Abstract
A multiplex PCR method incorporating primers flanking three variable-number tandem repeat (VNTR) loci (arbitrarily labeled TR1, TR2, and TR3) in the CT18 strain of Salmonella enterica serovar Typhi has been developed for molecular typing of S. enterica serovar Typhi clinical isolates from several Asian countries, including Singapore, Indonesia, India, Bangladesh, Malaysia, and Nepal. We have demonstrated that the multiplex PCR could be performed on crude cell lysates and that the VNTR banding profiles produced could be easily analyzed by visual inspection after conventional agarose gel electrophoresis. The assay was highly discriminative in identifying 49 distinct VNTR profiles among 59 individual isolates. A high level of VNTR profile heterogeneity was observed in isolates from within the same country and among countries. These VNTR profiles remained stable after the strains were passaged extensively under routine laboratory culture conditions. In contrast to the S. enterica serovar Typhi isolates, an absence of TR3 amplicons and a lack of length polymorphisms in TR1 and TR2 amplicons were observed for other S. enterica serovars, such as Salmonella enterica serovar Typhimurium, Salmonella enterica serovar Enteritidis, and Salmonella enterica serovar Paratyphi A, B, and C. DNA sequencing of the amplified VNTR regions substantiated these results, suggesting the high stability of the multiplex PCR assay. The multiplex-PCR-based VNTR profiling developed in this study provides a simple, rapid, reproducible, and high-resolution molecular tool for the epidemiological analysis of S. enterica serovar Typhi strains.
Salmonella enterica serovar Typhi is the etiological agent of typhoid fever, of which there are an estimated 16 million cases with 600,000 related deaths worldwide (15). The gram-negative rod-shaped bacterium is pathogenic only in humans, where it can be cultured from blood and stools. Infection occurs when water or food contaminated with S. enterica serovar Typhi is consumed. Most patients who recover from the infection are able to eliminate the bacterium completely from their bodies. However, some of them may remain as healthy carriers, continuously shedding S. enterica serovar Typhi in their stools (5).
Epidemiological studies of pathogens are of great importance in controlling their dissemination. The capability to strain type pathogens is a critical tool in epidemiological investigations. A number of strain-typing methods have been developed for S. enterica serovar Typhi. The classical methods of phage typing and isoenzyme analysis have been increasingly complemented by molecular techniques, such as pulsed-field gel electrophoresis (PFGE) (37, 38), ribotyping (2, 25), random amplification of polymorphic DNA (RAPD) (32), and DNA fingerprinting using the mobile genetic element IS200 (39), as well as the recent development of amplified fragment length polymorphism (AFLP) for discriminating S. enterica serovar Typhi strains (22).
Despite the availability of the above-mentioned methods, there is still a lack of methods with the right combination of rapidity, ease of use, reproducibility, and discriminatory power for typing S. enterica serovar Typhi. PFGE, ribotyping, and AFLP procedures are technically complicated and time-consuming. RAPD is rapid but is hampered by its poor reproducibility. IS200 fingerprinting has been reported to lack discriminative power (40).
Variable-number tandem repeats (VNTR) have been increasingly used as molecular markers for strain typing of various bacteria. VNTR, or short sequence repeats, consist of unique DNA elements that are repeated in tandem (41). Individual strains within a bacterial species often maintain the same sequence element but with different copy numbers. Such variation is often caused by slipped-strand mispairing during DNA replication (6, 43). Since sequence homology exists between strains in the flanking region of the VNTR locus, PCR amplification with flanking-sequence-specific primers can be used to determine the variations in copy numbers of repeat units that reflect the intraspecies genetic diversity. Therefore, individual strains could be easily identified by the amplicon sizes. This forms the basis for using VNTR for strain typing.
The availability of complete microbial genomic sequences has greatly facilitated the identification of VNTR for strain typing. Using software programs that rapidly scan the genomes for them, VNTR can be quickly located, and primers for PCR analysis can made based on the flanking sequences. We adopted this method of VNTR detection in this study, which resulted in the development of a multiplex PCR assay using three different VNTR loci as molecular markers to differentiate clinical S. enterica serovar Typhi isolates from several Asian countries. To our knowledge, this is the first report describing the use of multiplex-PCR-based VNTR profiling for the strain typing of S. enterica serovar Typhi isolates.
MATERIALS AND METHODS
Bacterial isolates and culture conditions.
The S. enterica serovar Typhi isolates used in this study are described in Table 1. A total of 61 S. enterica serovar Typhi isolates collected during the years 2000 and 2001 were obtained from the Singapore General Hospital. Four pairs of the 61 isolates were collected from four different patients (i.e., one pair per patient). Three of the four pairs were collected at different times over the course of infection, while one pair was collected from fresh whole blood versus a blood clot on the same day. Therefore, the total number of individual clinical isolates was 57 (Table 1). Of these 57 isolates, 13 were indigenous isolates obtained from sporadic cases in Singapore, while the rest were isolated from imported cases from several Asian countries, including Indonesia, India, Bangladesh, Malaysia, and Nepal. In addition, S. enterica serovar Typhi strains CT18 and TY2-b were obtained from the Salmonella Genetic Stock Centre (SGSC), University of Calgary, Calgary, Canada, to be used as reference strains in the study.
TABLE 1.
59 S. enterica serovar Typhi isolates used in this study
| Origin | Reference no. of isolatesa | Yr of collection |
|---|---|---|
| Singapore | 349, 578, 581 | 2001 |
| 156, 320, 326, 489, 493, 517, 541, 557, 587, 612 | 2000 | |
| Indonesia | 12, 31, 77, 117, 158, 182, 208, 219, 226, 256, 293, 303, 311, 343, 377, 425, 426, 447 | 2001 |
| 76, 191, 288, 308, 463, 609 | 2000 | |
| India | 10, 120, 138 | 2001 |
| 33, 72, 234, 261, 306, 354, 410 | 2000 | |
| Bangladesh | 283, 315, 446, 508 | 2001 |
| 136, 549 | 2000 | |
| Malaysia | 396 | 2001 |
| 470 | 2000 | |
| Nepal | 365, 367 | 2001 |
| SGSC | CT18, TY2-b | 2001 |
Paired isolates were obtained for isolates 493, 256, and 136. Each pair was collected from the corresponding patients 17, 35, and 38 days apart, respectively. A paired isolate was also obtained for 306 from fresh whole blood versus a blood clot of a patient on the same day.
Phage typing of the clinical isolates was carried out by the hospital, but some isolates did not produce conclusive results (Table 2).
TABLE 2.
VNTR profiles and phage types of 59 S. enterica serovar Typhi isolates
| VNTR profile (TR1/TR2/TR3)a | Isolate reference no. | VNTR profile designation | Phage typeb |
|---|---|---|---|
| 5x/36.9x/2.3x | 587 | S1 | E1 |
| 8x/35.9x/72.3x | 156 | S2 | NC |
| 10x/16.9x/2.3x | 557, 612 | S3 | D2 |
| 10x/23.9x/2.3x | 320, 326 | S4 | D2 |
| 12x/16.9x/2.3x | 578 | S5 | NC |
| 13x/6.9x/2.3x | 581 | S6 | NC |
| 13x/32.9x/3.3x | 541 | S7 | A1 |
| 15x/20.9x/2.3x | 349 | S8 | D1 |
| 16x/12.9x/2.3x | 517 | S9 | NC |
| 19x/10.9x/§2.3x | 493 | S10 | NC |
| 19x/15.9x/2.3x | 489 | S11 | NC |
| 6x/27.9x/2.3x | 77 | IDN1 | NC |
| 9x/11.9x/2.3x | 303 | IDN2 | K1 |
| 10x/11.9x/§2.3x | 226, 377 | IDN3 | A |
| 10x/30.9x/δ2.3x | 256 | IDN4 | NC |
| 11x/15.9x/2.3x | 12 | IDN5 | D2 |
| 12x/6.9x/2.3x | 31, 426, 470 | IDN6/MS2 | D2/NC |
| 12x/11.9x/2.3x | 343 | IDN7 | D2 |
| 12x/17.9x/2.3x | 208, 219, 293 | IDN8 | D2 |
| 12x/19.9x/2.3x | 609 | IDN9 | D2 |
| 12x/20.9x/2.3x | 182 | IDN10 | NC |
| 12x/26.9x/2.3x | 117 | IDN11 | NC |
| 12x/32.9x/3.3x | 447 | IDN12 | M1 |
| 13x/4.9x/2.3x | 158 | IDN13 | NC |
| 13x/12.9x/2.3x | 288 | IDN14 | D2 |
| 14x/14.9x/§2.3x | 191 | IDN15 | NC |
| 14x/21.9x/2.3x | 311 | IDN16 | NC |
| 14x/22.9x/§2.3x | 308 | IDN17 | UVS1 |
| 14x/24.9x/2.3x | 425 | IDN18 | D2 |
| 15x/5.9x/2.3x | 463 | IDN19 | NC |
| 16x/20.9x/2.3x | 76 | IDN20 | NC |
| 5x/29.9x/2.3x | 234, 261, 354 | IN1 | E1 |
| 5x/31.9x/2.3x | 10 | IN2 | E1 |
| 5x/32.9x/2.3x | 120 | IN3 | E1 |
| 6x/22.9x/1.3x | 72 | IN4 | J1 |
| 10x/15.9x/2.3x | 33 | IN5 | E1 |
| 12x/27.9x/2.3x | 138 | IN6 | NC |
| 14x/9.9x/4.3x | 410 | IN7 | A1 |
| 14x/14.9x/2.3x | 306 | IN8 | NC |
| 5x/33.9x/2.3x | 136 | B1 | NC |
| 8x/8.9x/2.3x | 549 | B2 | NC |
| 8x/24.9x/2.3x | 283 | B3 | NC |
| 9x/18.9x/2.3x | 315 | B4 | NC |
| 9x/27.9x/2.3x | 446 | B5 | NC |
| 18x/13.9x/2.3x | 508 | B6 | NC |
| 11x/8.9x/3.3x | 396 | MS1 | A |
| 10x/10.9x/3.3x | 365, 367 | N1 | M1 |
| 11x/9.9x/2.3x | TY2-b | TY2-b type | |
| 12x/27.9x/§3.3x | CT18 | CT18 type |
The VNTR profiles were defined based on the copy numbers of the respective repeats at the three different loci in each isolate. x represents the copy number of each VNTR; δ represents a 122-bp deletion; § represents a 61-bp deletion in the 5′ upstream sequences of the TR3 alleles.
NC, not confirmed.
Strains of other Salmonella enterica serovars were used as controls in the study. They included S. enterica serovar Typhimurium (strains TR7095 and SL1344), S. enterica serovar Enteritidis (strains LK5 and PT4) obtained from the SGSC, and S. enterica serovar Paratyphi A, B, and C from the Singapore General Hospital.
Preparation of crude bacterial DNA.
About one to five bacterial colonies from Luria-Bertani agar medium were suspended in 200 μl of distilled H2O. The cell suspension was boiled at 95°C for 7 min and then stored at 4°C before being used directly for PCR.
Selection of VNTR loci and PCR primers.
Five VNTR loci, designated TR1 to TR5, were selected by the Tandem Repeat Finder program (http://tandem.biomath.mssm.edu/trf/trf.html) (4) from the entire genomic sequence of S. enterica serovar Typhi CT18, a multiple-drug-resistant strain (26), under GenBank accession no. NC_003198. PCR primers were designed from sequences flanking the respective TR1 to TR5 loci (Table 3). These five loci, each containing a unique direct repeat, are located within intergenic regions, increasing the probability of copy number variation.
TABLE 3.
PCR primers flanking the five VNTR loci selected from the S. enterica serovar Typhi CT18 genome
| Primer | Nucleotide positions | Sequence (5′-3′) | VNTR sequence |
|---|---|---|---|
| TR1F1 | 2017115-2017136 | AGA ACC AGC AAT GCG CCA ACG A | (AGAAGAA)12 |
| TR1R1 | 2017354-2017375 | CAA GAA GTG CGC ATA CTA CAC C | |
| TR2F1 | 2556810-2556831 | CCC TGT TTT TCG TGC TGA TAC G | (CCAGTTCC)27 |
| TR2R2 | 2557299-2557320 | CAG AGG ATA TCG CAA CAA TCG G | |
| TR3F1 | 2926145-2926166 | CGA AGG CGG AAA AAA CGT CCT G | (CGCGGGGATCGGTTTATCCCCGCTGG)3.3 |
| TR3R1 | 2926668-2926689 | TGC GAT TGG TGT CGT TTC TAC C | |
| TR4F2 | 4396728-4396749 | AAA AGC CCG TCT AGT CTT GCA G | (GAAATAAAAAATG)2.1 |
| TR4R1 | 4397127-4397148 | ATC CTT CGG TAT CGG GGT ATC C | |
| TR5F1 | 4624169-4624190 | TGA AAA CCG GCT CGT AGC AGT G | (CGTCACG)4.7 |
| TR5R1 | 4624342-4624363 | CAT ACG GTT ACT GCG GGA TTG G |
Screening for length polymorphism via PCR of individual VNTR loci.
Prior to performing the multiplex PCR assay, the five VNTR loci were evaluated for the presence of allelic variations using PCR with the respective primer pairs on 20 different S. enterica serovar Typhi isolates. All PCR assays were performed with the Platinum PCR Supermix kit (Invitrogen Life Technologies). Each 25-μl reaction mixture contained a mixture of 1 μl of the suspension of the boiled bacterial cell lysate, 5 pmol of each primer, and 22.5 μl of the Supermix supplied with the kit. After an initial denaturation at 94°C for 2 min, the reaction was performed for 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by an extension step of 72°C for 7 min. In these 20 isolates, allelic variations were eventually detected in the TR1, TR2, and TR3 loci but not the TR4 and TR5 loci (see Results). Therefore, the TR1, TR2, and TR3 loci were further characterized in this study.
Multiplex PCR assay.
The primers flanking the TR1, TR2, and TR3 loci were selected for developing the multiplex PCR assay. Each 25-μl reaction mixture contained 1 μl of the bacterial lysate suspension and 10 pmol each of the forward and reverse primers for TR1 and TR3, as well as 12.5 pmol each of the corresponding primers for TR2, in addition to 22.5 μl of the Supermix. The same PCR cycling conditions described above were used. A 5-μl aliquot of the PCR products was resolved on a 1.5% (wt/vol) agarose gel containing 0.5 μg of ethidium bromide ml−1 in 0.5× TBE buffer (45 mM Tris-borate, 1 mM EDTA, pH 8.0). A 100-bp DNA marker (Promega Corp., Madison, Wis.) was used as a molecular size standard. The multiplex PCR was repeated more than five times using different thermocyclers and different preparations of the cell lysates to ascertain the reproducibility of the results. Finally, the reproducibility, discriminatory power, and typeability of this multiplex-PCR-based VNTR typing method, in comparison with other PCR-based typing methods, including RAPD, enterobacterial repetitive intergenic consensus PCR, and AFLP, were evaluated on 20 randomly selected S. enterica serovar Typhi isolates.
DNA sequencing and data analysis.
To confirm the identities of the amplicons and that any length polymorphism was due to variations in the VNTR copy number, DNA sequencing was performed on the nonmultiplexed PCR products from all 61 clinical isolates and the 2 reference strains following a purification process primarily by an enzyme digest method. An alternative method using the QIAquick PCR purification kit (Qiagen, Chatsworth, Calif.) was used only for PCRs with lower yields. The enzyme digest method involved treating 8 μl of PCR products with 0.3 U of exonuclease I and 1.5 U of shrimp alkaline phosphatase (Amersham) in a 10-μl reaction mixture at 37°C for 5 min to remove the excess primers and deoxynucleoside triphosphates, followed by the inactivation of the enzymes at 72°C for 15 min. A 2-μl aliquot of the enzyme-treated PCR products was used directly in the sequencing reaction. Each PCR fragment was sequenced from both directions using the same PCR primers with a DYEnamic ET dye terminator kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions. The sequencing reactions were resolved on a MegaBACE automated capillary sequencer. DNA sequences were analyzed with the LASERGENE software package (DNASTAR, Madison, Wis.) and Tandem Repeat Finder (4).
RESULTS
Screening for allelic variations at the TR1, TR2, and TR3 VNTR loci.
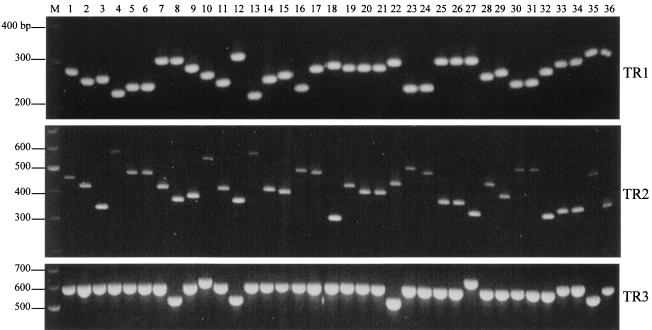
PCR was performed on 20 isolates using individual primer pairs to evaluate the potential use of the TR1 to TR5 VNTR loci as molecular markers for the strain typing of S. enterica serovar Typhi. Length polymorphisms of the amplified fragments among the 20 isolates were observed for the TR1, TR2, and TR3 loci but not for the TR4 and TR5 loci, for which amplicons of ∼420 and 190 bp, respectively, were observed (results not shown). The specificity of the primers could be seen from the single band obtained in each reaction. Length polymorphisms were especially pronounced for TR1 and TR2. Allelic variations at these three loci were also observed when the remaining isolates were screened. The results of the amplification of representative isolates can be seen in Fig. 1.
FIG. 1.
Gel electrophoresis of the TR1, TR2, and TR3 VNTR loci of 36 representative isolates amplified using the TR1, TR2, and TR3 primer pairs. Lanes: 1 to 14, Singapore isolates (lanes 8 and 12 are paired isolates collected 17 days apart); 15 to 22, Indonesian isolates; 23 to 27, Indian isolates; 28 to 32, Bangladeshi isolates (lanes 30 and 31 are paired isolates collected 38 days apart); 33 and 34, Nepalese isolates; 35 and 36, S. enterica serovar Typhi CT18 and TY2-b; M, 100-bp DNA marker.
It was also observed that there were no changes in the sizes of the TR1, TR2, and TR3 amplicons from four isolates that had been passaged for 40 rounds (results not shown), suggesting that the three loci have sufficient genetic stability for use as molecular strain-typing markers. In addition, no changes in the sizes and sequences of TR1, TR2, and TR3 were observed in the paired S. enterica serovar Typhi isolates, especially the three pairs that were collected from the same patients at different times (Fig. 1, lanes 8 and 12 and 30 and 31) over the course of infection. This implied that the VNTR markers in the same strain did not change over the course of an infection. This is a strong indication that these markers could cluster epidemiologically related strains instead of erroneously segregating them.
Sequence analysis of individual VNTR alleles.
The specificity of the PCR was confirmed by the sequencing data. The TR1, TR2, and TR3 sequences of the CT18 strain were identical to those from GenBank. Sequence analysis of all amplicons from hospital and reference strains revealed that the length polymorphisms observed among the TR1 and TR2 amplicons were indeed the result of different copy numbers of the respective repeats. As expected, paired isolates taken from the same patient had identical repetitive sequences despite the different times of collection or types of clinical specimens. Among the 59 individual isolates, including two reference strains, a total of 14 distinct alleles ranging between 2 and 19 copies of the 7-bp repeat unit were observed at the TR1 locus, while a total of 29 distinct alleles ranging between 4.9 and 36.9 copies of the 8-bp repeat were detected at the TR2 locus. In contrast to TR1 and TR2, there were only four alleles of 1.3, 2.3, 3.3, and 4.3 copies of the 26-bp repeat at the TR3 locus among the 59 isolates (Table 2).
Sequencing analysis of the TR3 amplicons also revealed deletions of 61- and 122-bp nucleotides in the 5′ upstream region of the TR3 repeats among seven different isolates (results not shown). The 61-bp deletion was found in six isolates (493, 226, 377, 191, 308, and CT18), while the 122-bp deletion was found only in the Indonesian isolate 256 (Table 2). Therefore, unlike the TR1 and TR2 amplicons, the length polymorphisms for the TR3 amplicons among these seven isolates is a result of variations in the copy number of the TR3 VNTR, as well as the presence of the nucleotide deletions in the 5′ upstream regions.
Multiplex-PCR-based VNTR analysis.
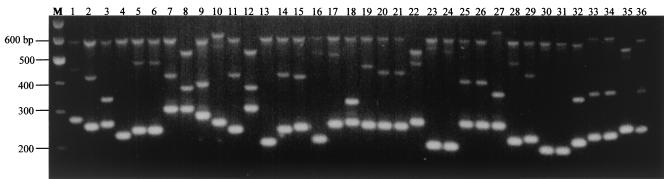
Amplicons of different sizes were observed for isolates when they were subjected to multiplex PCR with primers for TR1, TR2, and TR3 (Fig. 2). Based on visual observation, each of these bands was similar in size to its counterpart obtained during amplification with individual primer pairs (Fig. 1). A total of 49 arbitrarily assigned strain types based on the banding profiles could be identified among the 59 isolates (Table 2). The results showed that we were able to segregate 13 Singapore isolates into 11 VNTR types (S1 to S11), 24 Indonesian isolates into 20 VNTR types (IND1 to IND20), 10 Indian isolates into 8 VNTR types (IN1 to IN8), 6 Bangladeshi isolates into 6 VNTR types (B1 to B6), and 2 Malaysian isolates into 2 VNTR types (MS1 and MS2). Two Nepalese isolates were found to have identical VNTR types (N1). With the exception of the Malaysian isolate 470 (VNTR profile MS2), which is identical in profile to the Indonesian isolates 31 and 426 (VNTR profile IDN6), most of the isolates from each country were distinctive and different from the isolates from all other countries. Intracountry variation in the VNTR profiles was generally observed, although there were some isolates that had the same profile. The reference strains CT18 and TY2-b exhibited distinct banding profiles compared with one another and with the rest of the isolates (Table 2 and Fig. 2, lanes 35 and 36). Repetition of the multiplex PCR confirmed that the results were reproducible. Comparison of the VNTR profiles with the phage-typing data revealed that isolates with the same VNTR profile share the same phage type. However, isolates with the same phage types could exhibit different VNTR profiles (Table 2).
FIG. 2.
Agarose gel analysis of the VNTR banding profiles amplified from the 36 representative S. enterica serovar Typhi isolates by multiplex PCR containing TR1, TR2, and TR3 primer pairs. Lanes: 1 to 14, Singapore isolates (lanes 8 and 12 are paired isolates collected 17 days apart); 15 to 22, Indonesian isolates; 23 to 27, Indian isolates; 28 to 32, Bangladeshi isolates (lanes 30 and 31 are paired isolates collected 38 days apart); 33 and 34, Nepalese isolates; 35 and 36, S. enterica serovar Typhi CT18 and TY2-b; M, 100-bp DNA marker.
Finally, a comparison between the multiplex PCR assay and other PCR-based strain-typing methods, such as RAPD, enterobacterial repetitive intergenic consensus PCR, and AFLP, demonstrated that VNTR profiling had the best mix of discriminatory power, reproducibility, typeability, speed, ease of usage, and cost (data not shown).
Specificity of the multiplex PCR assay.
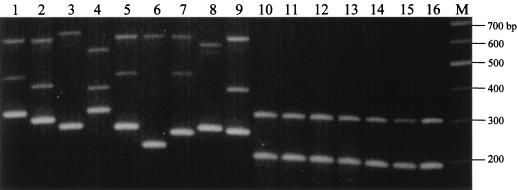
When the multiplex PCR was performed on S. enterica serovar Typhimurium, S. enterica serovar Enteritidis, and S. enterica serovar Paratyphi A, B, and C, two amplicons of ∼200 and 300 bp were observed for all reactions (Fig. 3). This is in sharp contrast to the highly polymorphic VNTR profiles in the S. enterica serovar Typhi isolates. A PCR assay using individual primer pairs confirmed that the 200-bp fragment was amplified by the TR1 primers while the 300-bp fragment was a product of the TR2 primers. However, no amplification was observed using the TR3 primers (data not shown), suggesting that sequences of the TR3 primer sites are conserved in S. enterica serovar Typhi.
FIG. 3.
Specificity of the multiplex PCR assay for strain typing S. enterica serovar Typhi isolates. Lanes: 1 to 9, various S. enterica serovar Typhi isolates; 10, S. enterica serovar Typhimurium TR7095; 11, S. enterica serovar Typhimurium SL1344; 12, S. enterica serovar Enteritidis LK5; 13, S. enterica serovar Enteritidis PT4; 14, S. enterica serovar Paratyphi A; 15, S. enterica serovar Paratyphi B; 16, S. enterica serovar Paratyphi C; M, 100-bp DNA marker.
DISCUSSION
In this study, we characterized three VNTR loci from S. enterica serovar Typhi strain CT18 and used them as molecular markers to discriminate among isolates from Singapore, Indonesia, India, Bangladesh, Malaysia, and Nepal. A multiplex PCR assay containing primers specific to the flanking sequences of these VNTR loci was developed. Our results have shown that this method is rapid, reproducible, and highly discriminative for the strain typing of S. enterica serovar Typhi isolates. The banding profiles could be easily interpreted by visual inspection after electrophoresis on conventional agarose gels.
Our study showed that substantial genetic heterogeneity at the VNTR loci exists among S. enterica serovar Typhi isolates within and among geographical areas. Previous studies using phenotypic markers, such as multilocus enzyme electrophoresis, had suggested that S. enterica serovar Typhi strains from different geographical regions were from two different clones (31). However, more recent studies using genotypic methods uncovered more variability than was previously observed (24, 35). These studies suggested that multiple S. enterica serovar Typhi clones exist within and among countries. One of the studies, of 75 multidrug-resistant S. enterica serovar Typhi isolates from four independent outbreaks of typhoid fever in southern Vietnam between 1993 and 1997, reported that each of these outbreaks was caused by a single bacterial strain (8). However, different outbreaks did not derive from the clonal expansion of a single S. enterica serovar Typhi strain, leading to the conclusion that different clones were in circulation. Franco et al. (13) also found genetic heterogeneity in 69 Indonesian and Peruvian isolates by using envelope protein profiles and chromosomal restriction endonuclease digestion patterns. A study of Chilean strains isolated between 1977 and 1986 revealed multiple S. enterica serovar Typhi ribotypes, suggesting that there were multiple sources of infection due to different strains being in circulation (12). Work on 73 Vietnamese and 217 Hong Kong strains using plasmid profile analysis, plasmid fingerprinting, ribotyping, and total-DNA fingerprinting revealed a high level of genetic heterogeneity within each country, as well as among the countries (21). A Malaysian study using PFGE on 158 S. enterica serovar Typhi isolates revealed that individual outbreaks were associated with closely related PFGE strains, whereas isolates from sporadic cases were very diverse (37). A more comprehensive PFGE analysis of 120 S. enterica serovar Typhi isolates from Southeast Asia (60 from Malaysia, 50 from Indonesia, and 10 from Thailand) also found considerable genetic diversity among them, although some PFGE profiles were shared between countries (38). Nair et al. (23) reported that many PFGE types are circulating in Asia. Our findings are therefore in concordance with these reports.
Given the high volume of human traffic among Singapore, Indonesia, and Malaysia, we would have expected more genetic homogeneity among isolates. This was not observed. Nonetheless, the probability of observing identical VNTR profiles in different countries is also dependent on the locations where the isolates were collected. It is not unreasonable to postulate that locations with a higher frequency of cross-border interactions (e.g., towns and cities where there are more foreign workers and visitors) are more likely to have isolates with identical strain profiles. Moreover, the possibility that increased sample size would reveal identical VNTR profiles among isolates of different countries could not be ruled out.
The globalization of international trade and travel has changed the epidemiological profiles of many diseases by making it easier for pathogens to cross geographical borders. Based upon a surveillance report by the Ministry of the Environment (http://www.env.gov.sg), 15 to 20% of the typhoid cases in Singapore from the years 1996 to 2000 were indigenous cases. These indigenous S. enterica serovar Typhi isolates had VNTR profiles that were distinctive and different from those of the other countries studied. This suggests that there is a cluster of strains unique to and circulating in Singapore. Whether the strains responsible for the sporadic cases were identical to those strains harbored by healthy S. enterica serovar Typhi carriers remains to be investigated.
In our study, we sequenced the TR1, TR2, and TR3 amplicons to verify PCR specificity and to confirm that any length polymorphism observed was due solely to variations in VNTR copy numbers. This was necessary, since these loci were being analyzed for the first time. Our results confirmed that the sequencing of the TR1 and TR2 amplicons would not be necessary for subsequent strain-typing attempts. Sequencing of TR3 might be needed, since the length polymorphism of the TR3 amplicons could be the result of deletions at the 5′ flanking regions. However, given the high copy number variability among isolates at the TR1 and TR2 loci, the sequencing of TR3 would be quite an infrequent event, since length polymorphisms at TR1 and TR2 are varied enough for strain separation.
We have yet to ascertain if the VNTR polymorphisms seen in the TR1, TR2, and TR3 loci, as well as the deletions in the TR3 upstream region, have any bearing on microbial physiology and virulence. All three VNTR loci are found in noncoding regions ∼200 to 700 bp upstream of hypothetical open reading frames. TR1 is adjacent to an open reading frame that codes for a hypothetical protein with hydrophobic membrane-spanning regions that is highly homologous to the Escherichia coli YedE putative permease protein. VNTR copy number variations in the promoter region or within the coding regions of virulence genes have been documented to have an effect on a pathogen's ability to adapt to a hostile host environment (14, 28, 29, 30, 41).
To our knowledge, PCR-based VNTR profiling has not been reported for studying the relationship between the S. enterica serovar Typhi isolates, although it has been used for the strain typing of a number of bacterial species, e.g., Mycoplasma pneumoniae (7), Staphylococcus aureus (10), Bacillus anthracis (3), Mycobacterium tuberculosis (16), Neisseria meningitidis (27), group B streptococci (19), and Yersinia pestis (1). This highly versatile method can be further improved by using primers tagged with fluorescent dyes, allowing accurate sizing of amplicons after electrophoresis on an automated DNA sequencer. New primers can also be added to increase the discriminative power. More recently, multiple-locus VNTR analysis, a scaled up version of VNTR amplification, has been used to increase discriminative power for differentiating among isolates from genetically highly homogenous bacterial species, such as Y. pestis (18), B. anthracis (17) and Francisella tularensis (11).
The high degree of reproducibility and discriminative power of our multiplex PCR method suggests that it could be used for both comparative typing and library typing. Comparative typing methods are used for outbreak investigations with the aim of segregating strains (34). These methods must therefore be highly typeable and discriminative. Library typing methods are used for epidemiological surveillance and hence must be highly reproducible within and between laboratories for an extended period. These techniques should also have some standard type nomenclature for easy comparison of results. Our VNTR-based multiplex PCR fulfils these requirements by giving fast, discriminative, and reproducible results with minimal reagent and manpower costs.
Acknowledgments
This work was supported by a grant from the Defense Research Committee, Ministry of Defence, Singapore.
REFERENCES
- 1.Adair, D. M., P. L. Worsham, K. K. Hill, A. M. Klevytska, P. J. Jackson, A. M. Friedlander, and P. Keim. 2000. Diversity in a variable-number tandem repeat from Yersinia pestis. J. Clin. Microbiol. 38:1516-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altwegg, M., F. W. Hickman-Brenner, and J. J. Farmer. 1989. Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J. Infect. Dis. 160:145-149. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, G. L., J. M. Simchock, and K. H. Wilson. 1996. Identification of a region of genetic variability among Bacillus anthracis strains and related species. J. Bacteriol. 178:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27:573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchwald, D. S., and M. J. Blaser. 1984. A review of human salmonellosis. II. Duration of excretion following infection with non-typhi Salmonella. Rev. Infect. Dis. 6:345-356. [DOI] [PubMed] [Google Scholar]
- 6.Bzymek, M., and S. T. Lovett. 2001. Instability of repetitive DNA sequences: the role of replication in multiple mechanisms. Proc. Natl. Acad. Sci. USA 98:8319-8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman, S. D., P. C. Hu, and K. F. Bott. 1990. Prevalence of novel repeat sequences in and around the P1 operon in the genome of Mycoplasma pneumoniae. Gene 87:91-96. [DOI] [PubMed] [Google Scholar]
- 8.Connerton, P., J. Wain, T. T. Hien, T. Ali, C. Parry, N. T. Chinh, H. Vinh, V. A. Ho, T. S. Diep, N. P. Day, N. J. White, G. Dougan, and J. J. Farrar. 2000. Epidemic typhoid in Vietnam: molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype typhi from four outbreaks. J. Clin. Microbiol. 38:895-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalla-Costa, L. M., K. Irino, J. Rodrigues, I. N. Rivera, and L. R. Trabulsi. 1998. Characterisation of diarrhoeagenic Escherichia coli clones by ribotyping and ERIC-PCR. J. Med. Microbiol. 47:227-234. [DOI] [PubMed] [Google Scholar]
- 10.Del Vecchio, V. G., J. M. Petroziello, M. J. Gress, F. K. McCleskey, G. P. Melcher, H. K. Crouch, and J. R. Lupski. 1995. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J. Clin. Microbiol. 33:2141-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farlow, J., K. L. Smith, J. Wong, M. Abrams, M. Lytle, and P. Keim. 2001. Francisella tularensis strain typing using multiple-locus, variable-number tandem repeat analysis. J. Clin. Microbiol. 39:3186-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fica, A. E., S. Prat Miranda, A. Fernandez Ricci, K. D'Ottone, and F. C. Cabello. 1996. Epidemic typhoid in Chile: analysis by molecular and conventional methods of Salmonella typhi strain diversity in epidemic (1977 and 1981) and nonepidemic (1990) years. J. Clin. Microbiol. 34:1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco, A., C. Gonzalez, O. S. Levine, R. Lagos, R. H. Hall, S. L. Hoffman, M. A. Moechtar, E. Gotuzzo, M. M. Levine, D. M. Hone, et al. 1992. Further consideration of the clonal nature of Salmonella typhi: evaluation of molecular and clinical characteristics of strains from Indonesia and Peru. J. Clin. Microbiol. 30:2187-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood, D. W., M. E. Deadman, M. P. Jennings, M. Bisercic, R. D. Fleischmann, J. C. Venter, and E. R. Moxon. 1996. DNA repeats identify novel virulence genes in Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 93:11121-11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanhoff, B. 1995. Typhoid fever, global situation and W. H. O. recommendations. Southeast Asian J. Trop. Med. Public Health 26(Suppl. 2):1-6. [Google Scholar]
- 16.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klevytska, A. M., L. B. Price, J. M. Schupp, P. J. Worsham, J. Wong, and P. Keim. 2001. Identification and characterization of variable-number tandem repeats in the Yersinia pestis genome. J. Clin. Microbiol. 39:3179-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachenauer, C. S., R. Creti, J. L. Michel, and L. C. Madoff. 2000. Mosaicism in the alpha-like protein genes of group B streptococci. Proc. Natl. Acad. Sci. USA 97:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinson, G., and G. A. Gutman. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol. Biol. Evol. 4:203-221. [DOI] [PubMed] [Google Scholar]
- 21.Ling, J. M., N. W. Lo, Y. M. Ho, K. M. Kam, N. T. Hoa, L. T. Phi, and A. F. Cheng. 2000. Molecular methods for the epidemiological typing of Salmonella enterica serotype Typhi from Hong Kong and Vietnam. J. Clin. Microbiol. 38:292-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair, S., E. Schreiber, K. L. Thong, T. Pang, and M. Altwegg. 2000. Genotypic characterization of Salmonella typhi by amplified fragment length polymorphism fingerprinting provides increased discrimination as compared to pulsed-field gel electrophoresis and ribotyping. J. Microbiol. Methods 41:35-43. [DOI] [PubMed] [Google Scholar]
- 23.Nair, S., C. L. Poh, Y. S. Lim, L. Tay, and K. T. Goh. 1994. Genome fingerprinting of Salmonella typhi by pulsed-field gel electrophoresis for subtyping common phage types. Epidemiol. Infect. 113:391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang, T. 1998. Genetic dynamics of Salmonella typhi-diversity in clonality. Trends Microbiol. 6:339-342. [DOI] [PubMed] [Google Scholar]
- 25.Pang, T., M. Altwegg, G. Martinetti, C. L. Koh, and S. Puthucheary. 1992. Genetic variation among Malaysian isolates of Salmonella typhi as detected by ribosomal RNA gene restriction patterns. Microbiol. Immunol. 36:539-543. [DOI] [PubMed] [Google Scholar]
- 26.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 27.Peak, I. R., M. P. Jennings, D. W. Hood, and E. R. Moxon. 1999. Tetranucleotide repeats identify novel virulence determinant homologues in Neisseria meningitidis. Microb. Pathog. 26:13-23. [DOI] [PubMed] [Google Scholar]
- 28.Peak, I. R., M. P. Jennings, D. W. Hood, M. Bisercic, and E. R. Moxon. 1996. Tetrameric repeat units associated with virulence factor phase variation in Haemophilus also occur in Neisseria spp. and Moraxella catarrhalis. FEMS Microbiol. Lett. 137:109-114. [DOI] [PubMed] [Google Scholar]
- 29.Renders, N., L. Licciardello, C. Ijsseldijk, M. Sijmons, L. van Alphen, H. Verbrugh, and A. van Belkum. 1999. Variable numbers of tandem repeat loci in genetically homogeneous Haemophilus influenzae strains alter during persistent colonisation of cystic fibrosis patients. FEMS Microbiol. Lett. 173:95-102. [DOI] [PubMed] [Google Scholar]
- 30.Seib, K. L., I. R. Peak, and M. P. Jennings. 2002. Phase variable restriction-modification systems in Moraxella catarrhalis. FEMS Immunol. Med. Microbiol. 32:159-165. [DOI] [PubMed] [Google Scholar]
- 31.Selander, R. K., P. Beltran, N. H. Smith, R. Helmuth, F. A. Rubin, D. J. Kopecko, K. Ferris, B. D. Tall, A. Cravioto, and J. M. Musser. 1990. Evolutionary genetic relationships of clones of Salmonella serovars that cause human typhoid and other enteric fevers. Infect. Immun. 58:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shangkuan, Y. H., and H. C. Lin. 1998. Application of random amplified polymorphic DNA analysis to differentiate different strains of Salmonella typhi and other Salmonella species. J. Appl. Microbiol. 85:693-702. [DOI] [PubMed] [Google Scholar]
- 33.Strand, M., T. A. Prolla, R. M. Liskay, and T. D. Petes. 1993. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365:274-276. [DOI] [PubMed] [Google Scholar]
- 34.Struelens, M. J., Y. De Gheldre, and A. Deplano. 1998. Comparative and library epidemiological typing systems: outbreak investigations versus surveillance systems. Infect. Control Hosp. Epidemiol. 19:565-569. [DOI] [PubMed] [Google Scholar]
- 35.Thong, K. L., Y. L. Goh, R. M. Yasin, M. G. Lau, M. Passey, G. Winston, M. Yoannes, T. Pang, and J. C. Reeder. 2002. Increasing genetic diversity of Salmonella enterica serovar typhi isolates from Papua New Guinea over the period from 1992 to 1999. J. Clin. Microbiol. 40:4156-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thong, K. L., A. M. Cordano, R. M. Yassin, and T. Pang. 1996. Molecular analysis of environmental and human isolates of Salmonella typhi. Appl. Environ. Microbiol. 62:271-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thong, K. L., Y. M. Cheong, S. Puthucheary, C. L. Koh, and T. Pang. 1994. Epidemiologic analysis of sporadic Salmonella typhi isolates and those from outbreaks by pulsed-field gel electrophoresis. J. Clin. Microbiol. 32:1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thong, K. L., S. Puthucheary, R. M. Yassin, P. Sudarmono, M. Padmidewi, E. Soewandojo, I. Handojo, S. Sarasombath, and T. Pang. 1995. Analysis of Salmonella typhi isolates from Southeast Asia by pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:1938-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Threlfall, E. J., E. Torre, L. R. Ward, B. Rowe, and I. Gibert. 1993. Insertion sequence IS200 can differentiate drug-resistant and drug-sensitive Salmonella typhi of Vi-phage types E1 and M1. J. Med. Microbiol. 39:454-458. [DOI] [PubMed] [Google Scholar]
- 40.Threlfall, E. J., E. Torre, L. R. Ward, A. Davalos-Perez, B. Rowe, and I. Gibert. 1994. Insertion sequence IS200 fingerprinting of Salmonella typhi: an assessment of epidemiological applicability. Epidemiol. Infect. 112:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Belkum, A. 1999. The role of short sequence repeats in epidemiologic typing. Curr. Opin. Microbiol. 2:306-311. [DOI] [PubMed] [Google Scholar]
- 42.van Belkum, A., S. Scherer, W. van Leeuwen, D. Willemse, L. van Alphen, and H. Verbrugh. 1997. Variable number of tandem repeats in clinical strains of Haemophilus influenzae. Infect. Immun. 65:5017-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Belkum, A., S. Scherer, L. van Alphen, and H. Verbrugh. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62:275-293. [DOI] [PMC free article] [PubMed] [Google Scholar]