Abstract
Salmonella enterica serovar Heidelberg is perhaps the second most frequent Salmonella serovar isolated from humans and the most common isolated from animals in Canada. This pathogen has shown increasing resistance to antimicrobial agents and mimics the multidrug resistance observed in S. enterica serovar Typhimurium strain DT 104. However, unlike for serovar Typhimurium, a rapid and inexpensive subtyping method has not been available for large-scale surveillance efforts. We developed a phage typing scheme and subtyped 2,523 strains of serovar Heidelberg from outbreaks, sporadic infections, and environmental sources in Canada between January 1991 and December 2000. All strains were sensitive to one or more phages and could be subdivided into 49 phage types. A total of 196 isolates from 13 major outbreaks could be subtyped into six phage types, while 86 strains from family outbreaks were assigned to seven phage types. All strains were typeable, and epidemiologically related strains isolated from patients and implicated foods had identical phage types, antibiograms, and pulsed-field gel electrophoresis (PFGE) patterns. Combining PFGE with phage typing increased the discriminatory power of the analysis beyond that of either method alone. We concluded that this phage typing scheme, in conjunction with PFGE, enhances subtyping of serovar Heidelberg strains. Furthermore, this phage typing scheme is a rapid, economical, stable, and reliable epidemiologic tool for tracing the origin of food-borne disease and for the surveillance of sporadic infections.
Salmonella enterica serovar Heidelberg is of significant public health concern in Canada and elsewhere. It has caused large outbreaks of food-borne illness in nursing homes, in hospitals, and within the community at large (14, 18, 19, 20). Although isolates of Salmonella enterica subsp. enterica serovar Heidelberg alternate with those of S. enterica serovar Enteritidis to be the second or third most prevalent Salmonella serotype found in human infections in Canada and the United States (15; http://www.cdc.gov/ncidod/dbmd/phlisdata/salmtab/2000/SalmonellaAnnualSummary2000.pdf), little is known about its infections in humans. Although this organism is often seen in North America, it was not among the top 15 serotypes seen in Africa (Senegal), Asia, Australia, Europe, Israel, or New Zealand in 2000 (World Health Organization Global Salmonella Survey website [http://www.who.int/emc/diseases/zoo/SALM-SURV/frameset:html]). In Canada, serovar Heidelberg is the most common Salmonella serovar obtained from nonhuman sources and is most often found in poultry, eggs (13, 15), and ground beef (24). Similarly, serovar Heidelberg is found most often in Danish turkeys, though it was not often the cause of human infections (23). Serovar Heidelberg infections have been associated with severe disease symptoms, including extraintestinal infections (28), septicemia, and myocarditis (11). According to reports submitted to the National Enteric Surveillance Program in Canada, this Salmonella serotype is most frequently isolated from blood (15). In addition to the emotional and physical distress of affected individuals, epidemic outbreaks of this organism impart significant economic burden on health care systems in Canada and elsewhere (8). Phenotypic and genotypic typing techniques such as serotyping, phage typing, biotyping, pulsed-field gel electrophoresis (PFGE), ribotyping, plasmid typing, IS200 sequence typing, and antibiotic resistance testing have been used to characterize various bacterial pathogens, including serovar Heidelberg (5, 6, 7, 9, 12, 25). Phage typing provides long-term and internationally comparable surveillance data when, because of their recent introduction, such information is not available for molecular techniques.
Phage typing of enteric pathogens has been successfully used to characterize disease-causing agents in epidemiological investigations and for surveillance (2, 3, 6). Small laboratories may have difficulties in maintaining expertise in phage typing; however, participation in quality control programs like Enter-Net External Quality Assurance, which has been in place for the last 5 years, may be helpful in alleviating such problems. Phage typing is a fast, economical, reliable, and reproducible technique requiring no specialized equipment (1, 3, 6). Lysogenic phages of serovar Heidelberg have been used as markers in tracing strains in Australia (17); however, they have been found to have limited value due to a large proportion of untypeable isolates (5).
We describe here the development of a phage typing scheme for serovar Heidelberg and the characterization of 2,523 strains isolated in Canada between 1991 and 2000. The use of PFGE as an accessory subtyping method allowed further discrimination of strains.
MATERIALS AND METHODS
Phage isolation and purification.
Typing phages 1 to 3, 5, and 6 were isolated from raw sewage received from the waste treatment plant serving Ottawa, Ontario, Canada. Phages 4 and 7 to 10 were isolated from chicken cecal contents and phage 11 was isolated from a serovar Heidelberg strain. Chicken cecal contents were kindly supplied by the Ontario Ministry of Agriculture, Food, and Rural Affairs in Guelph, Ontario, Canada. Phages were isolated, purified, and propagated according to standard methods described by Adams (1). Briefly, sewage samples were pooled and filtered through a 0.2-μm-pore-size bottle top filter with 150-ml capacity (Corning Costar, Corning, N.Y.). Five grams of pooled chicken cecal contents was suspended and mixed thoroughly in 10 ml of Difco phage broth (DPB) (Difco Laboratories, Baltimore, Md.). The mixture was centrifuged at 9,000 × g for 15 min to remove debris. The supernatant was filtered through a 0.2-μm-pore-size Super Acrodisc syringe filter (Gelman Sciences, Ann Arbor, Mich.) and stored at 4°C. One milliliter of filtrate and 0.1 ml of an overnight broth culture of serovar Heidelberg were added to a test tube containing 4.5 ml of DPB and incubated at 37°C in a shaking water bath for 6 to 7 h. The phage lysate was centrifuged at 9,000 × g for 15 min to pellet bacterial debris, and the supernatant was filtered through 0.2-μm-pore-size syringe filters.
Bacterial lawns were prepared by inoculating 4.5 ml of DPB and incubating at 37°C in a shaking water bath for 1.5 to 2 h to attain a bacterial turbidity equivalent to 0.5 McFarland standard (approximately 10 5 cells per ml). A Difco phage agar (DPA) plate was flooded with 2 ml of broth culture. The excess broth was removed using a Pasteur pipette and the plate was allowed to dry for 15 min. A volume of 20 μl of each filtrate was inoculated on the surface of seeded DPA plates. The plates were air dried for 10 min, inverted, and incubated for 18 h at 37°C. Filtrates showing lytic activity were diluted with DPB in 10-fold serial dilutions, and each dilution was inoculated onto a bacterial lawn of propagating strain to obtain isolated plaques. Single plaques with a small amount of adjacent bacterial growth were transferred to 4.5 ml of DPB and incubated for 6 h at 37°C in a shaking water bath for phage propagation. The final phage suspensions were filtered as described previously (2, 3). Phage purification was achieved by three or more consecutive single-plaque propagations on their respective propagating strains.
The typing phages were examined with an electron microscope to establish their identity and confirm the purity of phage lysates. All 11 phages were tailed and had icosahedral heads. Tails were contractile, long and noncontractile, or short. These phages were classified into the families Myoviridae (phage 10), Siphoviridae (phages 2, 3, 4, 5, and 8), and Podoviridae (phages 1, 6, 7, 9, and 11).
Typing phages were selected from 21 phage preparations on the basis of stability, host range, and shelf life. The routine test dilution of phages was determined as the highest dilution giving semiconfluent lysis on its propagating strain. The phages were stored in DPB at 4 to 6°C. The phage reactions were reproducible when tested at 3-month to 1-year intervals, and phage titers were stable at 10× routine test dilution after 5 years. Phages and hosts were deposited in the Félix d'Hérelle Center (accession numbers HER 427-438 and 1427-1438, respectively).
Phage typing.
Serovar Heidelberg isolates were maintained at room temperature on Dorset egg slants (QueLab, Montreal, Québec, Canada). For testing, isolates were plated on nutrient agar plates and incubated at 37°C for 18 h. A small portion of three smooth colonies was inoculated into 4.5 ml of DPB (pH 6.8) and incubated for 1.5 to 2 h in a shaking water bath at 37°C to attain a growth turbidity equivalent to 0.5 McFarland standard. The DPA plates were flooded with 2 ml of culture, and excess liquid was removed using a Pasteur pipette. Seeded plates were allowed to dry for 15 min at room temperature, and approximately 15 μl of each of the 11 typing phages was inoculated onto the bacterial lawn using a multiple-inoculating syringe method (16). The plates were incubated as described above, and lytic patterns were read and recorded (4, 5). Positive readings include confluent lysis, opaque lysis, semiconfluent lysis, and 71 to 100 plaques, and negative readings were 21 to 70 plaques, 5 to 20 plaques, <5 plaques, and no lysis.
Antimicrobial resistance typing.
Antimicrobial resistance patterns (i.e., R types) were determined for 2,403 strains using the Kirby-Bauer disk diffusion technique using BBL Sensi-Disk antimicrobial susceptibility disks (Becton Dickinson, Cockeysville, Md.). Escherichia coli strain ATCC 25922, Staphylococcus aureus strain ATCC 25023, and Pseudomonas aeruginosa strain ATCC 25853 were included as quality control strains in accordance with NCCLS guidelines (21, 22). The antibiotics employed in this study were ampicillin (10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), streptomycin (10 μg), sulfadiazine (0.25 mg), tetracycline (30 μg), and trimethoprim and sulfamethoxazole (1.25 and 23.75 μg, respectively). A part of the culture prepared for phage typing with a bacterial turbidity equivalent to a 0.5 McFarland standard was inoculated with a sterile cotton swab onto Mueller-Hinton agar plates (Oxoid, London, United Kingdom). Antibiotic disks were applied using an eight-place, self-tamping, BBL Sensi-Disk dispenser. Mueller-Hinton agar plates were incubated overnight at 37°C, and inhibition zones were measured (10, 22).
PFGE.
PFGE was done on 225 strains using the method of Barrett et al. (9), which had been modified according to the PulseNet standardized method (12, 26). Bacterial cell concentrations were adjusted to a reading of 0.68 to 0.72 as measured in #2057 Falcon tubes (Becton Dickinson., Franklin Lakes, N.J.) with a Dade Microscan turbidity meter (Dade Behring, West Sacramento, Calif.). Plugs were prepared, washed, and digested according to standard protocols. Electrophoresis was carried out in 1% SEAKEM GOLD agarose (Mandel Scientific, Guelph, Ontario, Canada). All isolates tested were analyzed after XbaI digestion, while selected isolates were analyzed using BlnI as the second enzyme. Patterns were labeled by designating the organism (SH, S. enterica serovar Heidelberg), the restriction enzyme (XAI, XbaI; BNI, BlnI), and a unique identifier (e.g., 0.0001). Any pattern with a difference of one or more bands was given a unique identification number. PFGE results were interpreted in accordance with the criteria described previously (7, 26).
RESULTS
Phage typing.
A panel of 11 bacteriophages was selected according to dissimilar lytic reactions, host range, titer stability, and lytic reaction reproducibility. All 2,523 serovar Heidelberg isolates from human and nonhuman sources belonging to epidemiologically related outbreaks and to sporadic cases were typeable and were divided into 49 different phage types. The lytic patterns that occurred only once and that did not correspond to the 49 phage types were termed atypical. Details of phage lytic reactions and numbers of isolates in each phage type are presented in Table 1. Phage type 19 (PT 19) was the most frequent phage type among human sporadic cases (55%), in outbreak cases (34%), and from nonhuman sources (44%). PT 47 was the second most common type in human sporadic cases (81 isolates [6%]), followed by PT 29 (71 isolates [5%]), PT 6 (64 isolates [5%]), and PT 8 (32 isolates [2%]). PT 47 was also the second most common phage type in outbreak cases (71 isolates [25%]) followed by PT 6 (46 isolates [16%]) and PT 29 (33 isolates [12%]) Further details regarding phage typing of outbreak isolates are provided in the following sections. The frequency of nonhuman phage types was as follows: PT 36 (67 isolates [8%]), PT 47 (59 isolates [7%]), PT 17 (44 isolates [5%]), and PT 6 (32 isolates [4%]). Thirty-three familial outbreaks other than those described in Table 2 produced 86 isolates which were subdivided into PT 19 (55 isolates), PT 8 (8 isolates), PT 6 (7 isolates), PT 29 (4 isolates), and PT 25 (3 isolates). PT 2 and 41 were represented by two isolates each. Five isolates were from two clusters of two different atypical patterns of three and two isolates, respectively.
TABLE 1.
S. enterica serovar Heidelberg phage types of sporadic and outbreak isolates from human and nonhuman sourcesa
| PT | Lysis type for bacteriophage:
|
No. of isolates that were type:
|
Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | H | NH | OB | ||
| 1 | OL | CL | CL | SCL | SCL | OL | OL | CL | SCL | SCL | − | 6 | 0 | 6 | |
| 2 | OL | − | − | CL | SCL | OL | OL | CL | CL | OL | − | 16 | 1 | 2 | 19 |
| 3 | OL | − | − | − | − | OL | OL | SCL | CL | SCL | − | 0 | 1 | 1 | |
| 4 | OL | − | − | − | − | OL | OL | − | CL | SCL | − | 2 | 5 | 7 | |
| 5 | − | − | − | CL | CL | OL | − | CL | CL | SCL | CL | 6 | 16 | 22 | |
| 6 | − | − | − | − | − | OL | OL | CL | CL | SCL | − | 64 | 32 | 46 | 142 |
| 7 | − | − | − | OL | − | OL | − | CL | − | − | SCL | 5 | 1 | 6 | |
| 8 | − | − | − | SCL | SCL | − | − | CL | CL | SCL | − | 32 | 8 | 16 | 56 |
| 9 | − | − | − | − | − | − | OL | − | CL | SCL | − | 11 | 5 | 16 | |
| 10 | − | − | CL | CL | CL | OL | OL | CL | CL | CL | − | 10 | 6 | 16 | |
| 11 | − | − | − | CL | − | − | − | CL | CL | SCL | − | 4 | 1 | 5 | |
| 12 | − | − | − | SCL | CL | OL | − | − | CL | SCL | − | 1 | 13 | 14 | |
| 13 | − | − | − | SCL | − | − | OL | CL | CL | SCL | − | 12 | 6 | 18 | |
| 14 | − | CL | CL | CL | CL | − | OL | CL | CL | SCL | − | 1 | 2 | 3 | |
| 15 | − | CL | CL | CL | − | − | OL | SCL | SCL | SCL | − | 1 | 0 | 1 | |
| 16 | − | − | − | SCL | − | OL | − | − | CL | SCL | − | 2 | 1 | 3 | |
| 17 | − | − | − | SCL | SCL | OL | OL | CL | CL | SCL | SCL | 23 | 44 | 6 | 73 |
| 18 | − | − | − | − | SCL | OL | OL | CL | CL | SCL | − | 27 | 20 | 47 | |
| 19 | − | − | − | SCL | SCL | OL | OL | CL | CL | SCL | − | 772 | 362 | 97 | 1,231 |
| 20 | − | − | − | SCL | SCL | − | OL | SCL | SCL | SCL | − | 29 | 20 | 49 | |
| 21 | − | − | − | − | CL | OL | OL | CL | SCL | − | − | 3 | 2 | 5 | |
| 22 | − | − | SCL | CL | CL | − | OL | CL | CL | SCL | − | 6 | 0 | 6 | |
| 23 | − | − | − | CL | − | − | − | CL | − | − | SCL | 3 | 9 | 12 | |
| 24 | − | − | − | − | − | OL | − | CL | CL | SCL | − | 11 | 2 | 13 | |
| 25 | − | − | − | SCL | − | − | OL | − | CL | SCL | − | 6 | 2 | 3 | 11 |
| 26 | − | − | − | CL | − | OL | OL | − | CL | SCL | − | 9 | 1 | 10 | |
| 27 | − | − | − | CL | − | − | − | CL | − | − | − | 5 | 0 | 5 | |
| 28 | OL | CL | SCL | CL | CL | OL | OL | CL | CL | − | − | 0 | 1 | 1 | |
| 29 | − | − | − | − | − | OL | OL | − | CL | SCL | − | 71 | 29 | 33 | 133 |
| 30 | − | − | − | − | − | − | OL | CL | CL | SCL | − | 3 | 1 | 4 | |
| 31 | OL | CL | CL | CL | − | OL | OL | − | − | SCL | − | 1 | 0 | 1 | |
| 32 | − | − | − | − | − | − | − | − | CL | SCL | − | 7 | 10 | 17 | |
| 33 | SCL | − | − | SCL | − | OL | OL | CL | SCL | SCL | − | 1 | 1 | 2 | |
| 34 | − | CL | CL | CL | − | − | OL | CL | − | SCL | − | 0 | 1 | 1 | |
| 35 | − | − | − | SCL | CL | OL | − | CL | − | − | CL | 25 | 13 | 38 | |
| 36 | − | − | − | SCL | CL | − | − | CL | − | − | CL | 17 | 67 | 84 | |
| 37 | − | − | − | − | − | − | OL | − | − | SCL | − | 7 | 0 | 7 | |
| 38 | OL | CL | CL | CL | − | − | OL | CL | CL | SCL | − | 2 | 0 | 2 | |
| 39 | − | − | − | − | − | − | − | − | − | SCL | − | 6 | 0 | 6 | |
| 40 | − | − | − | SCL | − | OL | − | − | − | − | − | 16 | 5 | 21 | |
| 41 | − | − | − | − | − | OL | − | − | − | − | − | 2 | 4 | 2 | 8 |
| 42 | − | − | − | OL | SCL | − | OL | SCL | CL | − | − | 6 | 0 | 6 | |
| 43 | − | − | − | CL | − | − | OL | − | − | OL | − | 4 | 0 | 4 | |
| 44 | − | − | − | CL | SCL | − | − | CL | − | OL | − | 2 | 2 | 4 | |
| 45 | − | − | − | − | SCL | − | − | CL | CL | SCL | − | 3 | 0 | 3 | |
| 46 | − | − | − | − | SCL | − | OL | SCL | SCL | SCL | − | 3 | 2 | 5 | |
| 47 | − | − | − | SCL | − | OL | OL | SCL | SCL | SCL | − | 81 | 59 | 71 | 211 |
| 48 | − | − | − | − | − | − | − | CL | − | SCL | − | 1 | 0 | 1 | |
| 49 | − | − | − | CL | CL | − | − | − | −/OL | SCL | − | 0 | 13 | 13 | |
| AT | 90 | 58 | 6 | 154 | |||||||||||
| Total | 1,415 | 826 | 282 | 2,523 | |||||||||||
AT, atypical lytic patterns appeared only once in this study and did not conform to any phage types; CL, confluent lysis; H, human; NH, nonhuman; OB, outbreak; OL, opaque; PT, phage type; SCL, semiconfluent; −, no lysis.
TABLE 2.
Characterization of selected outbreaks of S. enterica serovar Heidelberg by phage typing, PFGE, and R typing
| Outbreak no. | Date | Source of outbreak | Source of isolates | PTa | PFGE | R typeb | Total cases |
|---|---|---|---|---|---|---|---|
| 00.086 | December 2000 | Christmas party | Human | 19 | SHEXAI.0001 | Sensitive | 5 |
| 19 | SHEXAI.0008 | S | 1 | ||||
| 47 | SHEXAI.0001 | SSu | 1 | ||||
| 99.055 | December 1999 | Nursing home | Human | 6 | SHEXAI.0001 | Sensitive | 4 |
| Human | 6 | SHEXAI.0002 | Sensitive | 1 | |||
| 98.042 | December 1998 | Sandwich shop | Human | 29 | SHEXAI.0020 | ST | 3 |
| Human | 29 | Not done | ST | 4 | |||
| 98.041 | December 1998 | Christmas party | Human | 47 | SHEXAI.0001 | Sensitive | 22 |
| Human | 47 | SHEXAI.0015 | Sensitive | 2 | |||
| Human | 47 | SHEXAI.0034 | Sensitive | 1 | |||
| Human | 47 | SHEXAI.0045 | Sensitive | 1 | |||
| Human | 47 | Not done | Sensitive | 26 | |||
| Turkey/pork | 47 | SHEXAI.0001 | Sensitive | 2 | |||
| 98.039 | December 1998 | Christmas party | Human | 6 | SHEXAI.0052 | ASSuT | 9 |
| Human | 6 | Not done | ASSuT | 10 | |||
| 98.003 | April 1998 | Pot luck dinner | Human | 17 | SHEXAI.0001 | A | 5 |
| 17 | SHEXAI.0009 | A | 1 | ||||
| AT | SHEXAI.0033 | Su | 1 | ||||
| 97.02 | July 1997 | Restaurant | Human | 29 | SHEXAI.0001 | Sensitive | 8 |
| Human | 29 | SHEXAI.0016 | Sensitive | 1 | |||
| Human | 29 | Not done | Sensitive | 4 | |||
| 96.076 | December 1996 | Christmas party | Human | 29 | SHEXAI.0025 | SSuT | 4 |
| Human | 29 | SHEXAI.0026 | SSuT | 1 | |||
| 29 | SHEXAI.0027 | Sensitive | 1 | ||||
| 29 | SHEXAI.0028 | SSuT | 1 | ||||
| 29 | SHEXAI.0029 | SSuT | 1 | ||||
| 29 | SHEXAI.0030 | SSuT | 1 | ||||
| 94.047 | December 1994 | Family gathering | Human | 8 | SHEXAI.0015 | T | 8 |
| 94.002 | January 1994 | Family gathering | Human | 19 | SHEXAI.0001 | Sensitive | 7 |
| Human | 19 | Not done | Sensitive | 8 | |||
| Turkey | 19 | SHEXAI.0001 | Sensitive | 2 | |||
| Pork roast/ham | 19 | SHEXAI.0001 | ASSu | 2 | |||
| 92.059 | August 1992 | Family gathering | Human | 47 | SHEXAI.0001 | Sensitive | 7 |
| Human | 47 | Not done | Sensitive | 9 | |||
| 91.019 | June 1991 | Community dinner | Human | 19 | Not done | Sensitive | 14 |
| Turkey/stuffing | 19 | Not done | Sensitive | 3 | |||
| 91.059 | October 1991 | Social event | Human | 6 | Not done | Sensitive | 13 |
| Beef stew | 6 | Not done | Sensitive | 1 | |||
| Total | 195 |
PT, phage type.
R type represents antimicrobial resistance. Antimicrobials used: ampicillin (A), chloramphenicol (C), ciprofloxacin, streptomycin (S), sulfadiazine (Su), tetracycline (T), and sulfamethoxazole-trimethoprim.
R typing.
Antimicrobial resistance profiles for 2,403 strains were determined using ampicillin, chloramphenicol, streptomycin, sulfadiazine, tetracycline, ciprofloxacin, and trimethoprim and sulfamethoxazole. These strains were divided into 30 R types and 49 phage types. There was no correlation between R types and phage types. Resistance to one antibiotic was detected in 848 strains (35%); resistance to more than one antibiotic occurred in 649 strains (27%). Pentadrug resistance (i.e., resistance to ampicillin, chloramphenicol, streptomycin, sulfadiazine, and tetracycline) was found in 20 strains (0.8%) with PT 6, 19, 23, 32, 35, and 47. These phage types were also identified among 906 strains (38%) that were susceptible to all seven antibiotics (data not shown). Multiple-drug resistance was found in isolates of three outbreaks. Resistance to both streptomycin and tetracycline was noted in seven isolates from a 1998 outbreak associated with a sandwich shop (Table 2). Ampicillin, streptomycin, sulfadiazine, and tetracycline resistance was detected in 20 isolates from an outbreak related to a Christmas party in 1998. Eight isolates, from another Christmas party outbreak observed in 1996, were resistant to streptomycin, sulfadiazine, and tetracycline. Five outbreaks yielded one or two strains with R types different from those of the majority of strains, all epidemiologically linked, within the outbreak. In the case of outbreaks numbered 00.086 and 98.003, strains with different R types had different phage types as well. These isolates may represent sporadic cases occurring simultaneously with outbreaks.
PFGE typing compared to phage typing.
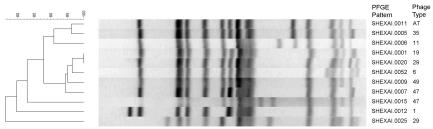
PFGE subdivided all suspected outbreak, sporadic, and reference strains into 52 XbaI PFGE types differing by at least one band. These patterns were designated SHEXAI.0001 to SHEXAI.0052 based on the number, position, and intensity of bands. Additional types are continuously added to the database as newly isolated strains arrive for analysis. A dendrogram of representative pattern types demonstrated that when a level of 90% relatedness was arbitrarily used as a cutoff, there were two major groups of related patterns and three patterns that showed a lower degree of relatedness with these two groups and with each other (Fig. 1). The three strains with very different PFGE patterns were phage typed and found to belong to PT 47, 1, and 29, respectively. Since not all isolates used for PFGE analysis were sent for phage typing, further analysis was done on a subset of strains for which phage typing data were available. SHEXAI.0001 was the most common PFGE pattern (124 of 225 isolates [55%]) and was found in isolates with 14 different phage types. Phage typing therefore provided an additional possibility to subdivide this group of strains. PT 29 strains with 10 PFGE XbaI patterns associated with both clusters on the dendrogram in Fig. 1 showed the most PFGE pattern diversity (data not shown). Pattern type SHEXAI.0001 accounted for 9 of 23 isolates, while 10 other PFGE patterns were seen with the remaining 14 isolates. Of the 66 PT 19 isolates, 60 were PFGE type SHEXAI.0001, while the remaining six isolates were associated with five other PFGE types. The endemic strains causing outbreaks or sporadic cases usually divided in large clusters regardless of the subtyping method used. Thirty-six PT 47 isolates were subdivided into PFGE type SHEXAI.0001 (32 isolates) and four isolates which belonged to three other PFGE types. Similarly, five of six PT 17 isolates were identified as SHEXAI.0001, with the remaining isolate having distinct PFGE pattern SHEXAI.0009. PFGE was not performed on strains associated with outbreaks 91.019 and 91.059 (Table 2).
FIG. 1.
Dendrogram of serovar Heidelberg generated by PFGE using restriction enzyme XbaI. At a level of 90% relatedness, there are two major groups of related patterns and three patterns that show a lower degree of relatedness with these two groups and with each other.
Use of typing schemes to characterize outbreaks of serovar Heidelberg.
A total of 196 epidemiologically related isolates from 13 major outbreaks were subdivided into six phage types, 11 PFGE patterns, and eight R types (Table 2). Identical phage types were observed among epidemiologically related human and food isolates. In four outbreaks, both food and human isolates were available for subtyping. Strains from the same outbreak always had identical phage types. PFGE patterns often correlated closely with phage types. In some cases, such as outbreaks 99.055, 98.041, and 96.076, the PFGE patterns exhibited a limited number of band differences in epidemiologically related strains and a single phage type. PFGE was useful for differentiating PT 29 strains that caused two different outbreaks, 96.076 and 97.020, in different years (Fig. 1).
During an investigation of an outbreak associated with a holiday party in December 1998, 52 human isolates and 2 food isolates were analyzed. All 54 isolates belonged to PT 47 and were sensitive to all antimicrobial agents tested. Twenty-two of the human isolates and both food isolates had PFGE patterns SHEXAI.0001. Two human isolates had PFGE pattern SHEXAI.0015, and the two others showed patterns SHEXAI.0034 and SHEXAI.0045, respectively. All patterns differed from SHEXAI.0001 by less than three bands. This established a link between consumed food and the illness of 26 people. The remaining 26 human cases were included in the outbreak without PFGE testing on the basis of phage type and R type.
In 1994, a community outbreak associated with a holiday banquet provided 15 human and 4 food isolates for testing. Two of the food isolates were from cooked turkey, one was from a pork roast, and one was from a ham. All 19 isolates from this outbreak were identified as PT 19. PFGE performed on seven human and four food isolates gave identical PFGE patterns (SHEXAI.0001). The human and two turkey isolates were sensitive to all antimicrobial agents tested, but the pork and ham isolates were resistant to ampicillin, streptomycin, and sulfadiazine. Since PT 19 and PFGE pattern SHEXAI.0001 were the most common strain classifications in Canada, the pork products were excluded as the likely source of infection on the basis of R typing.
Two outbreaks in 1991 were characterized by phage typing and R typing because of limited resources and time. A community dinner in June of 1991 yielded 14 human and three turkey and stuffing isolates, all with identical lytic patterns and R types. Similarly, beef stew was implicated as a source of infection in 13 human cases at a social event. All isolates from food and humans had identical phage types and R types.
DISCUSSION
All 2,523 strains were typeable with this typing scheme. The National Laboratory for Enteric Pathogens commonly uses a number of typing methods such as serotyping, biotyping, phage typing, antimicrobial resistance testing, PFGE, and ribotyping to determine etiology and relatedness of outbreak strains. As the characteristic markers of outbreak strains are ascertained, large numbers of the remaining strains are screened for established markers only. This strategy can allow phage typing to be used as a screening method to investigate large numbers of strains associated with outbreaks spreading across communities, cities, provinces, and countries. There was no correlation between phage types and PFGE patterns because two different markers were targeted. Correlation between phage types and R types was seen in serovar Typhimurium strain DT 104 and multidrug resistance (i.e., to ampicillin, chloramphenicol, streptomycin, sulfadiazine, and tetracycline). PFGE may be a method of choice for bacterial agents such as E. coli strain O157:H7, but it proved to be less effective for subtyping serovar Enteritidis, yielding fewer bands, which allows for less discrimination (4). Nevertheless, phage typing or the combination of phage typing and PFGE may necessitate characterization of fewer isolates by PFGE, resulting in substantial cost savings (3, 4, 27). On some occasions, phage typing provides refinement of epidemiologic investigations. One isolate thought to be epidemiologically associated with outbreak 00.086 and another from outbreak 98.003 represented phage types that differed from those of the outbreak strains. These isolates were possibly from sporadic cases occurring at the same time as the outbreaks. In several instances, phage typing grouped together isolates with different PFGE patterns. In most of those cases, the PFGE patterns did not differ by more than one or two bands, suggesting that the strains were actually related and that the PFGE patterns had changed during the outbreak (data not shown). Patterns SHEXAI.0001 and SHEXAI.0009, for example, differed only by the presence of a low-molecular-mass band that could have been a plasmid (Table 2).
Antibiograms could often be used to confirm the association of isolates with an outbreak even when PFGE patterns differed slightly. These results emphasize the need for the employment of multiple typing methods when investigating outbreaks. Strains from sporadic cases and nonhuman isolates were included in this study to help understand the distribution and epidemiology of serovar Heidelberg infection. However, due to the costs and logistics involved, not all isolates obtained by the provincial Public Health laboratories are forwarded to the National Laboratory for Enteric Pathogens for characterization by phage typing. It is therefore not possible to draw any firm conclusions about the geographical or temporal distributions of specific phage types or PFGE patterns in Canada. Further surveillance is required to establish whether PT 19 is a permanent aspect of the Canadian flora or whether its high prevalence is transitory. Isolates from other parts of the world may provide a substantially different phage type distribution. As more data are gathered, the typing databases for serovar Heidelberg will become more reliable, and outbreaks or other events of public health significance can be identified with greater precision and accuracy. Our study indicates that this phage typing scheme provides a new and valuable epidemiological tool in tracing the origin of serovar Heidelberg infections and will help in the development of control and prevention strategies for the Canadian population.
Acknowledgments
We thank Johanne Ismail for manuscript review and gratefully acknowledge receiving bacterial strains to be phage typed in this study from our colleagues in the provincial Public Health laboratories: Judith Isaac-Renton, Laboratory Services, BC Centre for Disease Control, Vancouver, British Columbia; Jutta K. Preiksaitis, Public Health Laboratory for Alberta, Edmonton, Alberta; Greg Horsman, Saskatchewan Health Laboratory and Disease Control Services Branch, Regina, Saskatchewan; Paul Van Caeseele, Manitoba Health Services Commission, Cadham Provincial Laboratory, Winnipeg, Manitoba; Frances Jamieson, Central Public Health Laboratory, Ontario Ministry of Health, Toronto, Ontario; Jean Joly, Laboratorie de la sante publique du Québec, Montreal, Québec; David Haldane, Bacteriology and Special Pathogens, Division of Microbiology, QE II Health Sciences Centre, Halifax, Nova Scotia; Lewis Abbott, Provincial Health Laboratory, Charlottetown, Prince Edward Island; Anne O'Brien, Department of Microbiology, Saint John, New Brunswick; and Sam Ratnam, Newfoundland Public Health Laboratories, St. John's, Newfoundland.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York, N.Y.
- 2.Ahmed, R., C. Bopp, A. Borczyk, and S. Kasatiya. 1987. Phage-typing scheme for Escherichia coli O157:H7. J. Infect. Dis. 155:806-809. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, R., P. Sankar-Mistry, S. Jackson, H.-W. Ackermann, and S. S. Kasatiya. 1995. Bacillus cereus phage typing as an epidemiological tool in outbreaks of food poisoning. J. Clin. Microbiol. 33:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed, R., G. Soule, W. Demczuk, R. Khakhria, S. Ratnam, S. Marshall, L.-K. Ng, D. Woodward, W. Johnson, and F. Rodgers. 2000. Epidemiologic typing of Salmonella enteritidis in a Canada-wide outbreak of gastroenteritis due to contaminated cheese. J. Clin. Microbiol. 38:2403-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amavisit, P., P. F. Markham, D. Lightfoot, K. G. Whithear, and G. F. Browning. 2001. Molecular epidemiology of Salmonella Heidelberg in an equine hospital. Vet. Microbiol. 80:85-98. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, E. S., and R. E. O. Williams. 1956. Bacteriophage typing of enteric pathogens and staphylococci and its use in epidemiology. J. Clin. Pathol. 9:94-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbeit, R. D. 1995. Laboratory procedures for epidemiologic analysis of microorganisms, p. 190-208. In P. R. Murray, E. J. Barron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 8.Barnass, S., M. O'Mahony, P. H. Sockett, J. Garner, J. Franklin, and S. Tabaqchali. 1989. The tangible cost implications of a hospital outbreak of multiply-resistant Salmonella. Epidemiol. Infect. 103:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett, T. J., H. Lior, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multi-state food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer, A. W., W. M. M. Kirby, J. C. Sherris, and M. Turck. 1996. Antibiotic testing by standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 11.Burt, C. R., J. C. Proudfoot, M. Roberts, and R. H. Horowitz. 1990. Fatal myocarditic secondary to Salmonella septicemia in a young adult. J. Emerg. Med. 8:295-297. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1998. Standardized molecular subtyping of foodborne bacterial pathogens by pulsed-field gel electrophoresis. The National Molecular Subtyping Network for Foodborne Disease Surveillance, Atlanta, Ga.
- 13.Chambers, J. R., J.-R. Bisaillon, Y. Labbé, C. Poppe, and C. F. Langford. 1998. Salmonella prevalence in crops of Ontario and Quebec broiler chickens at slaughter. Poult. Sci. 77:1497-1501. [DOI] [PubMed] [Google Scholar]
- 14.Choi, M., T. T. Yoshikawa, J. Bridge, A. Schlaifer, D. Osterweil, D. Reid, and D. C. Norman. 1990. Salmonella outbreak in a nursing home. J. Am. Geriatr. Soc. 38:531-534. [DOI] [PubMed] [Google Scholar]
- 15.Demczuk, W., R. Ahmed, D. Woodward, C. Clark, and F. Rodgers. 2000. Laboratory surveillance for enteric pathogens in Canada, 2000 annual summary. National Laboratory for Enteric Pathogens, National Microbiology Laboratory, Health Canada. The Canadian Science Centre for Human and Animal Health, Winnipeg, Manitoba, Canada.
- 16.Farmer, J. J., F. W. Hickman, and J. V. Sikes. 1975. Automation of Salmonella typhi phage-typing. Lancet ii:787-790. [DOI] [PubMed] [Google Scholar]
- 17.Harvey, D., C. Harrington, M. W. Heuzenroeder, and C. Murray. 1993. Lysogenic phage in Salmonella enterica serovar Heidelberg (Salmonella Heidelberg): implications for organism tracing. FEMS Microbiol. Lett. 103:291-295. [DOI] [PubMed] [Google Scholar]
- 18.Layton, M. C., S. G. Calliste, T. M. Gomez, C. Patton, and S. Brooks. 1997. A mixed foodborne outbreak with Salmonella heidelberg and Campylobacter jejuni in a nursing home. Infect. Control Hosp. Epidemiol. 18:115-121. [DOI] [PubMed] [Google Scholar]
- 19.Lintz, D., R. Kapila, E. Pilgrim, F. Tecson, R. Dorn, and D. Louria. 1976. Nosocomial Salmonella epidemic. Arch. Intern. Med. 136:968-973. [PubMed] [Google Scholar]
- 20.Lyons, R. W., C. L. Samples, H. N. Desilva, K. A. Ross, E. M. Julian, and P. J. Checko. 1980. An epidemic of resistant Salmonella in a nursery: animal-to-human spread. JAMA 243:546-547. [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1997. Approved standard M7-A4. Methods for dilution antimicrobial suseptibility tests for bacteria that grow aerobically, 4th ed., vol. 17, no. 2, M100-S7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.National Committee for Clinical Laboratory Standards. 1993. Approved standard M2-A5. Performance standards for antimicrobial disk susceptibility test, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Pedersen, K., H. C. Hansen, J. C. Jørgensen, and B. Borck. 2002. Serovars of Salmonella isolated from Danish turkeys between 1995 and 2000 and their antimicrobial resistance. Vet. Rec. 150:471-474. [DOI] [PubMed] [Google Scholar]
- 24.Sørensen, O., J. van Donkersgoed, M. McFall, K. Manninen, G. Gensler, and G. Ollis. 2002. Salmonella spp. shedding by Alberta beef cattle and the detection of Salmonella spp. in ground beef. J. Food Prot. 65:484-491. [DOI] [PubMed] [Google Scholar]
- 25.Stanley, J., A. Burnens, N. Powell, N. Chowdry, and C. Jones. 1992. The insertion sequence IS200 fingerprints chromosomal genotypes and epidemiological relationships in Salmonella heidelberg. J. Gen. Microbiol. 138:2329-2336. [DOI] [PubMed] [Google Scholar]
- 26.Tenover, F. C., R. Arbeit, R. Goering, et al. 1997. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect. Control Hosp. Epidemiol. 18:426-439. [DOI] [PubMed] [Google Scholar]
- 27.Warshawsky, B., I. Gumanis, B. Henry, J. Dow, J. Reffle, G. Pollett, R. Ahmed, J. Aldom, D. Alves, A. Chagla, B. Ciebin, F. Kolbe, F. Jamieson, and F. Rodgers. 2002. Outbreak of Escherichia coli O157:H7 related to animal contact at a petting zoo. Can. J. Infect. Dis. 13:175-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilmshurst, P., and H. Sutcliffe. 1995. Splenic abscess due to Salmonella heidelberg. Clin. Infect. Dis. 21:1065. [DOI] [PubMed] [Google Scholar]