Abstract
Serotype VIII group B streptococcus has only rarely been described outside Japan. The Streptococcus Unit, Statens Serum Institut, performed national surveillance of invasive group B streptococcal (GBS) diseases in Denmark in 1999 to 2002 and identified seven clinical GBS isolates of serotype VIII in blood from seven patients admitted to different hospitals.
Group B streptococci (GBS) are a cause of mortality and morbidity in neonates, besides being an emerging cause of invasive infection in elderly patients with underlying diseases (9, 16). GBS are classified into nine serotypes, Ia, Ib, and II to VIII, based on type-specific capsular polysaccharides, and surveillance studies have shown that serotype VIII predominates among GBS isolated from pregnant women in Japan. Outside Japan, serotype VIII is rare and only a few isolates have been described (4, 6, 10, 13, 17). The Streptococcus Unit, Statens Serum Institut, Copenhagen, Denmark, has surveyed the serotype distribution in invasive GBS infections in Denmark since 1999. We present seven clinical GBS isolates with serotype VIII from seven patients admitted to hospitals in Denmark during 2001 and 2002.
The Streptococcus Unit of the Statens Serum Institut serves as the National Streptococcus Reference Center and receives the majority of invasive GBS isolates as pure cultures from local clinical microbiological departments for national surveillance. Included in this study were GBS blood isolates collected from patients admitted to Danish hospitals from 1 January 1999 to 31 December 2002 and two serotype VIII GBS reference strains, JM9 Prague no. 130013 (CNCTC 1/92) and JM9 Prague no. 130669, which were kindly provided by J. Motlova, National Streptococcus and Enterococcus Reference Laboratory, National Institute of Public Health, Prague, Czech Republic.
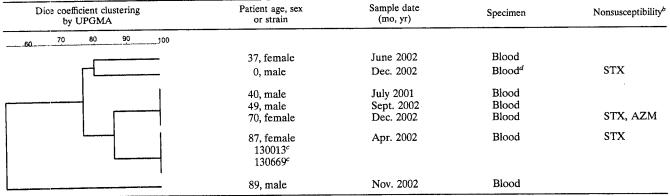
All isolates were serotyped by precipitin test as described by Lancefield with specific rabbit anti-capsular polysaccharide antibodies (Ia, Ib, and II to VIII; Statens Serum Institut). Nonserotypeable isolates were designated NT (8). Both 0.1 and 0.2 N hydrochloric acid (HCl) solutions were used to extract the capsular polysaccharide antigens (H.-C. Slotved, S. Sauer, and H. B. Konradsen, Letter, J. Clin. Microbiol. 40:1882-1883, 2002). MICs of benzylpenicillin, gentamicin, moxifloxacin, trimethoprim-sulfamethoxazole, tetracycline, clindamycin, and azithromycin were determined by E test (AB-Biodisk, Solna, Sweden) on Mueller-Hinton agar with 5% lysed horse blood (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Breakpoints were defined in accordance with NCCLS guidelines, except for trimethoprim-sulfamethoxazole and moxifloxacin, for which the breakpoints of the Swedish Reference Group for Antibiotics were used (12). The Swedish Reference Group for Antibiotics breakpoints were obtained at http://www.ltkronoberg.se/ext/raf/raf.htm. The clinical and reference strains were analyzed by pulsed-field gel electrophoresis (PFGE) as described elsewhere (15) with supplementation of the lysis buffer with 22 μl of mutanolysin (Sigma, St. Louis, Mo.) per ml (14). Calculation of similarity between PFGE patterns with the Dice coefficient and clustering by the unweighted pair group method using mathematical averages (UPGMA) was done in BioNummerics (Applied Maths, Kortrijk, Belgium) after the image was captured by Chemi Doc (Bio-Rad Laboratories). Strains were considered to be clonal if the Dice coefficients were greater than 85%.
In the study period, we received 386 invasive GBS isolates from patients admitted to hospitals in Denmark, and seven GBS isolates of serotype VIII were identified from 31 July 2001 to 20 December 2002 in blood from seven patients admitted to different hospitals, which constituted 1 and 6% of the total number of invasive GBS isolates in 2001 and 2002, respectively. The median age of the patients (three female [one pregnant] and four male) was 49.3 (range, 0.0 to 88.7) years. None of the patients had been outside Denmark in the last 3 weeks before hospital admission. Besides bacteremia, three of the patients presented erysipelas and one had clinical cystitis. In two of the patients, skin lesions were reported as a predisposing factor, one patient received immunosuppressive treatment, and one patient had no predisposing factors reported.
Table 1 shows the similarity of the SmaI PFGE patterns of the two reference strains and the seven epidemiologically unrelated isolates based on the Dice coefficient and clustering by UPGMA, in addition to the results of the antibiotic susceptibility tests. Four of the clinical isolates belonged to the same clone as the reference strains. The remaining three clinical isolates had distinct PGFE patterns.
TABLE 1.
Cluster analysis of SmaI PFGE of seven clinical serotype VIII GBS strains identified in Denmark is 2001 and 2002 in comparison with two reference strainsa
Similarities are based on the Dice coefficient, and cluster analysis was performed in according to UPGMA in BioNumerics.
STX, trimethoprim-sulfamethoxazole; AZM, azithromycin.
JM9 reference strains CNCTC Prague no. 130013 and Prague no. 130669 were used.
Taken from umbilical veins.
In Japan, GBS serotype VIII constitutes 36% of the serotypes identified in pregnant women (7), but outside Japan, isolates of serotype VIII have only been sporadically reported. In 2001 and 2002, serotype VIII constituted 1 and 6% of all of the invasive GBS infections in Denmark, respectively. The pathogenicity of serotype VIII has been described to be weaker than that of serotypes III and Ia because serotype VIII is rarely identified in neonatal GBS infections (5, 10). In Maryland, Harrison found one GBS isolate with serotype VIII among 816 invasive isolates from children and adults (4), while Paoletti identified one serotype VIII isolate among 114 GBS isolates colonizing women and children in Boston, Mass. (13). Setting up a group- and serotype-specific PCR, Kong et al. used one serotype VIII strain isolated from Australia (6), and two invasive isolates of serotype VIII were recently identified in Perugia, Italy, in a GBS surveillance of neonates (3). In a surveillance study in Korea, four noninvasive isolates of serotype VIII were detected in urine, female genital, and pus specimens (10). Other GBS surveillance studies have looked for but not identified serotype VIII (18, 19). GBS surveillance limited to serotypes Ia to V has also been performed (1, 11). Our study emphasized the importance of using antisera specific for serotypes Ia to VIII when describing the complete serotype distribution in GBS surveillance. In addition, we have been able to reduce the number of strains originally determined as NT by supplementing 0.2 N HCl with 0.1 N HCl in the extraction of capsular antigen (Slotved et al., letter, 2002).
The susceptibility testing revealed only minor differences between the two reference strains and the seven clinical isolates. The PFGE results showed that several lineages of GBS serotype VIII are causing invasive disease in Denmark. Four of the clinical isolates showed relatedness to the reference strains at a similarity level of greater than 85%, while three isolates belonged to separate lineages. In comparison Benson et al. found great homology among eight strains of serotype VIII (2). We conclude that serotype VIII is causing invasive infections outside Japan; therefore, monitoring of the serotype distribution including all nine serotypes (Ia to VIII) is necessary to sustain complete surveillance of invasive GBS infections.
REFERENCES
- 1.Amin, A., Y. M. Abdulrazzaq, and S. Uduman. 2002. Group B streptococcal serotype distribution of isolates from colonized pregnant women at the time of delivery in United Arab Emirates. J. Infect. 45:42-46. [DOI] [PubMed] [Google Scholar]
- 2.Benson, J. A., and P. Ferrieri. 2001. Rapid pulsed-field gel electrophoresis method for group B streptococcus isolates. J. Clin. Microbiol. 39:3006-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung, M., H. de Lencastre, P. Matthews, A. Tomasz, I. Adamsson, D. S. Aries, T. Camou, C. Cocuzza, A. Corso, I. Couto, A. Dominguez, M. Gniadkowski, R. Goering, A. Gomes, K. Kikuchi, A. Marchese, R. Mato, O. Melter, D. Oliveira, R. Palacio, R. Sa-Leao, S. Santos, I. J. H. Song, P. T. Tassios, and Multilaboratory Project Collaborators. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb. Drug Resist. 6:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Harrison, L. H., J. A. Elliott, D. M. Dwyer, J. P. Libonati, P. Ferrieri, L. Billmann, and A. Schuchat. 1998. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J. Infect. Dis. 177:998-1002. [DOI] [PubMed] [Google Scholar]
- 5.Hoshina, K., Y. Suzuki, H. Nishida, K. Kaneko, S. Matsuda, M. Kobayashi, and N. Kadoi. 2002. Trend of neonatal group B streptococcal infection during the last 15 years. Pediatr. Int. 44:641-646. [DOI] [PubMed] [Google Scholar]
- 6.Kong, F., S. Gowan, D. Martin, G. James, and G. L. Gilbert. 2002. Serotype identification of group B streptococci by PCR and sequencing. J. Clin. Microbiol. 40:216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachenauer, C. S., D. L. Kasper, J. Shimada, Y. Ichiman, H. Ohtsuka, M. Kaku, L. C. Paoletti, P. Ferrieri, and L. C. Madoff. 1999. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J. Infect. Dis. 179:1030-1033. [DOI] [PubMed] [Google Scholar]
- 8.Lancefield, R. C. 1933. A serological differentiation of specific types of bovine hemolytic streptococci (group B). J. Exp. Med. 59:441-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laursen, S. B., and H. B. Konradsen. 1997. Group B streptococcal infection in adults. Ugeskr. Laeg. 159:732-735. [PubMed] [Google Scholar]
- 10.Lee, K., J. W. Shin, Y. Chong, and H. Mikamo. 2000. Trends in serotypes and antimicrobial susceptibility of group B streptococci isolated in Korea. J. Infect. Chemother. 6:93-97. [DOI] [PubMed] [Google Scholar]
- 11.Moyo, S. R., J. A. Maeland, and K. Bergh. 2002. Typing of human isolates of Streptococcus agalactiae (group B streptococcus, GBS) strains from Zimbabwe. J. Med. Microbiol. 51:595-600. [DOI] [PubMed] [Google Scholar]
- 12.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing; twelfth informational supplement. NCCLS, Wayne, Pa.
- 13.Paoletti, L. J., J. Bradford, and L. C. Paoletti. 1999. A serotype VIII strain among colonizing group B streptococcal isolates in Boston, Massachusetts. J. Clin. Microbiol. 37:3759-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen, J. P., M. S. Kaltoft, J. C. Misfeldt, H. Schumacher, and H. C. Schonheyder. 2003. A community outbreak of perianal group A streptococcal infection in Denmark. Pediatr. Infect. Dis. J. 22:105-109. [DOI] [PubMed]
- 15.Sa-Leao, R., A. Tomasz, I. S. Sanches, S. Nunes, C. R. Alves, A. B. Avo, J. Saldanha, K. G. Kristinsson, and H. de Lencastre. 2000. Genetic diversity and clonal patterns among antibiotic-susceptible and -resistant Streptococcus pneumoniae colonizing children: day care centers as autonomous epidemiological units. J. Clin. Microbiol. 38:4137-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuchat, A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11:497-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sensini, A., L. Tissi, N. Verducci, M. Orofino, C. von Hunolstein, B. Brunelli, G. L. Mala, F. Perocchi, R. Brunelli, V. Lauro, R. Ferrarese, and G. Gilardi. 1997. Carriage of group B Streptococcus in pregnant women and newborns: a 2-year study at Perugia General Hospital. Clin. Microbiol. Infect. 3:324-328. [DOI] [PubMed] [Google Scholar]
- 18.Tyrrell, G. J., L. D. Senzilet, J. S. Spika, D. A. Kertesz, M. Alagaratnam, M. Lovgren, and J. A. Talbot. 2000. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study—1996. J. Infect. Dis. 182:168-173. [DOI] [PubMed] [Google Scholar]
- 19.Zaleznik, D. F., M. A. Rench, S. Hillier, M. A. Krohn, R. Platt, M. L. Lee, A. E. Flores, P. Ferrieri, and C. J. Baker. 2000. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30:276-281. [DOI] [PubMed] [Google Scholar]