Abstract
Molecular typing of normal (n = 456) and small-colony-variant (SCV; n = 239) Staphylococcus aureus isolates cultured from the airways of 52 of 72 cystic fibrosis (CF) patients (72.2%) during a 6-year prospective study revealed a median long-term persistence of 37 months (range, 6 to 70). SCV persisted longer in the airways than the normal S. aureus (statistically not significant). Pulsed-field gel electrophoresis identified six prevalent clonal lineages, which were cultured from more than one patient (3 to 12 patients), and 39 individual clones, which were isolated only from single patients. The SCV phenotype was not restricted to a distinct clonal lineage but occurred in many different clones. Most patients (33 of 52, 63.46%) harbored single clones. This study provides a basis for improved understanding of S. aureus colonization and infection dynamics in CF patients.
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator gene, leading to chronic respiratory infection with deteriorating lung function in spite of antibiotic treatment (13). Only a limited number of bacterial species have been found to be major pathogens chronically infecting the airways of CF patients: Staphylococcus aureus and Haemophilus influenzae are mainly present in children, and Pseudomonas aeruginosa is the leading pathogen in adolescents and adults (4, 5).
Persistence of S. aureus in CF and other invasive infections has been associated with the isolation of a subpopulation of S. aureus with the small-colony-variant (SCV) phenotype (10, 15, 23, 25). In contrast to normal S. aureus, with typical colony size, pigmentation, and hemolysis on Columbia blood agar, SCV S. aureus strains can be altered in their electron transport activity or thymidine synthesis, thus growing as nonhemolytic, nonpigmented, very small colonies or as colonies with a fried-egg appearance (11). Decreased expression of α-hemolysin allows SCV isolates to persist intracellularly in in vitro systems (1, 24).
Enhanced understanding of both bronchopulmonary pathophysiology and consequent microbial colonization and adaptivity in CF has been hampered by limited longitudinal studies on S. aureus infection as a function of colonization and infection dynamics of the pathogen (3, 16). Therefore, the goals of our study were to determine the duration and dynamics of S. aureus (normal and SCV) persistence in the airways of individual CF patients (same clone or new infection) over an extended period and concurrently to assess the population structure of chronic S. aureus colonization and infection in this defined patient group.
(Parts of this work were presented at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla., 20 to 24 May 2001 [B. C. Kahl, A. Duebbers, G. Lubritz, B. Ritzerfeld, M. Reilly, E. Harms, M. Herrmann, and G. Peters, Abstr. Gen. Meet. Am. Soc. Microbiol., abstr. D-41, 2001], and at the 24th European CF Conference, Vienna, Austria, 2001 [B. C. Kahl, A. Duebbers, H. G. Koch, G. Lubritz, B. Ritzerfeld, M. Reilly, E. Harms, M. Herrmann, and G. Peters, Abstr. 24th Eur. CF Conf., abstr. P-197, 2001].)
Specimens were obtained from CF patients attending our institution from April 1994 to January 2000. Only patients with an observation period of more than 12 months were included into the study (n = 72). The study group consisted of 41 male and 31 female patients with a median age of 9.5 years (range, 1 to 33) at the beginning of the study period and 14.5 years at the end (range, 1 to 36). Sputum or deep throat swabs (DTS) from children who did not produce sputum were processed. S. aureus was cultured from 185 of 391 (47.3%) throat swabs from 25 patients with a median age of 9 years (range, 2 to 18) and from 272 of 518 sputum samples (52.5%) of 22 patients with a median age of 20.5 years (range, 12 to 36). For three patients, nearly half of the samples consisted of DTS and sputum cultures. For two patients, insufficient information about the type of samples was available. Persistent infection was suggested if isolation of S. aureus (normal and/or SCV) occurred for more than 6 months.
The airways of 52 of 72 (72.2%) patients monitored for a median of 59.5 months (range, 21 to 72) were colonized or infected persistently by normal and SCV S. aureus. In particular, 28 patients harbored only normal S. aureus, 22 patients were infected by isogenic normal and/or SCV S. aureus, 2 patients had only SCV S. aureus, and from the specimens of 20 patients no S. aureus was cultured. Most patients with SCV (22 of 24) were treated for more than 18 months continuously with trimethoprim-sulfamethoxazole (SXT). Auxotrophism testing by analysis of supplemented growth of SCV on chemically defined medium agar around disks impregnated with thymidine, hemin, and menadione (10) revealed that 122 SCV isolates were thymidine dependent, 27 were hemin dependent, 26 had combined thymidine and hemin dependence, and 1 was menadione dependent. While 27 SCV isolates reverted before auxotrophism testing, it was not possible to determine the auxotrophism of 36 SCV isolates by this method. Thus, most SCV isolates were thymidine dependent (148 of 212; 69.8%). All thymidine-dependent SCV isolates were SXT resistant, while the respective isogenic normal isolates were SXT susceptible. In some patients, it was possible to culture SCV S. aureus 4.5 years after withdrawal of SXT. Therefore, while antibiotic pressure led to the induction or selection especially of thymidine-dependent SCV, once SCV isolates emerged, they were persistent and difficult to eradicate. In two patients who previously had only normal S aureus infection, SCV S. aureus emerged after 3 and 4 years of observation, indicating adaptation to antibiotic therapy and/or the hostile environment by the emergence of SCV. Furthermore, six patients who harbored normal and SCV S. aureus at the beginning for a median persistence of 30 months (range, 6 to 40) lost the normal phenotype. However, the SCV phenotype was still present, with an additional median persistence of 18.5 months (range, 6 to 40) so far. Therefore, while the normal S. aureus was eliminated from the airways, the longer persistence of the SCV phenotype indicates a survival advantage of the SCV versus the normal phenotype in the hostile milieu of the airways, possibly due to increased adaptation of the SCV.
To determine clonal identity and relatedness of consecutive normal and SCV S. aureus, all isolates were analyzed by pulsed-field gel electrophoresis (PFGE) after SmaI restriction of whole chromosomal DNA as described previously (6) except that SCV isolates were cultured in brain heart infusion broth. The gels were analyzed both visually and with the computer program Quantity One (Bio-Rad Laboratories, Hercules, Calif.) and interpreted according to suggested guidelines (20, 21). Isolates from different patients, or consecutive isolates from the same patient, with identical or only minor differences in fragment patterns were assigned to the same clone or clonal lineage if the Dice coefficient was higher than 85% (17). Isolates with a similarity index below 85% were considered to belong to different clones. The dendrograms are based on the Dice coefficient pattern similarity.
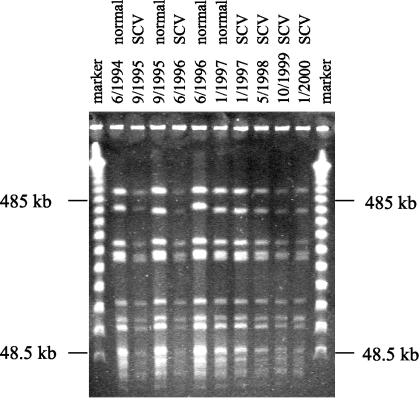
A total of 695 S. aureus isolates were typed by PFGE, yielding the following phenotypes: 456 normal S. aureus isolates, 212 SCV isolates, and 27 SCV isolates which reverted to normal S. aureus upon subculture. Patients with normal S. aureus or normal and SCV S. aureus harbored clonal isolates for up to 70 months in their airways, as shown in Fig. 1. SCV isolates from individual patients were isogenic to the respective normal S. aureus phenotype, as shown in Fig. 1, in which consecutive normal and SCV isolates are identical by PFGE analysis. The SCV phenotype was found in many different clones and in all prevalent clonal lineages except one. Therefore, the emergence of SCV phenotypes was not related to a distinct clone but was especially dependent on antibiotic pressure and probably on other, as-yet-unknown factors.
FIG. 1.
Persistence of normal and SCV S. aureus in a single CF patient. Consecutive isolates were cultured from the sputum of a CF patient from June 1994 until January 2000. Results of PFGE after SmaI digestion of chromosomal DNA from normal and SCV S. aureus isolates are shown. The marker is a molecular weight marker of concatemers of lambda phage DNA.
The median persistence of all S. aureus phenotypes (normal and SCV) was 37 months (range, 6 to 70). Specifically, the median persistence of normal and SCV S. aureus was 49.5 months, compared to 25 months for normal S aureus only. Although there was a trend of longer persistence in patients with SCV S. aureus, Kaplan-Meier analysis of S. aureus persistence (SCV plus normal versus normal only) did not reach statistical significance (P = 0.076). A mechanism which may contribute to the persistence of normal and SCV S. aureus in CF and in other persistent infections, such as osteomyelitis, is the internalization of bacteria by epithelial cells (2). The intracellular location provides a niche for the bacteria, where they are protected against host defense and antibiotic therapy. Internalization of S. aureus has been demonstrated in several in vitro studies for various eukaryotic cells (2, 9, 19), and it has been shown that SCV persisted to higher degrees intracellularly (22). Therefore, it is tempting to speculate that part of the persistence of normal and SCV S. aureus in CF patients may be caused by internalization of the bacteria by airway epithelial cells, as shown in in vitro studies (12).
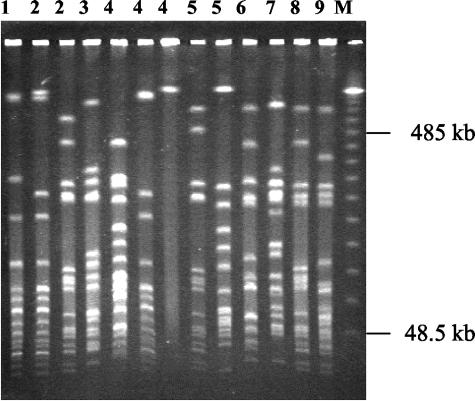
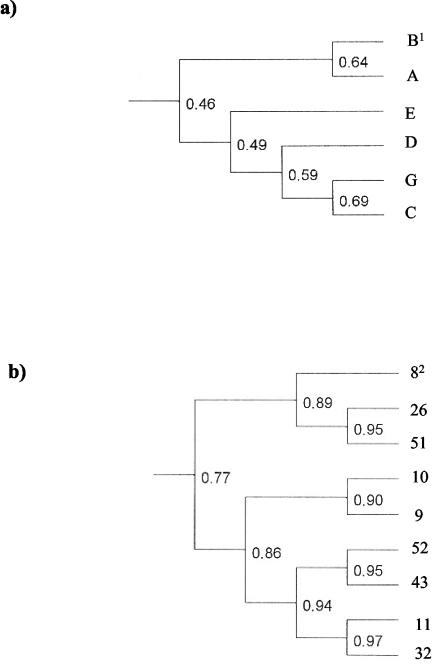
Molecular typing by PFGE of normal (n = 456) and SCV (n = 239) S. aureus isolates allowed the distinction of 45 different S. aureus clones. Six prevalent clonal lineages were cultured from more than one patient (3 to 12 patients), while 39 clones were individual clones which were isolated only from single patients. An example of the variability of S. aureus clones is given in Fig. 2. Dendrograms of the six prevalent clonal lineages (Fig. 3a) and the different isolates cultured from 9 patients belonging to clonal lineage B (Fig. 3b) were generated by analysis of the similarity of the PFGE fragment patterns. The dendrograms revealed the close relatedness of the isolates within a clonal lineage in contrast to the isolates belonging to the different clonal lineages. Theoretically, as patients colonized or infected by isolates of the same clonal lineage came from different living areas and did not have social contact, direct patient-to-patient transmission through social contact appears to be unlikely. However, direct or indirect nosocomial transmission during hospitalization cannot be excluded. In 30 of 52 patients, consecutive strains showed changes in fragment patterns by PFGE analysis (data not shown). These changes occurred in both the normal and SCV phenotypes. Such genome rearrangements have been observed in other chronically infecting organisms such as P. aeruginosa (14).
FIG. 2.
PFGE of persistent clones from nine CF patients. The numbers above the lanes are patient numbers and indicate clones isolated from the airways of individual patients. The marker (M) is a molecular weight marker of concatemers of lambda phage DNA.
FIG. 3.
Dendrograms of the six prevalent clonal lineages and of the isolates belonging to clonal lineage B from nine patients, showing the levels of similarity between SmaI restriction fragment patterns determined by PFGE and subsequent analysis of the Dice coefficient by the software Quantity One. The values show the genetic relatedness of the clones and isolates. (A) Relatedness of the six prevalent clonal lineages, which were isolated from more than one patient (3 to 12 patients). Superscript 1, capital letters indicate clonal lineages. (B) Relatedness of different isolates cultured from nine patients persistently, which belong to clonal lineage B. Superscript 2, each number identifies one patient.
All cultured S. aureus isolates (n = 9) from the sputum of seven young patients who only occasionally produced sputum were clonally identical to the respective throat isolates by PFGE. Similarly, 21 of 22 S. aureus isolates from DTS from 10 patients with sputum cultures were clonally identical to the respective sputum strains. Nearly 40% (185 of 457 positive specimens) of S. aureus strains in our study were cultured from DTS. Although the reliability of S. aureus cultured from DTS as a marker for lower-airway infection in children who do not produce sputum is questionable, it was the best material that was obtainable. However, in a prospective study, Rosenfeld et al. determined a positive predictive value of 59% for S. aureus isolated from the throat as a marker for lower-airway infection in children older than 18 months by comparison of positive cultures from DTS and bronchoalveolar lavage specimens (18). Accordingly, about 40% (74 of 185) of the S. aureus cultures isolated from DTS in our study may represent colonizing bacteria, while nearly 60% (111 of 185) of the pathogens presumably were cultured from patients with lower-airway infections. Consistent with this assumption is the finding that the few sputum isolates (9 of 9) available from young patients usually with DTS cultures were all clonally identical to their respective throat isolates.
During the study period, 33 of 52 (63.5%) patients with persistent S. aureus colonization or infection harbored one clone, 8 (15.4%) had two clones, 9 (17.3%) had three clones, and 2 (3.8%) had multiple changing clones. A turnover of clones occurred in six patients, parallel isolation of different clones occurred in eight patients, and two patients harbored initially two clones which were later replaced by a third clone. These results indicate that long-term colonization or infection with S. aureus prevents colonization by other S. aureus strains, suggesting a possible role of bacterial interference in the bronchial milieu.
The persistence of the prevalent clonal lineages did not differ from the persistence of the individual clones with the exception of one prevalent clonal lineage, which was recovered for extended periods, with a median persistence of 54 months, compared to a median persistence ranging from 17 to 33 months in the other clones. Therefore, it could be that selective mechanisms favored distinct S. aureus clonal lineages in the airways of CF patients. The results of our study indicate that the infection caused by S. aureus in CF is extremely persistent rather than recurrent, as described in a study from Denmark, which used phage typing as the typing method (8).
Our results for long-term persisting S. aureus in the airways of CF patients are different from the results of Goerke et al. (7), who analyzed the molecular epidemiology of nasal S. aureus carriage in CF patients and healthy persons. In addition to nasal isolates, they included isolates from sputum samples from 22 patients, which were identical to nasal isolates by PFGE in 19 of 22 patients. In their study, only 21% of nasal S. aureus carriers harbored the same clone in the nares after 19 months. The authors concluded that S. aureus persistence is short and inferred that short persistence also holds true for the isolates of the lower airways. However, in our study, in which we analyzed the persistence of S. aureus in the upper and lower airways, 63.5% of the patients were still colonized or infected by the same clone after 24 months. Therefore, we assume that the dynamics of nasal colonization differs from the dynamics of S. aureus colonization and infection of the upper and lower respiratory tract, indicating a survival advantage of the bacteria in the airways and/or adaptation of the pathogen to the bronchial milieu. Further longitudinal studies are required to confirm this observation.
To our knowledge, this is the first prospective longitudinal study in CF patients which reveals highly prevalent and long-term persisting infection associated with the SCV or normal S. aureus phenotype. This study provides a basis for improved understanding of S. aureus colonization and infection dynamics in CF patients. Further studies are necessary to determine the pathogenic role of S. aureus for airway infection related to CF.
Acknowledgments
This work was supported by the Innovative Medical Research Grant of the Medical Faculty, University of Muenster, Ka-1-6-I/98-45 (to B.C.K.), by a grant from the German Minister of Education and Research for the Interdisciplinary Center for Clinical Research, C8 (to M.H.), and by National Institute of Allergy and Infectious Diseases grant R01 AI 42072 (to R.A.P.).
We thank B. Gruenastel and the staff of the microbiology laboratory for their assistance in the collection of isolates, S. Weber for her help in performing PFGE, and B. Sinha and K. Becker for fruitful discussions.
REFERENCES
- 1.Balwit, J. M., P. van Langefelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 2.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branger C., C. Gardye, and N. Lambert-Zechovsky. 1996. Persistence of Staphylococcus aureus strains among cystic fibrosis patients over extended periods of time. J. Med. Microbiol. 45:294-301. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. 1999. Patient registry 1998 annual data report. Cystic Fibrosis Foundation, Bethesda, Md.
- 6.Goering, R. V., and M. A. Winters. 1992. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J. Clin. Microbiol. 30:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goerke, C., K. Kraning, M. Stern, G. Döring, K. Botzenhart, and C. Wolz. 2000. Molecular epidemiology of community-acquired Staphylococcus aureus in families with and without cystic fibrosis. J. Infect. Dis. 181:984-989. [DOI] [PubMed] [Google Scholar]
- 8.Hoff, G. E., and N. Hoiby. 1975. Staphylococcus aureus in cystic fibrosis: antibiotic sensitivity and phage types during the latest decade. Acta Pathol. Microbiol. Scand. Sect. B 83:219-225. [PubMed] [Google Scholar]
- 9.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 10.Kahl, B., M. Herrmann, A. Schulze Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 11.Kahl, B. C., G. Belling, R. Reichelt, M. Herrmann, R. A. Proctor, and G. Peters. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles, M. R., P. H. Gilligan, and R. C. Boucher. 2000. Cystic fibrosis, p. 767-772. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases 5th ed. Churchill Livingstone, New York, N.Y.
- 14.Mahenthiralingam, E., M. E. Campbell, J. Foster, J. S. Lam, and D. P. Speert. 1996. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor, R. A., P. van Langefelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persisting and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95-102. [DOI] [PubMed] [Google Scholar]
- 16.Renders, N. H. M., A. van Belkum, S. E. Overbeek, J. W. Mouton, and H. A. Verbrugh. 1997. Molecular epidemiology of Staphylococcus aureus strains colonizing the lungs of related and unrelated cystic fibrosis patients. Clin. Microbiol. Infect. 3:216-221. [DOI] [PubMed] [Google Scholar]
- 17.Roemling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tuemmler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 170:1616-1621. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld, M., J. Emerson, F. Accurso, D. Armstrong, R. Castile, K. Grimwood, P. Hiatt, K. McCoy, S. McNamara, B. Ramsey, and J. Wagener. 1999. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr. Pulmonol. 28:321-328. [DOI] [PubMed] [Google Scholar]
- 19.Sinha, B., P. Francois, P. Vaudaux, M. Foti, O. M. Hartford, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 20.Struelens, M. J. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2:2-11. [DOI] [PubMed] [Google Scholar]
- 21.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643-1647. [DOI] [PubMed] [Google Scholar]
- 23.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolaufs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25:1250-1251. [DOI] [PubMed] [Google Scholar]
- 24.von Eiff, C., C. Heilmann, R. A. Proctor, C. Wolz, G. Peters, and F. Goetz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Eiff, C., P. E. Vaudaux, B. C. Kahl, D. P. Lew, S. Emler, A. Schmidt, G. Peters, and R. A. Proctor. 1999. Bloodstream infections caused by small-colony variants of coagulase-negative staphylococci following pacemaker implantation. Clin. Infect. Dis. 29:932-934. [DOI] [PubMed] [Google Scholar]