Abstract
In this study we describe a multiplex PCR assay for the detection of nine clinically relevant antibiotic resistance genes of Staphylococcus aureus. Conditions were optimized to amplify fragments of mecA (encoding methicillin resistance), aacA-aphD (aminoglycoside resistance), tetK, tetM (tetracycline resistance), erm(A), erm(C) (macrolide-lincosamide-streptogramin B resistance), vat(A), vat(B), and vat(C) (streptogramin A resistance) simultaneously in one PCR amplification. An additional primer pair for the amplification of a fragment of the staphylococcal 16S rDNA was included as a positive control. The multiplex PCR assay was evaluated on 30 different S. aureus isolates, and the PCR results correlated with the phenotypic antibiotic resistance data obtained by the broth microdilution assay. The multiplex PCR assay offers a rapid, simple, and accurate identification of antibiotic resistance profiles and could be used in clinical diagnosis as well as for the surveillance of the spread of antibiotic resistance determinants in epidemiological studies.
During the past 15 years, the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) has increased in many parts of the world (38). Severe MRSA infections such as bacteremia are associated with higher mortality than one methicillin-susceptible S. aureus infections (see reference 10 for a meta-analysis). It is well established that rapid identification and antimicrobial susceptibility testing have an impact on the clinical outcome of severe infections (6, 7). Rapid detection of MRSA by PCR for the mecA gene coding for methicillin resistance via penicillin binding protein 2a (PBP2a) is well established (14). Furthermore, this PCR became the “gold standard” for detection of methicillin resistance because methicillin (oxacillin) resistance is often heterogeneously expressed in vitro (8).
Until the mid-1990s the majority of MRSA isolates exhibited a multiresistance phenotype. Therefore, demonstration of mecA as basic marker was regarded as sufficient for choosing glycopeptides as a therapeutic alternative. However, we have observed the dynamics of MRSA clones during the past 8 years and detected an increased prevalence of newly emerging epidemic strains with less broad resistance patterns, at least in Europe (20, 39). On the other hand, outbreaks with “old” epidemic strains that had acquired resistance to quinupristin-dalfopristin and also exhibited reduced susceptibility to glycopeptides have been reported (15, 36). There are also observations of sporadic cases of linezolid resistance in S. aureus (33, 37) and of acquisition of transferable glycopeptide resistance (vanA [30]). Therefore, detection of genes conferring resistance to the “old” antibiotics like macrolides, lincosamidines, gentamicin, and tetracyclines has become clinically relevant again (18). For these reasons, rapid diagnostic tests for MRSA should include tests of resistances to older standard antibiotics as well as to recently introduced compounds.
Here we report a multiplex PCR based on demonstration of the most frequent resistance genes besides mecA for different groups of antibiotics. For aminoglycoside resistance, this is the aacA-aphD gene, coding for a bifunctional enzyme (26, 34) and conferring cross-resistance to clinically used aminoglycosides such as gentamicin, tobramycin, kanamycin, and, when overexpressed, amikacin (31). In the vast majority of S. aureus isolates, resistance to macrolides is due to N6-dimethylation of the adenine at position 2058 of 23S rRNA. Recent clinical isolates possess the erm(A) and/or the erm(C) gene coding for rRNA methylases; hospital strains carrying erm(B) are rather infrequent (29). When expressed constitutively, methylases also confer resistance to lincosamidines and to streptogramin B compounds. For full resistance to the streptogramin combination quinupristin-dalfopristin, strains need to carry additional resistance to streptogramin A compounds, which is basically mediated by acetylation [acetyltransferase genes vat(A), vat(B), and vat(C) (1, 3, 5)], supplemented by efflux pumps encoded by vga(A) and vga(B) (2, 4). Tetracycline resistance in S. aureus is either based on modification of the ribosome encoded by the widely disseminated tetM gene or mediated by tetK encoded efflux. Of the variety of different tet genes coding for efflux mechanisms, tetK is most often found in S. aureus (27, 32). Tetracycline-resistant MRSA isolates (up to 50% of MRSA isolates [27]) carrying the tetK gene are susceptible to minocycline, whereas minocycline-resistant strains harbor tetM or both tetK and tetM (32).
MATERIALS AND METHODS
Bacterial strains.
S. aureus isolates investigated in this study originated from the strain collection of our laboratory at the National Reference Center for Staphylococci in Germany and are representatives of the major clonal groups of MRSA disseminated in Central Europe (12). S. aureus ES 1767 [the reference strain for vat(A)], ES 1768 [vat(B)] and ES 1877 [vat(C)] were kindly provided by N. El Solh, Paris, France.
Antimicrobial susceptibility testing.
Isolates were tested by the broth microdilution assay as described in the NCCLS standard (25), except that Iso-Sensitest broth (Oxoid, Wesel, Germany) was used. The antibiotic panel included penicillin G, oxacillin, gentamicin, erythromycin, clindamycin, oxytetracycline, ciprofloxacin, phosphomycin, chloramphenicol, trimethoprim-sulfamethoxazole, fusidic acid, rifampin, quinupristin-dalfopristin, vancomycin, teicoplanin, and linezolid. The breakpoints were those described in NCCLS standards (21) except for those antibacterials not included in the standards, such as phosphomycin (resistance, ≥128 mg/liter), fusidic acid (≥4 mg/liter), and linezolid (≥8 mg/liter).
DNA extraction.
DNA was extracted from about 10 single colonies with the DNeasy tissue kit (Qiagen, Hilden, Germany) as specified by the manufacturer and using lysostaphin (100 μg/ml; Sigma, Munich, Germany) to achieve bacterial lysis.
Multiplex PCR for the detection of selected antibiotic resistance genes.
The PCR primers used to detect 10 different loci in a multiplex PCR approach are listed in Table 1. All primers were selected from public databases and synthesized by TibMolbiol (Berlin, Germany). Primer length and G+C content were kept as uniform as possible to minimize differences in annealing temperature and were checked for specificity in a BLAST search available through the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). The primers were tested in single PCR amplifications with DNA from genotypically defined isolates before being used in the multiplex assay. Single PCR amplifications were performed with Ready-to-Go-PCR beads (Amersham Pharmacia Biotech, Freiburg, Germany) in a 25-μl reaction mixture containing approximately 10 ng of template DNA and 2.5 pmol of each primer. Initial denaturation at 94°C for 3 min was followed by 30 cycles of amplification with 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s (except for the final cycle, which had an extension step of 4 min). The PCR products were analyzed on a 1.5% agarose gel and further controlled by sequencing.
TABLE 1.
Characteristics of the primers used in this study
| Primer pair | Target gene | Resistance phenotypea | Reference strain | Sequence (5′→3′) | Amplicon size (bp) | GenBank accession no. (reference) |
|---|---|---|---|---|---|---|
| mecA 1 | mecA | PEN, OXA | 694/01 | AAA ATC GAT GGT AAA GGT TGG C | 532 | Y00688, bp 1282-1303 |
| mecA 2 | AGT TCT GCA GTA CCG GAT TTG C | Y00688, bp 1814-1793 | ||||
| aacA-aphD 1 | aacA-aphD | GEN | 694/01 | TAA TCC AAG AGC AAT AAG GGC | 227 | M18086, bp 2144-2164 |
| aacA-aphD 2 | GCC ACA CTA TCA TAA CCA CTA | M18086, bp 2371-2350 | ||||
| ermA 1 | erm(A) | ERY, CLI | 694/01 | AAG CGG TAA ACC CCT CTG A | 190 | X03216, bp 5074-5056 |
| ermA 2 | TTC GCA AAT CCC TTC TCA AC | X03216, bp 4885-4904 | ||||
| ermC 1 | erm(C) | ERY, CLI | 694/01 | AAT CGT CAA TTC CTG CAT GT | 299 | V01278, bp 2068-2088 |
| ermC 2 | TAA TCG TGG AAT ACG GGT TTG | V01278, bp 2365-2345 | ||||
| tetK 1 | tetK | OTE | 694/01 | GTA GCG ACA ATA GGT AAT AGT | 360 | S67449, bp 871-891 |
| tetK 2 | GTA GTG ACA ATA AAC CTC CTA | S67449, bp 1231-1211 | ||||
| tetM 1 | tetM | OTE | 694/01 | AGT GGA GCG ATT ACA GAA | 158 | X56353, bp 301-318 |
| tetM 2 | CAT ATG TCC TGG CGT GTC TA | X56353, bp 459-440 | ||||
| vatA 1 | vat(A)b | SYN | ES 1767 | TGG TCC CGG AAC AAC ATT TAT | 268 | AF117258, bp 2642-2622 |
| vatA 2 | TCC ACC GAC AAT AGA ATA GGG | AF117258, bp 2375-239 | ||||
| vatB 1 | vat(B)b | SYN | ES 1768 | GCT GCG AAT TCA GTT GTT ACA | 136 | U19459, bp 496-519 |
| vatB 2 | CTG ACC AAT CCC ACC ATT TTA | U19459, bp 631-611 | ||||
| vatC 1 | vat(C)b | SYN | ES 1877 | AAG GCC CCA ATC CAG AAG AA | 467 | AF015628, bp 1326-1345 |
| vatC 2 | TCA ACG TTC TTT GTC ACA ACC | AF015628, bp 1753-1773 | ||||
| 16s 1 | 16S rDNA | Amplification control | Ubiquitous (Staphylococcus) | CAG CTC GTG TCG TGA GAT GT | 420 | Y15856, bp 1058-1077 |
| 16s 2 | AAT CAT TTG TCC CAC CTT CG | Y15856, bp 1477-1458 (11) | ||||
| sau1 | S. aureus-specific sequence | Amplification control | S. aureus specific | AAT CTT TGT CGG TAC ACG ATA TTC TTC ACG | 107 | AF033191, bp 5-34 |
| sau2 | CGT AAT GAG ATT TCA GTA GAT AAT ACA ACA | AF033191, bp 112-83 (24) |
PEN, penicillin; OXA, oxacillin; GEN, gentamicin; ERY, erythromycin; CLI, clindamycin; OTE, oxytetracyline; SYN, quinupristin-dalfopristin.
The constitutive erm gene is present.
Sequencing reactions were carried out using the ABI PRISM BigDye Terminator cycle-sequencing ready reaction kit (Applied Biosystems, Foster City, Calif.) as specified by the manufacturer. Sequence comparison to the published sequence data was performed with the DNASTAR software package (DNASTAR Inc., Madison, Wis.).
Multiplex PCR amplifications were carried out in a 100-μl volume comprising approximately 40 ng of template DNA, 10 pmol of each of the 20 primers, a final concentration of 0.4 mM each deoxyribonucleoside triphosphate, and 5 U of Taq DNA polymerase (Amersham Pharmacia Biotech) in 1× PCR buffer supplied by the manufacturer; the MgCl2 final concentration in the PCR mixture was adjusted to 4 mM. Cycling conditions were the same as described above. Amplification products were analyzed on a 2.5% agarose gel (80 V for 200 min) to separate the different amplification products efficiently. To accelerate the analysis, amplification products were separated using the 2100 Bioanalyzer together with the DNA 1000 LabChip kit (Agilent Technologies, Böblingen, Germany).
Southern hybridization.
Southern blotting and hybridizations were performed by standard procedures. To verify the identity of the amplification products, the products of the multiplex reaction as well as the single PCR amplification (positive control) were transferred to nylon membranes (Boehringer GmbH, Mannheim, Germany) and hybridized with the different digoxigenin-labeled PCR products as probes.
RESULTS
Multiplex PCR for the detection of selected antibiotic resistance genes.
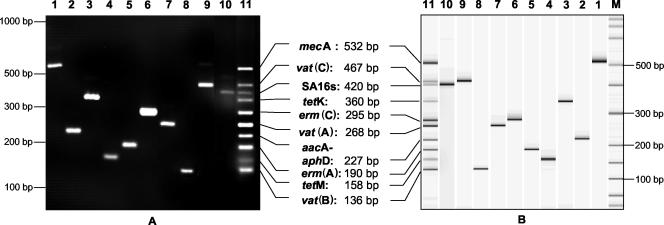
Before the optimization of the multiplex reaction, we ensured that the single PCR amplifications yielded amplicons of the expected sizes. Amplicon sizes ranged from 107 to 532 bp, differing by at least 20 bp to facilitate electrophoretic separation (Table 1). The reaction conditions for the multiplex PCR were optimized using a DNA mixture (containing all resistance gene targets) and following the general principles described by Henegariu et al. (16) to amplify the 10 targets almost equally. Therefore, we tested different concentrations of primer, oligonucleotides, and MgCl2 until we had adjusted the optimal conditions as described in Materials and Methods. Figure 1A shows an agarose gel stained with ethidium bromide to illustrate the typical results obtained with the single PCR amplifications and the optimized multiplex PCR assay, respectively. To accelerate the analysis of the amplification product, we separated the PCR fragments by use of the Agilent Bioanalyzer together with the Agilent DNA 1000 LabChip kit. The separation process takes only 30 min by this technique, in contrast to 3 h by conventional gel electrophoresis (Fig. 1B). To verify the efficiency of the amplification and the absence of significant PCR inhibition, an internal control primer pair targeting the staphylococcal 16S rDNA (16s 1 plus 16s 2 [Table 1]) was included in the Multiplex PCR assay. Using this primer pair, we were able to amplify a PCR product of the expected size from template DNA of 10 different staphylococcal species (including S. aureus and coagulase-negative staphylococci [CNS]) but not from DNA of other bacterial species. Another internal control primer pair targeting an S. aureus-specific DNA fragment (24) was included as an alternative (data not shown). Including this primer pair facilitates the definite identification of S. aureus isolates as well as the determination of their resistance genotypes. To determine the sensitivity of the assay, we performed multiplex PCR using different amounts of template DNA ranging from 40 ng to 100 fg in a 50-μl reaction mixture. We were able to amplify all expected targets from a minimum of 100 pg of target DNA (data not shown). To verify the identity of the PCR products, the single amplicons were sequenced; all sequences obtained were identical to those obtained from the databases. Furthermore, digoxigenin-labeled amplicons were used as probes in a Southern blot hybridization. All probes hybridized to the expected fragments of the multiplex PCR amplification, indicating that all targets are amplified efficiently and correctly in the multiplex approach (data not shown).
FIG. 1.
Single and multiplex PCR amplification products for selected staphylococcal genes. Lanes: 1, mecA; 2, aacA-aphD; 3, tetK; 4, tetM; 5, erm(A); 6, erm(C); 7, vat(A); 8, vat(B); 9, vat(C); 10, 16S rRNA; 11, multiplex; M, molecular size standard. (A) DNA fragments were visualized on a 2.5% agarose gel and stained with ethidium bromide. (B) Fragment analysis was performed with the Agilent Bioanalyzer and the DNA 1000 LabChip kit.
Correlation between susceptibility testing and the multiplex PCR.
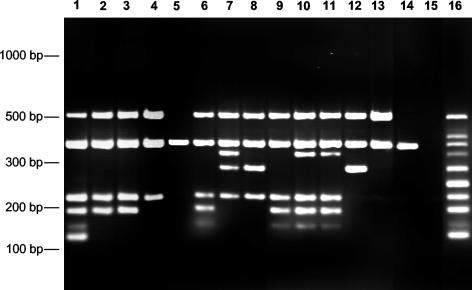
For 30 Staphylococcus isolates, we compared susceptibility results determined by the broth microdilution assay with the results of the multiplex PCR assay for the simultaneous detection of antibiotic resistance genes (Fig. 2; Table 2). For these isolates, we found a correlation between the results of the multiplex PCR and those of classical resistance testing (Table 2). The amplification control could be amplified in all Multiplex PCR amplifications, indicating that the PCR had been performed efficiently. Of 28 oxacillin-resistant strains, all carried a mecA gene. All gentamicin-resistant isolates were shown to have the aacA-aphD gene. A total of 23 strains were resistant to erythromycin and/or clindamycin. In 15 of these isolates, the erm(A) gene was present; in 8, erm(C) could be detected. One isolate carried both erm(A) and erm(C). Ten isolates were resistant to oxytetracycline and carried either tetK or tetM or both resistance genes. The only quinupristin-dalfopristin-resistant isolate in our collection was shown to carry the vat(B) gene. Thus, the results of the multiplex PCR assay correlated with the results of the phenotypic antibiotic resistance determination.
FIG. 2.
Examples of multiplex PCR amplifications on template DNA of 14 different S. aureus isolates (lanes 1 to 14). Lanes: 1, 1719/00; 2, 3932/02; 3, 3940/02; 4, 3887/02; 5, 3211/02; 6, 3516/02; 7, 2513/02; 8, 2569/02; 9, 1450/93; 10, 3396/01; 11, 163/01; 12, 3980/01; 13, 1150/93; 14, 8325; 15, no template DNA; 16, DNA mixture with all targets.
TABLE 2.
Correlation between phenotypic antibiotic resistance and PCR results
| S. aureus strain | Resistance phenotypea | Presence of fragment:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16S | mecA | aacA-aphD | erm(A) | erm(C) | tetK | tetM | vat(A) | vat(B) | vat(C) | ||
| 994/93 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, RAM, FUS | + | + | + | + | − | − | + | − | − | − |
| 1150/93 | PEN, OXA, CIP | + | + | − | − | − | − | − | − | − | − |
| 1450/93 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, RAM | + | + | + | + | − | − | + | − | − | − |
| 1678/96 | PEN, OXA, CIP | + | + | − | − | − | − | − | − | − | − |
| 1719/00 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, SXT, FUS, RAM, SYN | + | + | + | + | − | − | + | − | + | − |
| 163/01 | PEN, OXA, GEN, ERY, OTE, CIP | + | + | + | + | − | + | + | − | − | − |
| 694/01 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, RAM | + | + | + | + | + | + | + | − | − | − |
| 2437/01 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, RAM | + | + | + | + | − | − | + | − | − | − |
| 3396/01 | PEN, OXA, GEN, ERY, CLI, OTE, CIP | + | + | + | + | − | + | + | − | − | − |
| 3980/01 | PEN, OXA, ERY, CLI, CIP | + | + | − | − | + | − | − | − | − | − |
| 2392/02 | PEN, OXA, CIP | + | + | − | − | − | − | − | − | − | − |
| 2513/02 | PEN, OXA, GEN, ERY, CLI, OTE, CIP, SXT | + | + | + | − | + | + | − | − | − | − |
| 2514/02 | PEN, OXA, GEN, ERY, CLI, CMP, CIP | + | + | + | − | + | − | − | − | − | − |
| 2569/02 | PEN, OXA, GEN, ERY, CMP, CIP, SXT | + | + | + | − | + | − | − | − | − | − |
| 3052/02 | PEN, OXA, GEN, ERY, CLI, CIP | + | + | + | + | − | − | − | − | − | − |
| 3057/02 | PEN, OXA, GEN, ERY, CLI, OTE, CMP, CIP, SXT | + | + | + | − | + | + | − | − | − | − |
| 3096/02 | PEN, OXA, GEN, ERY, CLI, CIP | + | + | + | + | − | − | − | − | − | − |
| 3099/02 | PEN, OXA, CIP | + | + | − | − | − | − | − | − | − | − |
| 3178/02 | PEN, OXA, ERY, CLI, CMP | + | + | − | − | + | − | − | − | − | − |
| 3179/02 | PEN, OXA, GEN, ERY, CLI, CIP | + | + | + | + | − | − | − | − | − | − |
| 3211/02 | PEN, CMP, CIP | + | − | − | − | − | − | − | − | − | − |
| 3516/02 | PEN, OXA, PHO, GEN, ERY, CLI, OTE, CIP, SXT, RAM | + | + | + | + | − | − | + | − | − | − |
| 3712/02 | PEN, OXA, ERY, CLI, CMP, CIP | + | + | − | + | − | − | − | − | − | − |
| 3887/02 | PEN, OXA, GEN, CIP | + | + | + | − | − | − | − | − | − | − |
| 3892/02 | PEN, OXA, PHO i, GEN, ERY, CLI, CMP, CIP, SXT | + | + | + | + | − | − | − | − | − | − |
| 3893/02 | PEN, OXA, PHO i, ERY, CLI, CIP | + | + | − | + | − | − | − | − | − | − |
| 3932/02 | PEN, OXA, PHO i, GEN, ERY, CLI, CIP, SXT, RAM | + | + | + | + | − | − | − | − | − | − |
| 3940/02 | PEN, OXA, PHO i, GEN, ERY, CLI, CIP, MUP | + | + | + | + | − | − | − | − | − | − |
| 4073/02 | PEN, OXA, ERY, CLI, CMP, CIP | + | + | − | − | + | − | − | − | − | − |
| 8325 | Susceptible | + | − | − | − | − | − | − | − | − | − |
PEN, penicillin; OXA, oxacillin; GEN, gentamicin; ERY, erythromycin; CLI, clindamycin; OTE, oxytetracyline; SYN, quinupristin/dalfopristin; CMP, chloramphenicol; RAM, rifampin; CIP, ciprofloxacin; FUS, fusidic acid; MUP, mupirocin; PHO, phosphomycin (i, intermediate); SXT, trimethoprim-sulfamethoxazole.
DISCUSSION
The use of multiplex PCR for the species identification of S. aureus and for the detection of antibiotic resistance genes has been described previously (17, 22, 28, 35). Those reports deal with the detection of the methicillin resistance gene mecA, along with the genes nuc, coa, 16S rDNA, and femA as species specific genes; these assays therefore amplify a maximum of three fragments simultaneously. A recent study describes a PCR assay system for the detection of the antibiotic resistance genes mecA, aacA-aphD, blaZ, erm(A), erm(B), erm(C), and msr(A) (23). This PCR system consists of seven different multiplex PCR assays, all including two species-specific primer pairs and a primer pair for the specific detection of one antibiotic resistance gene. The multiplex PCR assay described in our study includes the detection of nine different relevant resistance genes in one reaction, including resistance to older compounds and new antibiotics. It is easy to perform and much more cost-effective.
Resistance determinants to be included were selected based on clinical and technical considerations. Since the number of fragments to be amplified simultaneously in a single PCR amplification is limited, we chose genes most frequently associated with resistance of S. aureus to clinically relevant antibiotics (21, 26, 27, 29). Accessory resistance genes (e.g., vga genes [streptogramin A resistance], which are detected along with vat or erm genes in the majority of cases [21]) and/or contribute only to a minor increase in MIC, were not considered.
The technique presented here takes about 6 h for multiplex PCR and analysis of the amplification products. Using the Agilent Bioanalyzer technology, we drastically reduced the time for analysis to a total of approximately 2.5 h. Further work will focus on the combination of the multiplex PCR assay with DNA microarray technology to (i) further accelerate the analysis of amplification products, (ii) enhance the sensitivity of amplimer detection, and (iii) include a specificity control by hybridization to gene-specific oligonucleotide capture probes. Furthermore, detection of resistance due to mutations affecting the target of antimicrobials as DNA topoisomerases for fluoroquinolones and the β-subunit of RNA polymerase for rifampin will be facilitated by the use of capture probes for the relevant single-nucleotide polymorphisms. Microarrays for the detection of different virulence factors and various β-lactam antibiotic resistance genes in members of the Enterobacteriaceae have been described previously (9, 19).
The smallest amount of template DNA which can be detected by the multiplex PCR described here, i.e., 100 pg, corresponds to 104 staphylococcal cells (calculation according to www.molbiol.ru/ger/scripts). Therefore, this assay could be adapted to use in direct detection from positive blood cultures or from normally stable clinical samples such as blood or urine. However, it is less useful for direct detection from specimens in which mixed cultures with CNS are more frequent, such as wound swabs or nasal swabs. Since S. aureus and CNS have the same resistance genes (23), attribution of detected genes to a particular species is not possible. This can be overcome by using species-specific separation of S. aureus cells before performing DNA extractions, as described recently (13). A definite discrimination between S. aureus and CNS from pure cultures becomes possible by using the alternative control primer pair targeting the S. aureus-specific DNA fragment (24).
Acknowledgments
This study was performed as a part of the competence network “GenoMik”, funded by the Federal Ministry for Education and Research (BMBF).
REFERENCES
- 1.Allignet, J., and N. el Solh. 1995. Diversity among the gram-positive acetyltransferases inactivating streptogramin A and structurally related compounds and characterization of a new staphylococcal determinant, vatB. Antimicrob. Agents Chemother. 39:2027-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allignet, J., and N. el Solh. 1997. Characterization of a new staphylococcal gene, vgaB, encoding a putative ABC transporter conferring resistance to streptogramin A and related compounds. Gene 202:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Allignet, J., N. Liassine, and N. el Solh. 1998. Characterization of a staphylococcal plasmid related to pUB110 and carrying two novel genes, vatC and vgbB, encoding resistance to streptogramins A and B and similar antibiotics. Antimicrob. Agents Chemother. 42:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allignet, J., V. Loncle, and N. el Sohl. 1992. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene 117:45-51. [DOI] [PubMed] [Google Scholar]
- 5.Allignet, J., V. Loncle, C. Simenel, M. Delepierre, and N. el Solh. 1993. Sequence of a staphylococcal gene, vat, encoding an acetyltransferase inactivating the A-type compounds of virginiamycin-like antibiotics. Gene 130:91-98. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron, M. G., and M. Ouellette. 1998. Preventing antibiotic resistance through rapid genotypic identification of bacteria and of their antibiotic resistance genes in the clinical microbiology laboratory. J. Clin. Microbiol. 36:2169-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byl, B., P. Clevenbergh, F. Jacobs, M. J. Struelens, F. Zech, A. Kentos, and J. P. Thys. 1999. Impact of infectious diseases specialists and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin. Infect. Dis. 29:60-66. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chizhikov, V., A. Rasooly, K. Chumakov, and D. D. Levy. 2001. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 67:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 11.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francois, P., D. Pittet, M. Bento, B. Pepey, P. Vaudaux, D. Lew, and J. Schrenzel. 2003. Rapid detection of methicillin-resistant Staphylococcus aureus directly from sterile or nonsterile clinical samples by a new molecular assay. J. Clin. Microbiol. 41:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geha, D. J., J. R. Uhl, C. A. Gustaferro, and D. H. Persing. 1994. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J. Clin. Microbiol. 32:1768-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerin, F., A. Buu-Hoi, J. L. Mainardi, G. Kac, N. Colardelle, S. Vaupre, L. Gutmann, and I. Podglajen. 2000. Outbreak of methicillin-resistant Staphylococcus aureus with reduced susceptibility to glycopeptides in a Parisian hospital. J. Clin. Microbiol. 38:2985-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henegariu, O., N. A. Heerema, S. R. Dlouhy, G. H. Vance, and P. H. Vogt. 1997. Multiplex PCR: critical parameters and step-by-step protocol. BioTechniques 23:504-511. [DOI] [PubMed] [Google Scholar]
- 17.Kearns, A. M., P. R. Seiders, J. Wheeler, R. Freeman, and M. Steward. 1999. Rapid detection of methicillin-resistant staphylococci by multiplex PCR. J. Hosp. Infect. 43:33-37. [DOI] [PubMed] [Google Scholar]
- 18.Klein, N. C., and B. A. Cunha. 2001. New uses of older antibiotics. Med. Clin. North Am. 85:125-132. [DOI] [PubMed] [Google Scholar]
- 19.Lee, Y., C. S. Lee, Y. J. Kim, S. Chun, S. Park, Y. S. Kim, and B. D. Han. 2002. Development of DNA chip for the simultaneous detection of various beta-lactam antibiotic-resistant genes. Mol. Cell 14:192-197. [PubMed] [Google Scholar]
- 20.Lelievre, H., G. Lina, M. E. Jones, C. Olive, F. Forey, M. Roussel-Delvallez, M. H. Nicolas-Chanoine, C. M. Bebear, V. Jarlier, A. Andremont, F. Vandenesch, and J. Etienne. 1999. Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics. J. Clin. Microbiol. 37:3452-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lina, G., A. Quaglia, M. E. Reverdy, R. Leclercq, F. Vandenesch, and J. Etienne. 1999. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 43:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louie, L., J. Goodfellow, P. Mathieu, A. Glatt, M. Louie, and A. E. Simor. 2002. Rapid detection of methicillin-resistant staphylococci from blood culture bottles by using a multiplex PCR assay. J. Clin. Microbiol. 40:2786-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martineau, F., F. J. Picard, N. Lansac, C. Ménard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 2000. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau, F., F. J. Picard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 1998. Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 36:618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4, 4th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Schmitz, F. J., A. C. Fluit, M. Gondolf, R. Beyrau, E. Lindenlauf, J. Verhoef, H. P. Heinz, and M. E. Jones. 1999. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J. Antimicrob. Chemother. 43:253-259. [PubMed] [Google Scholar]
- 27.Schmitz, F. J., A. Krey, R. Sadurski, J. Verhoef, D. Milatovic, and A. C. Fluit. 2001. Resistance to tetracycline and distribution of tetracycline resistance genes in European Staphylococcus aureus isolates. J. Antimicrob. Chemother. 47:239-240. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, F. J., C. R. Mackenzie, B. Hofmann, J. Verhoef, M. Finken-Eigen, H. P. Heinz, and K. Kohrer. 1997. Specific information concerning taxonomy, pathogenicity and methicillin resistance of staphylococci obtained by a multiplex PCR. J. Med. Microbiol. 46:773-778. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz, F. J., J. Petridou, A. C. Fluit, U. Hadding, G. Peters, C. von Eiff, and Multicentre Study on Antibiotic Resistance in Staphylococci Study Group. 2000. Distribution of macrolide-resistance genes in Staphylococcus aureus blood-culture isolates from fifteen German university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 19:385-387. [DOI] [PubMed] [Google Scholar]
- 30.Sievert, M. 2002. Staphylococcus aureus resistant to vancomycin — United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 31.Torres, G. M., M. T. Tejedor Junco, M. M. Gonzalez, and L. Z. Gonzalez. 1996. Selection of subpopulations resistant to amikacin and netilmicin of gentamicin-resistant clinical strains of Staphylococcus aureus and Staphylococcus epidermidis. Zentbl. Bakteriol. 284:58-66. [DOI] [PubMed] [Google Scholar]
- 32.Trzcinski, K., B. S. Cooper, W. Hryniewicz, and C. G. Dowson. 2000. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 45:763-770. [DOI] [PubMed] [Google Scholar]
- 33.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 34.Vanhoof, R., C. Godard, J. Content, H. J. Nyssen, E. Hannecart-Pokorni, and Belgian Study Group of Hospital Infections (GDEPIH/GOSPIZ). 1994. Detection by polymerase chain reaction of genes encoding aminoglycoside-modifying enzymes in methicillin-resistant Staphylococcus aureus isolates of epidemic phage types. J. Med. Microbiol. 41:282-290. [DOI] [PubMed] [Google Scholar]
- 35.Vannuffel, P., J. Gigi, H. Ezzedine, B. Vandercam, M. Delmee, G. Wauters, and J. L. Gala. 1995. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J. Clin. Microbiol. 33:2864-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner, G., C. Cuny, F. J. Schmitz, and W. Witte. 2001. Methicillin-resistant, quinupristin-dalfopristin-resistant Staphylococcus aureus with reduced sensitivity to glycopeptides. J. Clin. Microbiol. 39:3586-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 38.Witte, W. 1999. Antibiotic resistance in gram-positive bacteria: epidemiological aspects. J. Antimicrob. Chemother. 44(Suppl. A):1-9. [DOI] [PubMed] [Google Scholar]
- 39.Witte, W., C. Braulke, C. Cuny, D. Heuck, and M. Kresken. 2001. Changing pattern of antibiotic resistance in methicillin-resistant Staphylococcus aureus from German hospitals. Infect. Control Hosp. Epidemiol. 22:683-686. [DOI] [PubMed] [Google Scholar]