Abstract
In order to gain precise data on the actual epidemiology of tuberculosis (TB) in Belgrade, central Serbia, we conducted the molecular epidemiological investigation described herein. IS6110 restriction fragment length polymorphism (RFLP) typing of 176 Mycobacterium tuberculosis isolates was performed. These strains were obtained from 48.4% of all patients diagnosed with culture-proven pulmonary TB from April through September 1998 and from May through October 1999. Clusters containing strains with identical RFLP IS6110 patterns were assumed to have arisen from recent transmission. Of the 176 cases, 55 (31.2%) were grouped into 23 clusters ranging in size from two to six patients. Nearly 80% of clustered patients were directly interviewed, and transmission between family-unrelated contacts was found to be predominant in the study population. Classical contact investigation identified only 2 (3.6%) of the 55 clustered patients. The clustering of TB patients was not associated with any demographic or clinical characteristic other than infection with multidrug-resistant (MDR) M. tuberculosis strains. Nearly 70% of MDR strains were clustered, which indicates active transmission of MDR TB in Belgrade. However, this was not observed by conventional epidemiologic surveillance. In conclusion, the first molecular epidemiologic analysis of TB in the region revealed frequent recent transmission of TB and pointed out significant shortcomings of the current concept for conventional contact tracing. The results presented also demonstrate that transmission of MDR TB in Belgrade is not optimally controlled, and they provide support for the development of improved control strategies, including application of molecular methods.
Tuberculosis (TB) remains a major health problem worldwide, but it is more prevalent in underdeveloped and developing countries, in which over 95% of cases occur (8). In central Serbia, which is a low-income region located in southeastern Europe, a national TB control program was introduced in 1952, the implementation of which resulted in significant decline in the incidence of TB over the past decades. Between 1956 and 1996, the patient notification rate decreased from 324 to 34.8 cases per 100,000 people (13). Although there was a growing concern that the outbreak of armed conflicts in the republics of former Yugoslavia could rapidly cause the TB problem in the region to worsen, no significant changes in the patient notification rates over the last decade were noted (13, 23). Current incidence rates, e.g., 35.9 cases per 100,000 people in 1998 and 31.4 per 100,000 in 2000, fall within the European average but are still significantly higher than rates in western European countries (8, 8a). For comparison, the incidence rate of TB in 1999 was 12.1 cases per 100,000 people in Germany, 9.8 cases per 100,000 people in The Netherlands, and as low as 6.1 cases per 100,000 people in Norway (8a).
Despite the significant decline of TB incidence rates over the past decades, the persistence of the disease in the region at a relatively high level indicates that some elements of the TB surveillance program are still ineffective. One of the program's main approaches to controlling TB in the region is tracing the transmission of the disease, primarily through contact investigation. Although DNA typing of clinical isolates of Mycobacterium tuberculosis has become an essential tool for reliable monitoring of TB transmission (24, 26, 27), this has not been included in the national program, and thus no molecular epidemiology study of TB in the region has so far been carried out.
Therefore, the present study aimed to provide the first insight into the status of TB in the region based on implementation of molecular methods. M. tuberculosis strains isolated from patients with pulmonary TB in Belgrade, the capital of Serbia, were analyzed by DNA fingerprinting using the insertion sequence IS6110 as a probe.
MATERIALS AND METHODS
Study population.
The study population included 176 randomly chosen patients with permanent residence in Belgrade who were diagnosed with culture-proven pulmonary TB from April through September 1998 and from May through October 1999. The patients ranged from 17 to 84 years old with a median age of 44.3 years, and 68% (n = 120) of the patients were male. These patients represented 48.4% of the total cases of culture-confirmed pulmonary TB that were reported in Belgrade during the periods of the survey. Basic demographic and clinical data, including sex, age, residence in the city, occupation, address at work, refugee status, date of diagnosis, and clinical diagnosis, were obtained by review of medical and laboratory records.
Bacterial strains.
A total of 176 M. tuberculosis strain isolates were analyzed in this study; they were isolated at the Municipal Institute for Lung Disease and Protection against Tuberculosis and the Institute for Lung Diseases, Clinical Center of Serbia. The former keeps the TB register and provides implementation of the TB control program in the city, while the latter serves as the National Reference Center. All the isolates were identified as M. tuberculosis by standard methods (i.e., bacterial and colony morphology, lack of growth at 22°C, and production of niacin) applied in the mycobacteriology laboratories participating in the survey and were obtained on Löwenstein-Jensen slants. Susceptibility of the isolates to isoniazid, rifampin, streptomycin, and ethambutol was examined by proportion method. Strains resistant to at least one drug were considered to be drug resistant, while strains resistant to at least isoniazid and rifampin were considered to be multidrug resistant (MDR). Information about the strains, such as date of sputum collection, acid-fast smear results, and drug susceptibility profiles, was obtained from laboratory records. M. tuberculosis isolates were repeatedly subcultured on Löwenstein-Jensen medium, and 4- to 6-week-old cultures were subsequently genotyped at the National Reference Center for Mycobacteria, Forschungszentrum Borstel, Borstel, Germany.
IS6110 RFLP typing.
Extraction of mycobacterial DNA and IS6110 restriction fragment length polymorphism (RFLP) analysis was performed by using the standardized protocol described by van Embden et al. (24). PvuII-digested total DNA of reference strain Mt. 14323 was included in each Southern blot experiment as an external size standard and was used for accuracy control of IS6110 RFLP experiments. The RFLP patterns of mycobacterial strains were compared visually and by using the Gelcompar software (Windows 98, version 4.2; Applied Maths, Kortrijk, Belgium) as described previously (19, 25). Clusters were defined as groups of patients with M. tuberculosis strains exhibiting identical IS6110 fingerprint patterns (the same number of insertions at identical positions [position tolerance, 1.3%]).
Statistical analysis.
The chi-square test and Student's t test were performed to compare the demographic characteristics (sex and age) of patients whose isolates were fingerprinted with those of culture-confirmed patients without DNA fingerprint data. The association of clustering with demographic and epidemiologic characteristics of the patients as well as the association with susceptibility patterns of M. tuberculosis isolates was evaluated by the chi-square test and Student's t test. Categorical variables were compared by the chi-square test or, when expected values were 5 or less, by the two-tailed Fisher's exact test, while continuous variables were compared by Student's t test. All risk factors for clustering identified by univariate analysis were further included in a multivariate logistic-regression model. P values that were less than 0.05 were considered significant.
RESULTS
DNA polymorphism of M. tuberculosis strains.
The M. tuberculosis isolates from 176 patients were included in the study, and RFLP analyses were done with the IS6110 insertion sequence used as a genetic marker. The survey included nearly 50% of the patients with newly diagnosed culture-confirmed pulmonary TB in Belgrade over the 12-month study period. The characteristics of the patients whose M. tuberculosis isolates were fingerprinted were similar to those of patients without DNA fingerprint data (data not shown). Thus, we considered that the randomly chosen sample was most likely representative of the entire population of TB patients in Belgrade. The IS6110 patterns were digitized and analyzed for similarity by using the Dice coefficient. To display the degree of relatedness, a dendrogram was calculated by the unweighted pair group method using average linkage.
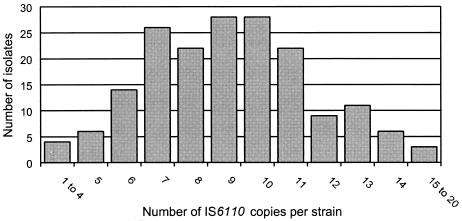
The number of IS6110 copies per isolate ranged from 1 to 20 (Fig. 1), with the great majority of strains (163 [92.6%]) having 6 to 15 copies. The strains contained a mean of 9.2 IS6110 insertions. Only four (2.2%) strains had less than five IS6110 copies: three strains each had four and one strain contained a single copy of the element. No strains lacking IS6110 were found. Strains displaying a typical Beijing-type pattern (25) were not present in the study population.
FIG. 1.
Number of IS6110 copies exhibited by 176 M. tuberculosis strains isolated from patients in Belgrade.
DNA fingerprinting of M. tuberculosis isolates revealed 144 different IS6110 patterns. Isolates from 121 patients (68.8%) presented unique RFLP fingerprint patterns, while the remaining 55 (31.2%) had clustered patterns and were classified into 23 distinct clusters. The clustering index, as defined by Small et al. (22), was 18.2%. All strains with identical fingerprints were isolated from patients with clinically proven TB and at different times (intervals of at least 17 to 31 days), which most probably excluded the possibility that clustering was due to laboratory cross-contamination. Only in one cluster comprising two patients did the submission dates of first samples differ by five days, but these two patients also had clear clinical symptoms of TB.
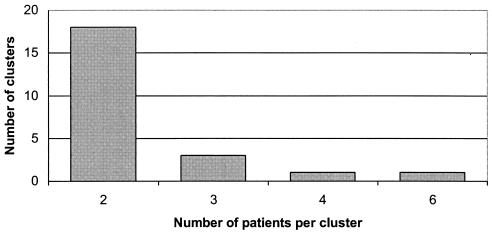
The number of patients with identical strains per cluster varied from two to six (Fig. 2). Only one cluster included the maximal number of patients, while the majority of them (n = 18, 78.3%) were groups of two patients.
FIG. 2.
Cluster sizes and numbers of clusters among tuberculosis patients in Belgrade.
Epidemiologic investigation of IS6110 clusters.
To identify the factors associated with clustering, demographic and epidemiologic characteristics of the 55 patients in clusters were compared with those of the 121 nonclustered patients (Table 1). Univariate analysis showed that clustering of M. tuberculosis isolates was not significantly associated with the following characteristics: sex, age, refugee status, and positive sputum smear. Although the male-to-female ratios in groups of clustered and nonclustered patients were notably different, namely 1.4 in the population of patients in clusters and 2.7 among nonclustered patients, the difference remained above the level of significance (Table 1). Multivariate analysis confirmed the univariate results and found no significant association for any of the characteristics tested.
TABLE 1.
Characteristics of the 176 clustered and nonclustered patients with tuberculosis from Belgrade
| Characteristic | No. (%) of patients
|
P valuea | P valueb | ||
|---|---|---|---|---|---|
| Clustered (n = 55) | Nonclustered (n = 121) | Total (n = 176) | |||
| Sex | |||||
| Male | 32 (58.2) | 88 (72.7) | 120 (68.2) | 0.06 | 0.06 |
| Female | 23 (41.8) | 33 (27.3) | 56 (31.8) | ||
| Median age (yr) | 43.4 | 45.4 | 44.3 | 0.6 | 0.6 |
| Refugee | |||||
| Yes | 1 (1.8) | 4 (3.3) | 5 (2.9) | ||
| No | 48 (87.3) | 102 (84.3) | 150 (85.2) | 0.8 | 0.7 |
| Unknown | 6 (10.9) | 15 (12.4) | 21 (11.9) | ||
| Sputum smear | |||||
| Positive | 36 (65.4) | 75 (62.0) | 111 (63.1) | ||
| Negative | 10 (18.2) | 22 (18.2) | 32 (18.2) | 0.9 | 0.9 |
| Unknown | 9 (16.4) | 24 (19.8) | 33 (18.7) | ||
Results of univariate analysis.
Results of multivariate analysis.
For the purposes of investigation into possible epidemiologic linkages, direct interviews of clustered patients were performed. The interviews were conducted with 42 (76.4%) of 55 clustered patients, while the remaining patients within this category could not be located. All patients were investigated in 10 clusters comprising two members, one cluster comprising three members, and one cluster comprising six members, which resulted in 52.2% of clusters being analyzed completely. Only one cluster, comprising two patients, was not investigated at all. There were no identifiable epidemiologic links among 20 of the 42 (47.6%) interviewed patients. In four groups of strains with identical RFLP patterns (two clusters containing two patients and two clusters containing three patients), the isolates originated from neighbors living on the same streets. One group of identical multiple banding patterns was found in M. tuberculosis strains isolated from patients who were family related. The strains were isolated from a grandmother and granddaughter living in a common household. The largest group of clustered isolates originated from six patients for whom no direct epidemiologic links were found. However, four of these had similar socioeconomic backgrounds (highly educated persons with high incomes) and, although they were not working together, were possibly connected since they were spending a substantial amount of time in an environment of the same financial institution. Diagnosis of TB in these patients was not suspected until they developed the cavitary form of the disease, and they were all diagnosed over a period of 12 months. Although epidemiologic links among these patients were not fully elucidated, indirect linkages among them through a common index source of infection, which was not included in the study, or transmission through short-term casual contacts are reasonable assumptions. No identifiable links were found between the remaining two individuals in this cluster and any of its other members. A pair of clustered strains was isolated from two construction workers who were working together for a couple of months. One group of two clustered isolates originated from a construction worker and a woman who employed him for a 1-month period.
Results of routine contact investigation were available for 40 (72.7%) clustered patients. No contacts were identified for nine patients, while the investigation of 72 family contacts for the remaining 31 patients revealed eight cases of active TB. Out of these eight patients, only one was included in our randomly chosen study population. Thus, conventional contact investigation identified a connection only between two family-related patients of the 55 patients (3.6%) found to be connected as shown by RFLP analysis. All other transmission links found by molecular typing remained unrecognized by classical contact tracing.
Drug susceptibility analysis.
In accordance with recommendations of the TB control program, drug susceptibility testing by the proportion method is routinely performed for all culture-confirmed cases of TB in Belgrade. Results of susceptibility testing for the 176 M. tuberculosis strain isolates analyzed here are presented in Table 2. Apart from one cluster that included one susceptible and one MDR isolate, a good correlation between IS6110 fingerprints and drug susceptibility patterns was found. In this discrepant cluster, the susceptible strain was isolated 11 months prior to isolation of the MDR strain. Since a clear epidemiological link was established for the two patients, we considered them to be clustered. The drug resistance of the MDR strain might have been developed after transmission not resulting in a change of the IS6110 pattern. Of the 23 drug-resistant isolates, 10 (43.5%) were clustered. As far as MDR strains are concerned, the proportion of clustered strains was even higher (67.7%). Comparison of susceptibility patterns between clustered and nonclustered M. tuberculosis strains, both by univariate and multivariate analysis (Table 3), showed that only MDR strains were significantly more likely to be clustered (odds ratio, 4.8; 95% confidence interval, 1.2 to 20.0; P = 0.03).
TABLE 2.
Resistance to antituberculosis drugs of M. tuberculosis strains isolated from 176 patients from Belgrade, central Serbia
| Resistance | n (%) |
|---|---|
| Fully susceptible | 153 (86.9) |
| Resistant to one or more drugs | 23 (13.1) |
| Monoresistant to: | |
| Isoniazid | 1 (0.6) |
| Rifampin | 1 (0.6) |
| Ethambutol | 1 (0.6) |
| Streptomycin | 6 (3.4) |
| Resistant to at least two drugs (not MDR) | 5 (2.8) |
| MDR | 9 (5.1) |
TABLE 3.
Drug susceptibility patterns of M. tuberculosis strains isolated from clustered and nonclustered patients
| Resistance | No. (%) of patients
|
P valuea | P valueb | |
|---|---|---|---|---|
| Clustered (n = 55) | Nonclustered (n = 121) | |||
| Fully susceptible | 45 (81.8) | 108 (89.3) | 0.2 | 0.2 |
| Resistant to one or more drugs | 10 (18.2) | 13 (10.7) | ||
| Monoresistant to: | ||||
| Isoniazid | 0 (0) | 1 (0.8) | 0.5 | 0.9 |
| Rifampin | 0 (0) | 1 (0.8) | 0.5 | 0.9 |
| Ethambutol | 0 (0) | 1 (0.8) | 0.5 | 0.9 |
| Streptomycin | 2 (3.6) | 4 (3.3) | 0.9 | 0.9 |
| Resistant to at least two drugs (not MDR) | 2 (3.6) | 3 (2.5) | 0.7 | 0.7 |
| MDRc | 6 (10.9) | 3 (2.5) | 0.02 | 0.03 |
Results of univariate analysis.
Results of multivariate analysis.
DISCUSSION
Our study presents for the first time a precise picture of the epidemiology of TB in central Serbia by applying both molecular and classical epidemiological methods. It was performed in Belgrade because it is the most densely populated urban area in central Serbia, with 1.6 million inhabitants, and has an incidence rate of TB comparable to the national average (13, 8a). In 2000, the notification rate of all clinical forms of TB was 38.8 cases per 100,000 people, while the rate of pulmonary TB was 32.9 cases per 100,000 people (data obtained from the Municipal Institute for Lung Disease and Protection Against Tuberculosis). TB surveillance in the city is provided in accordance with national TB control policy and includes Mycobacterium bovis BCG vaccination at birth, supervision of the chemotherapy, routine contact investigation, and follow-up of the patients and treatment outcome. Application of directly observed therapy has not been adopted as a part of regional TB control policy. Diagnosis of TB is based on results of acid-fast microscopy of sputum specimens and culture of M. tuberculosis. All patients diagnosed with TB are registered in the TB register at the Municipal Institute for Lung Disease and Protection against Tuberculosis in Belgrade. However, we found that approximately 10% of culture-positive cases were not notified. The omissions in notification of TB could have been expected since the prewar health care system, including the TB surveillance program, has been disrupted by specific situations in the region.
The first DNA typing of M. tuberculosis strains isolated in the region showed that the great majority of the isolates had 6 to 15 IS6110 copies, which is in agreement with results reported for isolates from other European countries (7, 15, 17, 19, 20, 28). The small proportion of strains containing less than five copies of IS6110 (2%), which was established in our study, is also typical for series of European isolates (15, 17, 19, 20, 28). These findings indicate that M. tuberculosis strains isolated in Belgrade are similar to those found in other regions of Europe. They also showed that IS6110, the most commonly used genetic marker for typing of M. tuberculosis, had sufficient discriminatory power for DNA fingerprinting of strains isolated in the study population and, therefore, may be used as the foundation of future molecular epidemiological studies of TB in the region.
The analyzed strains exhibited a high degree of DNA polymorphism, as 144 different RFLP patterns were observed among the 176 isolates analyzed. A factor inversely associated with strain diversity is a higher incidence of TB (16). Thus, based on an incidence of over 30 cases per 100,000 people, a somewhat lower level of diversity might have been expected among M. tuberculosis strains from Belgrade. On the other hand, a higher degree of M. tuberculosis strain diversity has already been observed in large, mixed urban populations into which new strains of heterogeneous geographical origin are frequently introduced (5, 16).
It is generally assumed that the level of clustering among M. tuberculosis isolates from a certain region is associated with the level of recent transmission. On the contrary, nonclustered cases are considered to indicate TB resulting from reactivation of latent infection. Although the validity of this assumption has been questioned (3, 4, 6), epidemiological data in most urban populations tested strongly suggest that the rate of clustering reflects the level of recent transmission of TB (1, 10, 12, 15, 17, 20, 22, 23). According to the rate of clustering we found, 31% of cases of newly diagnosed TB in Belgrade were due to recent transmission. Similar proportions of clustered cases were reported for other urban settings with substantially different incidence rates of TB: 28% in Berne (12), 38% in Seville (20), 35% in Amsterdam (23), 29% in Prague (17), 36% in Paris (15), 33.9% in Hamburg (7), 37.5% in New York City (1), 25% in Sao Paulo (10), and 40% in San Francisco (22). The clustering index (22), a more restrictive criterion that excludes an index case from each cluster, was approximately 18% among the strains analyzed here. However, in accordance with the novel recommendations for design of molecular epidemiology studies of TB (18), we considered that the sum of all clustered patients rather than clustering index was more appropriate for our study, which aimed to estimate the number of persons involved in active transmission of TB. It should be noted that the true amount of recent transmission of TB in the study population may have been underestimated for at least two reasons. First, our 12-month study period may have been too short to capture all possible cases of recent transmission, and the study sample did not include all patients with newly diagnosed TB in Belgrade. Second, the great majority of the clusters identified in our study were pairs of patients, which is of possible importance since it has been shown that small size of the clusters may cause an underestimation of the amount of recent transmission (14). The predominance of small clusters in our sample suggests that TB transmission in the study region is probably due to small outbreaks involving different M. tuberculosis strains. One family of strains with similar RFLP patterns was noted in the study sample, but without further analysis using additional genetic markers, this observation remains purely speculative.
Nearly 80% of clustered patients were directly interviewed, and definite or possible epidemiologic links between them were established in eight clusters. Although this investigation did not identify or fully elucidate relationships among all clustered patients, it did demonstrate that transmission between family-unrelated contacts, i.e., between neighbors and coworkers, was predominant in the study population. The transmission of this kind is hard to detect with the contact tracing concept currently implemented in Belgrade, which is primarily focused on identifying transmission among close family contacts of a patient diagnosed with TB. Thus, it is not surprising that classical contact investigation identified only one link between patients found to be connected as shown by RFLP analysis. Similarly low efficiency of conventional contact investigation has already been reported (7, 11, 22, 23). Only large-scale DNA fingerprinting might provide accurate identification of TB transmission pathways, which is, however, clearly not practical in a region with scarce TB resources and a TB incidence rate of over 30 cases per 100,000 people. In view of the mode of transmission we found to be predominant in the study population, this study indicated that certain modifications of the current concept for conventional contact tracing in Belgrade are needed. In addition to the members of a family or a common household, family-unrelated contacts should be included as well. Although it is clear that even an expanded concept of contact tracing would not disclose all cases of transmission, such an approach is a feasible one for our TB resources and should result in more efficient detection of new TB cases.
A number of previous studies identified various factors associated with recent TB infection, including infection with human immunodeficiency virus (HIV), low household income, drug use, alcohol use, and homelessness (1, 7, 10, 15, 22). All patients enrolled in our study were considered HIV seronegative since the incidence of HIV infection in the general population is very low, namely, 0.6 cases per 100,000 people in 2001 (9), while information on the other above-noted factors was for the most part unavailable. The direct interviews of clustered patients revealed no instances of alcohol and/or drug use or homelessness apart from one cluster that included two alcoholics. Socioeconomic backgrounds of the clustered patients were quite dissimilar. Considering that only one clustered patient was a refugee, this subgroup of the population was also not at high risk for recent infection. These incomplete data suggest that identification of specific groups at high risk of contracting TB in Belgrade would require extensive prospective investigation.
The statistical analysis in the present study showed that clustering of tested TB patients was not strongly associated with any of the demographic or clinical characteristics analyzed. Nearly 70% of MDR strains were clustered, which indicates that active transmission of MDR TB is taking place in Belgrade. However, the officially registered incidence rates of resistant and MDR TB remained at stable and low levels over the last 5 years. The rate of isolation of drug-resistant M. tuberculosis strains ranged from 4.8 to 5.4%, while the rates of MDR TB varied from 0.6 to 1% (data obtained from the Municipal Institute for Lung Disease and Protection against Tuberculosis). There is obvious discordance between these values and the rates of drug-resistant (13%) and MDR (5%) strains found in our study sample. Although the observed discordance may reflect sample size and selection bias, the similar differences have already been noted in several local studies and can thus be attributed to the inadequate monitoring and recording of drug-resistant and MDR TB in the city. Currently under way in Belgrade are rigorous retrospective control of M. tuberculosis drug susceptibility data and interlaboratory control of drug susceptibility testing procedures, an undertaking which is partially due to the results of this study. The established active transmission of MDR TB is of considerable importance in terms of Belgrade's position as the only metropolitan area in Serbia and the high mobility of its population. In general, mobility of populations facilitates the spread of TB, including resistant and MDR TB. It has been shown that MDR M. tuberculosis strains first identified in New York City later appeared in many different parts of the United States (2). No epidemiologic links were identified among patients with clustered MDR isolates in our study. Thus, the true magnitude of MDR TB as well as specific pathways of its transmission in Belgrade remain to be fully explored.
Nevertheless, the results we obtained indicate that transmission of MDR TB in Belgrade is not optimally controlled and suggest the need for the development of novel approaches to the problem. The recommended strategy for regions which currently have a low prevalence of resistant and MDR TB is continuous and extensive use of directly observed therapy (21), which is, however, not included in our TB control program. Therefore, more effective control measures specifically aimed at detecting the transmission of MDR TB in Belgrade are needed. The present study revealed active transmission of MDR M. tuberculosis strains in Belgrade and thus provided information that would not have been available had the molecular analysis not been performed. This suggests that molecular epidemiology techniques, as an adjunctive approach to conventional epidemiologic techniques, would be of considerable benefit for reliable surveillance of resistant and MDR TB in Belgrade.
Acknowledgments
D. Vuković acknowledges the Federation of European Microbiological Societies for providing a research fellowship during the period of the study. Parts of this work were supported by the Robert-Koch Institute, Berlin, Germany, and the EU Concerted Action project “New generation genetic markers and techniques for the epidemiology and control of tuberculosis” (QLK2-CT-2000-00630).
We thank G. Stefanović, Institute for Lung Diseases, Clinical Center of Serbia, and R. Pavlica, Municipal Institute for Lung Disease and Protection against Tuberculosis, Belgrade, for assistance with the collection of clinical isolates and information on patients analyzed in the study. We thank I. Radzio and A. Zyzik, Borstel, Germany, for helpful technical assistance.
REFERENCES
- 1.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Drucker, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 2.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275:452-457. [PubMed] [Google Scholar]
- 3.Braden, C. R., G. L. Templeton, M. D. Cave, S. Valway, I. M. Onorato, K. G. Castro, D. Moers, Z. Yang, W. W. Stead, and J. H. Bates. 1997. Interpretation of restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from a state with a large rural population. J. Infect. Dis. 175:1446-1452. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., R. R. Reves, A. P. Hawkes, C. A. Rietmeijer, Z. Yang, H. El-Hajj, J. H. Bates, and M. D. Cave. 1997. DNA fingerprinting with two probes decreases clustering of Mycobacterium tuberculosis. J. Respir. Crit. Care Med. 155:1140-1146. [DOI] [PubMed] [Google Scholar]
- 5.Chevrel-Dellagi, D., A. Abderrahman, R. Haltiti, H. Koubaji, B. Gicquel, and K. Dellagi. 1993. Large-scale DNA fingerprinting of Mycobacterium tuberculosis strains as a tool for epidemiological studies of tuberculosis. J. Clin. Microbiol. 31:2446-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale, J. W., R. Mat Nor, S. Ramayah, T. Hock Tang, and Z. F. Zainuddin. 1999. Molecular epidemiology of tuberculosis in Malaysia. J. Clin. Microbiol. 37:1265-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diel, R., S. Schneider, K. Meywald-Walter, C. Ruf, S. Rüsch-Gerdes, and S. Niemann. 2002. Epidemiology of tuberculosis in Hamburg, Germany: long-term population-based analysis applying classical and molecular epidemiological techniques. J. Clin. Microbiol. 40:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement—global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 8a.Euro TB and the National Coordinators for Tuberculosis Surveillance in the WHO European Region. 2002. Surveillance of tuberculosis in Europe. Report on tuberculosis cases notified in 1999. WHO Collaborating Centre for the Surveillance of Tuberculosis in Europe, Saint-Maurice, France.
- 9.Federal Bureau of Health Care. 2002. Report on HIV cases notified in Yugoslavia from 1985 to 30.06.2002. Federal Bureau of Health Care, Belgrade, Serbia.
- 10.Ferrazoli, L., M. Palaci, L. R. M. Marques, L. F. Jamal, J. B. Afiune, E. Chimara, M. C. Martins, M. A. da Silva Telles, C. A. F. Oliveira, M. C. Palhares, D. T. A. Spada, and L. W. Riley. 2000. Transmission of tuberculosis in an endemic urban setting in Brazil. Int. J. Tuberc. Lung. Dis. 4:18-25. [PubMed] [Google Scholar]
- 11.French, A. L., S. F. Welbel, S. E. Dietrich, L. B. Mosher, P. S. Breall, W. S. Paul, F. E. Kocka, and R. A. Weinstein. 1998. Use of DNA fingerprinting to assess tuberculosis infection control. Ann. Intern. Med. 129:856-861. [DOI] [PubMed] [Google Scholar]
- 12.Genewein, A., A. Telenti, C. Bernasconi, C. Mordasini, S. Weiss, A. Maurer, H. L. Rieder, K. Schopfer, and T. Bodmer. 1993. Molecular approach to identifying route of transmission of tuberculosis in the community. Lancet 342:841-844. [DOI] [PubMed] [Google Scholar]
- 13.Gledović, Z., M. Jovanović, and T. Pekmezović. 2000. Tuberculosis trends in central Serbia in the period 1956-1996. Int. J. Tuberc. Lung Dis. 4:32-35. [PubMed] [Google Scholar]
- 14.Glynn, J. R., E. Vynnycky, and P. E. M. Fine. 1999. Influence of sampling on estimates of clustering and recent transmission of Mycobacterium tuberculosis derived from DNA fingerprinting techniques. Am. J. Epidemiol. 149:366-371. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez, M. C., V. Vincent, D. Aubert, J. Bizet, O. Gaillot, L. Lebrun, C. Le Pendeven, M. P. Le Pennec, D. Mathieu, C. Offredo, B. Pangon, and C. Pierre-Audigier. 1998. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J. Clin. Microbiol. 36:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermans, P. W. M., F. Messadi, H. Guebrexabher, D. Van Soolingen, P. E. W. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, D. Frommel, M. Zribi, and J. D. A. Van Embden. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and the Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 17.Kubin, M., L. W. Riley, M. Havelková, N. Poltoratskaia, and A. Koèová. 1998. Molecular epidemiology of tuberculosis in Prague: analysis by restriction fragment length polymorphism. Int. J. Infect. Dis. 2:155-158. [DOI] [PubMed] [Google Scholar]
- 18.Murray, M., and D. Alland. 2002. Methodological problems in the molecular epidemiology of tuberculosis. Am. J. Epidemiol. 155:565-571. [DOI] [PubMed] [Google Scholar]
- 19.Niemann, S., S. Rüsch-Gerdes, and E. Richter. 1997. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J. Clin. Microbiol. 35:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safi, H., J. Aznar, and J. C. Palomares. 1997. Molecular epidemiology of Mycobacterium tuberculosis strains isolated during a 3-year period (1993-1995) in Seville, Spain. J. Clin. Microbiol. 35:2472-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schluger, N. W. 2000. The impact of drug resistance on the global tuberculosis epidemic. Int. J. Tuberc. Lung Dis. 4:S71-S75. [PubMed] [Google Scholar]
- 22.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 23.Van Deutekom, H., J. J. J. Gerritsen, D. Van Soolingen, E. J. C. Van Ameijden, J. D. A. Van Embden, and R. A. Coutinho. 1997. Molecular epidemiological approach to studying the transmission of tuberculosis in Amsterdam. Clin. Infect. Dis. 25:1071-1077. [DOI] [PubMed] [Google Scholar]
- 24.Van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Van Solingen, D. L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. A van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Sooligen, D., P. W. M. Hermans, P. E. W. de Haas, D. R. Soll, andJ. D. A. Van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 28.Yang, Z. H., P. E. W. de Haas, C. H. Wachmann, D. Van Soolingen, J. D. A. Van Embden, and Å. B. Andersen. 1995. Molecular epidemiology of tuberculosis in Denmark in 1992: evidence of tuberculosis transmission between Greenland and Denmark. J. Clin. Microbiol. 33:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]