver tissue.
In vitro.
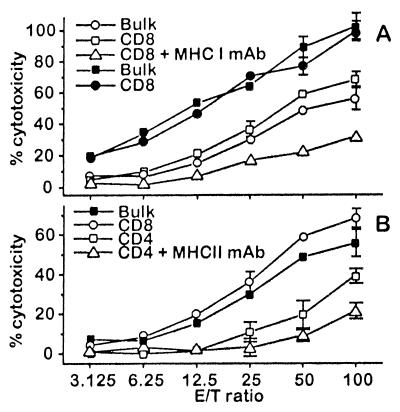
Splenocytes of mice previously vaccinated by two s.c. injections of 10 × 106 cells of the high scIL-12-producing NXS2 clone at 1-week intervals induced a strong lysis against NXS2 target cells in an 18-h chromium release assay. The highest lysis was produced by the CD8+ T cell population. However, this lysis was clearly suppressed after the addition of 25 μg/ml H2Kk MHC class I antibody (Fig. 7A), indicating an MHC class I specific restriction. Importantly, the same preparation of CD8+ T cells effectively lysed C1300 cells that were originally used to generate the hybrid neuroblastoma cell line NXS2, indicating that CD8+ T cells recognized an antigen shared by these two related cell lines. The use of CD4+ T cells in this same assay also induced lysis of NXS2 targets, but to a somewhat lesser extent than that of CD8+ T cells. This effect was specifically inhibited by I-Ak anti-MHC class II antibodies in the stimulation phase of the assay (Fig. 7B); however, this did not affect CD8+ T cell-mediated lysis, a well known feature of TH1 CD4+ cytotoxic T cells. Controls with effector cells from naïve mice revealed no detectable lysis (data not shown).
Figure 7.
Characterization of effector cells involved in the anti-tumor response observed following vaccination with NXS2 cells genetically engineered to produce scIL-12. Mice were vaccinated by two subcutaneous injections of 10 × 106 cells of the high scIL-12-producing NXS2 clone at 1-week intervals. (A) Splenocyte bulk cultures and purified CD8+ T cells from previously vaccinated mice were incubated with NXS2 (open symbols) and C1300 (closed symbols) target cells in either the presence or absence of anti-MHC class I antibodies. Cytotoxicity was determined in an 18-h 51Cr release assay. (B) Splenocyte bulk cultures (filled symbols) and purified T cell subsets (open symbols) were incubated with NXS2 target cells in either the presence or absence of anti-MHC class II antibodies in the stimulation phase. Cytotoxicity was determined in an 18-h 51Cr release assay.
DISCUSSION
The induction of an effective cellular immune response against syngeneic tumors by a local increase of inflammatory cytokines in the tumor microenvironment followed by a protective immunity is a promising approach for cancer immunotherapy. This can be achieved among other means by the use of tumor cells genetically engineered ex vivo to produce various cytokines (17), including IL-12 (6), which proved to be a very effective immunomodulator in this regard. However, most of the expression systems used thus far had to rely on the separate transduction of the two genes encoding for p35 and p40 chains and were forced to use separate expression units that complicated the design of vectors used for IL-12 gene therapy.
Here, we demonstrate the efficacy of an IL-12 gene therapy approach using a genetically engineered single chain IL-12 fusion protein, constructed by linkage of the p35 and p40 genes with DNA encoding for a flexible protein linker commonly used in bioengineering of single chain antibodies. Due to the monomeric nature of this single chain IL-12 fusion protein, it assures equimolar expression of each subunit, thus avoiding the formation of inhibitory p40 dimers and facilitating its use in vectors with only one expression unit. The bioactivity and efficacy for this type of gene therapy was established in a novel, immunocompetent syngeneic model for murine neuroblastoma using NXS2 cells that naturally express the disialoganglioside GD2. This model also features experimental metastases to bone marrow and liver, expresses the neuroblastoma tumor marker tyrosine hydroxylase, and represents many pathophysiological similarities to human neuroblastoma (15).
The immune response induced by genetically engineered NXS2 cells, which produce such single chain IL-12 constructs, is highly effective, as it was clearly mediated by CD8+ T cells followed by a protective immunity against a subsequent challenge with NXS2 wild-type cells. This finding is in contrast to a natural killer cell-mediated immune mechanism observed in the same animal model induced by a recombinant anti-disialoganglioside GD2–interleukin 2 fusion protein, which directs IL-2 to the tumor microenvironment (16). Because such recombinant IL-2 fusion proteins were demonstrated to induce T cell-mediated immune responses in other animal models, e.g., melanoma (18) and colon carcinoma (19), we concluded that poor immunogenicity and immunosuppressive factors secreted by NXS2 cells, e.g., IL-10 and transforming growth factor-β, could account for T cell anergy and preferential natural killer cell-mediated responses in this model (16). However, this T cell anergy is apparently overcome by the use of IL-12 gene therapy.
One of the major differences between the two proinflammatory TH1 cytokines IL-2 and IL-12, both capable of potentiating T and natural killer cell-mediated cytotoxic responses, is that IL-12 is a much stronger stimulator of TH1 CD4+ T cells (20). These cells are involved in the maturation of CD8+ T cells, the main effector cells observed in our experimental system. In fact, we were able to demonstrate the presence of CD4+ T cells in cellular infiltrates observed in livers from mice previously vaccinated with scIL-12-producing NXS2 cells. Interestingly, this is in contrast to previous work with a recombinant antibody IL-2 fusion protein in this same tumor model, where such CD4+ T cells were completely absent (16). The contention that an increased TH1 CD4+ T cell stimulation is achieved by using IL-12 instead of IL-2 is supported by the observation of CD4+ T cell-mediated killing in vitro after vaccination with scIL-12-producing NXS2 cells.
In summary, we demonstrate here that CD8+ T cells, induced by subcutaneous vaccination with NXS2 cells, genetically engineered to produce a single chain IL-12 fusion protein, effectively protect syngeneic A/J mice from disseminated neuroblastoma tumor growth, while eradicating established metastases to liver and markedly suppressing bone marrow metastases. A possible role for CD4+ T cells, as helper or effector cells, was proposed due to their presence in cellular infiltrates and cytotoxic activity observed in vitro. The use of monomerized IL-12, constructed by genetic linkage of the p35 and p40 chains with a flexible protein linker, proved to be highly effective in this regard. This IL-12 fusion protein will also simplify future vector designs for gene therapy approaches. Taken together, the data described here suggest that application of IL-12 gene therapy may overcome the poor immunogenicity of neuroblastoma and therefore lead to further improvement in the treatment of patients with minimal residual disease in an adjuvant setting.
Acknowledgments
We thank Carrie Dolman for her excellent technical assistance. We also express our appreciation to Lynne Kottel for preparation of this manuscript. This work was supported by the National Institutes of Health, Outstanding Investigator’s Grant, CA-42508 (R.A.R.). H.N.L. and T.D. were supported by a training grant of the Deutsche Forschungsgemeinschaft. This is The Scripps Research Institute Manuscript 11204-IMM.
ABBREVIATIONS
- scIL-12
single chain interleukin 12
- IL
interleukin
- RT
reverse transcription
- MHC
major histocompatibility complex
References
- 1.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern A S, Podlaski F J, Hulmes J D, Pan Y C, Quinn P M, Wolitzky A G, Familletti P C, Stremlo D L, Truitt T, Chizzonite R, et al. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall E. Science. 1995;268:1555–1555. [Google Scholar]
- 4.Rakhmilevich A L, Turner J, Ford M J, McCabe D, Sun W H, Sondel P M, Grota K, Yang N S. Proc Natl Acad Sci USA. 1996;93:6291–6296. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahara H, Zitvogel L, Storkus W J, Zeh H J, III, McKinney T G, Schreiber R D, Gubler U, Robbins P D, Lotze M T. J Immunol. 1995;154:6466–6474. [PubMed] [Google Scholar]
- 6.Lotze M T, Zitvogel L, Campbell R, Robbins P D, Elder E, Haluszczak C, Martin D, Whiteside T L, Storkus W J, Tahara H. Ann NY Acad Sci. 1996;795:440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- 7.Tahara H, Zeh H J, III, Storkus W J, Pappo I, Watkins S C, Gubler U, Wolf S F, Robbins P D, Lotze M T. Cancer Res. 1994;54:182–189. [PubMed] [Google Scholar]
- 8.Zitvogel L, Tahara H, Robbins P D, Storkus W J, Clarke M R, Nalesnik M A, Lotze M T. J Immunol. 1995;155:1393–1403. [PubMed] [Google Scholar]
- 9.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 10.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 11.Huston J S, Levinson D, Mudgett-Hunter M, Tai M S, Novotny J, Margolies M N, Ridge R J, Bruccoleri R E, Haber E, Crea R, et al. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieschke G J, Rao P K, Gately M K, Mulligan R C. Nat Biotechnol. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 13.Gately M K, Chizzonite R, Presky D H. In: Measurements of Human and Mouse Interleukin 12. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: John Wiley; 1995. pp. 6.16.1–6.16.15. [Google Scholar]
- 14.Greene L A, Shain W, Chalazonitis A, Breakfield X, Minna J, Coon H G, Nirenberg M. Proc Natl Acad Sci USA. 1975;72:4923–4927. doi: 10.1073/pnas.72.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lode H N, Xiang R, Varki N M, Dolman C S, Gillies S D, Reisfeld R A. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 16.Lode, H. N., Xiang, R., Dreier, T., Varki, N. M., Gillies, S. D. & Reisfeld, R. A. (1997) Blood, in press. [PubMed]
- 17.Golumbek P T, Azhari R, Jaffee E M, Levitsky H I, Lazenby A, Leong K, Pardoll D M. Cancer Res. 1993;53:5841–5844. [PubMed] [Google Scholar]
- 18.Becker J C, Pancook J D, Gillies S D, Furukawa K, Reisfeld R A. J Exp Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang R, Lode H N, Dolman C S, Dreier T, Varki N M, Qian X, Lo K, Lan Y, Super M, Gillies S D, Reisfeld R A. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 20.Trinchieri G. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]