Abstract
We used tick cell culture to isolate a bacterium previously referred to as the “white-tailed deer (WTD) agent” from two captive fawns inoculated with blood from wild WTD (Odocoileus virginianus). Buffy coat cells were added to ISE6 tick cell cultures and incubated at 34°C, and 8 days later, Anaplasma-like inclusions were demonstrated in Giemsa-stained culture samples. The microbes became established and could be continuously passaged in tick cells. The identity of a culture isolate designated WTD76 was verified as the WTD agent by using specific PCR primers and by DNA sequencing. Comparison with sequences available in GenBank indicated that the isolate was most closely related first to Anaplasma platys and second to Anaplasma phagocytophilum, supporting its placement in the genus Anaplasma. Transmission electron microscopy of this Anaplasma sp. organism in tick cell cultures revealed large inclusions filled with pleomorphic and rod-shaped bacteria. Tick cells infected with the Anaplasma sp. organism were used to successfully infect a naive deer, thereby proving the infectivity of the isolate for deer.
The genus Anaplasma comprises obligate intracellular rickettsial pathogens that are biologically transmitted by ixodid ticks (15, 20, 32, 36, 54). They target circulating blood cells of wild and domestic animals, as well as of humans, and are of global veterinary and human health importance. Although the type species, Anaplasma marginale, was described early last century (56) and has been intensively studied (33), it was isolated in continuous culture only recently (46). A continuous culture system for this obligate intraerythrocytic parasite became available with the development of cell lines from the black-legged tick, Ixodes scapularis (45), the vector for a range of different pathogens from viruses to protozoa (52, 54, 55). Subsequently, I. scapularis cell lines proved to be competent host cells for the cultivation of the closely related Ehrlichia canis (S. A. Ewing, U. G. Munderloh, E. F. Blouin, K. M. Kocan, and T. J. Kurtti, Proc. 76th Conf. Res. Workers Anim. Dis., abstr. 165, 1995) and Anaplasma phagocytophilum (47, 48), formerly known as Ehrlichia equi, Ehrlichia phagocytophila, Cytoecetes phagocytophila, or the human granulocytic ehrlichiosis agent (17). Tick cell culture has allowed not only primary isolation and continuous in vitro maintenance of these pathogens but also analysis of differential expression of specific antigens in the vector and vertebrate host (28). Other, as yet uncultivable, tick-borne pathogens might be candidates for isolation in ixodid tick cell lines.
White-tailed deer (WTD; Odocoileus virginianus) are proven vertebrate reservoirs for Ehrlichia chaffeensis (40, 41) and are known to be naturally infected with at least two related zoonotic rickettsiae, Ehrlichia ewingii (60) and A. phagocytophilum (6, 39). Although some wild WTD have been shown to carry antibodies that recognize A. marginale, their role in the epizootiology of bovine anaplasmosis is debated (31, 42, 44). WTD also are important hosts for Lone Star ticks, Amblyomma americanum, and the black-legged tick, I. scapularis, which are proven vectors of these zoonotic disease agents (2, 18, 36, 54). Examination of wild WTD also has disclosed molecular evidence of another rickettsial agent, termed WTD agent, which is closely related to, but not identical with, Anaplasma platys (7, 14, 38). Thus far, the only known vertebrate hosts of the WTD agent are wild WTD, as well as possibly mule deer and black-tailed deer (21). The public or veterinary health importance of this WTD agent has not been determined.
Here, we report the cultivation of the WTD agent from WTD in a cell line from I. scapularis and its molecular identification as an undescribed Anaplasma sp. strain based on PCR and nucleotide sequencing, describe its morphological characteristics by light and electron microscopy, and demonstrate its ability to infect a naive WTD fawn.
MATERIALS AND METHODS
Tick cell culture.
Cell line ISE6, derived from I. scapularis embryos (48), was used throughout these studies. Uninfected and infected cultures were maintained at 34°C in closed flasks (Greiner America, Longwood, N.C.), with L15B300 (48) supplemented with 5% tryptose phosphate broth (Difco Laboratories, Detroit, Mich.), 5% heat-inactivated fetal bovine serum (Harlan, Indianapolis, Ind.), and 0.1% bovine lipoprotein concentrate (ICN, Irvine, Calif.), pH 7.2. Medium for infected cultures was additionally supplemented with 25 mM HEPES and 0.25% NaHCO3, and the pH was adjusted to 7.5 to 7.7 with 1 N NaOH.
Experimental fawns and source of infected deer blood.
Hand-raised WTD fawns were housed in a vector-free building at the College of Veterinary Medicine, University of Georgia. Fawns were determined to be free of Ehrlichia and Anaplasma prior to inoculation by PCR with oligonucleotide primers specific for 16S ribosomal DNA (rDNA) of E. chaffeensis (HE1 and HE3 [13]), E. ewingii (Eew forward and Eew reverse [51]), A. phagocytophilum (p44-1 and p44-2 [26]), and the WTD agent (38) (Table 1). Anticoagulated blood was obtained by sterile venipuncture from five WTD at the Piedmont National Wildlife Refuge, Jasper and Jones counties, Georgia. This WTD population is known to be naturally coinfected with multiple species in the family Anaplasmataceae as emended by Dumler et al. (17), specifically the WTD agent, E. chaffeensis, and E. ewingii (38, 41, 60). Two captive, 4-month-old WTD fawns, WTD76 and WTD81, were injected intradermally, subcutaneously, intravenously, and intraperitoneally with 2 ml of blood by each route (8 ml total) from blood pooled from three of the wild deer. A nested 16S rDNA PCR was used to determine with which agents the fawns became infected (see below). Fawns were bled under aseptic conditions by venipuncture at regular intervals postinoculation (p.i.), and total RNA was extracted using a QIAamp RNA Blood minikit (Qiagen, Valencia, Calif.).
TABLE 1.
Oligonucleotide sequences of primers used in this study
| Primer designation | Specificity and target gene | Nucleotide sequence (5′→3′) | Product size (bp) | Refer- ence |
|---|---|---|---|---|
| BAP-2 | Anaplasma marginale msp1β | GTA TGG CAC GTA GTC TTG GGA TCA | 407 | 53 |
| AL34S | CAG CAG CAG CAA GAC CTT CA | |||
| ECB | Anaplasma and Ehrlichia-wide 16S rDNA | CGT ATT ACC GCG GCT GCT GGC A | 490 | 39 |
| ECC | AGA ACG AAC GCT GGC GGC AAG CC | |||
| DGA | WTD-Anaplasma 16S rDNA | TTA TCT CTG TAG CTT GCT ACG | 405 | 38 |
| GA1UR | GAG TTT GCC GGG ACT TCT TCT | |||
| p44-1 | Anaplasma phagocytophilum msp2 | AGC GTA ATG ATG TCT ATG GC | 1,279 | 26 |
| p44-2 | ACC TAA CAC CAA ATT CCC | |||
| PER1 | Anaplasma and Ehrlichia-wide 16S rDNA | TTT ATC GCT ATT AGA TGA GCC TAT G | 451 | 23 |
| PER2 | CTC TAC ACT AGG AAT TCC GCT AT | |||
| HE1 | Ehrlichia chaffeensis 16S rDNA | CAA TTG CTT ATA ACC TTT TGG TTA TAA AT | 389 | 13 |
| HE3 | TAT AGG TAC CGT CAT TAT CTT CCC TAT | |||
| Eew forward | Ehrlichia ewingii 16S rDNA | AAC GAA CAA TTC CTA AAT AGT CTC TGA CT | 917 | 51 |
| Eew reverse | TCT GTT AAA AGG GAT ACG ACC TTC |
WTD blood culture.
Blood for culturing (5 ml) was drawn from WTD76 and WTD81 into EDTA Vacutainer tubes (Becton Dickinson, Rutherford, N.J.) on day 82 p.i. and shipped to Minnesota by overnight delivery in insulated containers with gel ice. A second shipment of blood from WTD76 was sent on day 131 p.i. All procedures were carried out under sterile conditions, and antibiotics were not used at any time. Blood was pipetted into 15-ml centrifuge tubes and centrifuged for 15 min at 1,000 × g, 4°C, and the buffy coat was transferred to 10 ml of L15B300 with 10% fetal bovine serum. This was repeated twice, and the buffy coat was recovered each time into medium. After the final wash, the white cell-enriched fraction was resuspended in 5 ml of L15B300 supplemented as stated above for infected cells and added to one confluent, 25-cm2 culture of ISE6 cells. Cultures were fed a complete change of medium twice weekly.
Light microscopy.
Thin blood films were prepared from blood used as culture inoculum and fixed and stained the same way as cell samples were. To screen cultures for the presence of bacteria, samples were centrifuged onto microscope slides, dried briefly, and fixed for 5 min in absolute methanol. Slides were stained in a 4% buffered (pH 6.8) solution of Giemsa's stain (Karyomax; Gibco, Grand Island, N.Y.) for 30 min at 37°C. Slides were viewed and photographed under ×100 magnification with a Nikon 400 Eclipse microscope fitted with a DMX 1200 digital camera.
Electron microscopy.
A culture of ISE6 determined to be ≥70% infected with the WTD76 isolate was fixed for transmission electron microscopy. Five hundred microliters of cell suspension was pipetted into 1 ml of Ito's modified fixative in a 1.5-ml microcentrifuge tube (34), pH 7.3, and fixed for 60 min on ice. Cells were then centrifuged at 275 × g for 5 min, and the supernatant was replaced with fresh fixative to fill the tube. Cells were postfixed in osmium tetroxide and dehydrated in graded changes of an ascending alcohol series. The pellet was embedded in Spurr epoxy resin, and thin sections were stained with methanolic uranyl acetate and Reynold's lead citrate.
nRT-PCR and PCR assays.
To increase sensitivity of detection of the target bacterial 16S RNA in blood samples, a nested reverse transcriptase (nRT) assay was used (19). RNA was extracted from 300 μl of EDTA-anticoagulated whole blood in an RNase-free environment. Reverse transcription and primary amplification were carried out in a 50-μl single-tube reaction mixture with 2 μl of RNA in a solution containing 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, 0.1% Triton X-100, 1.6 mM β-mercaptoethanol, 0.25 mM deoxynucleoside triphosphates, 1.5 U of Taq DNA polymerase (Promega Corp., Madison, Wis.), 3 U of avian myeloblastosis virus reverse transcriptase (Promega Corp.), and 0.4 μM Anaplasma-Ehrlichia-wide primers ECC and ECB (Table 1). Following reverse transcription at 42°C for 15 min, cDNA was amplified for 31 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min by using a PTC-100TM Thermal Cycler (MJ Research, Inc., Waltham, Mass.). For the nested, secondary PCR, 1 μl of the first-stage products was further amplified by use of specific primers DGA and GA1UR (38) (Table 1) for 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. RNA extracted from the blood of a WTD known to be infected with the WTD agent was used as a positive control, and as a negative control, water was extracted and amplified in parallel with all samples.
The PCR was used to establish the identity of the intracellular bacterium isolated in tick cell culture. DNA was extracted from a 400-μl portion of the blood used as culture inoculum and from infected tick cells. Erythrocytes were first lysed in buffered ammonium chloride (erythrocyte lysis solution; Gentra Systems, Minneapolis, Minn.), and the remaining cells were then processed as described elsewhere for cultured Anaplasma (24). Tick cell culture-derived WTD agent was separated from host cells and solubilized in lysis buffer, and DNA was extracted using the PureGene kit (Gentra Systems). DNA was dissolved in sterile water (100 μl for DNA from blood and 500 μl for each DNA pellet harvested from one 25-cm2 culture) and stored at −20°C. Five microliters (blood samples) or 2 μl (culture samples) of DNA was used in each 50-μl reaction mixture containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) deoxynucleotides, 0.5 μM (each) oligonucleotide primer, and 1.25 U of Taq DNA polymerase (Promega). Primers used for identification were those listed in Table 1, and PER1 and PER2 (23) were used instead of ECC and ECB. DNA was initially denatured for 3 min at 95°C and then amplified in a Robocycler (Stratagene, La Jolla, Calif.) for 40 cycles consisting of a denaturation step of 30 s at 94°C, annealing for 30 s at 45°C, and elongation for 45 s at 72°C, with a final elongation step of 5 min. DNA extracted from tick cell cultures of A. marginale, A. phagocytophilum, and E. chaffeensis (29, 46, 47; S. D. Jauron, S. A. Ewing, J. E. Dawson, K. M. Kocan, and U. G. Munderloh, Proc. 80th Conf. Res. Workers Anim. Dis., abstr. 144, 1999) and blood from a WTD known to be infected with E. ewingii served as positive controls. PCR products were separated by electrophoresis in 1% (PCR products) or 2% (nRT-PCR products) agarose gels and visualized by ethidium bromide staining and UV transillumination.
Nucleotide sequence analysis.
DNA was extracted from the second and fifth tick cell culture passages of the WTD agent from WTD76. The 16S rDNA fragment, amplified by use of the WTD agent-specific primer combination DGA-GA1UR, was purified by agarose gel electrophoresis, and the band was extracted using the QIAquick gel extraction kit (Qiagen). DNA was sequenced twice in both the forward and reverse directions with the same primers and an ABI 377 automated sequencer at the Advanced Genetic Analysis Center, University of Minnesota, St. Paul.
Cell culture inoculation of naive fawn.
A 13-month-old WTD (WTD86) was chosen for inoculation with the seventh tick cell (ISE6) culture passage of the WTD agent isolated from WTD76 after 5 months in vitro. Infected tick cells in growth medium were shipped chilled to the University of Georgia overnight. Cells were collected by centrifugation at 2,000 × g at 4°C for 15 min and resuspended in sterile phosphate-buffered saline to a final volume of 8 ml. WTD86 was injected with 2-ml portions of this inoculum by each of four routes, i.e., intradermal, subcutaneous, intravenous, and intraperitoneal. Blood samples from WTD86 were collected at 33 days p.i. and assayed for infection by cell culture, PCR, and light microscopy as described above.
Nucleotide sequence accession number.
The WTD76 isolate was sequenced, and its nucleotide sequence was deposited in GenBank under accession number AY208945.
RESULTS
Infection in experimental fawns.
WTD76 and WTD81 became infected with the WTD agent as shown by PCR assays with DNA from their blood at every sampling time between 12 and 131 days p.i. (C. M. Tate et al., unpublished data). Blood samples from both fawns were PCR positive on days when tick cell culture was attempted, specifically day 82 p.i. (WTD76 and WTD81) and day 131 p.i. (WTD76). In addition, WTD76 tested positive for E. ewingii on day 68, while WTD81 was positive for E. chaffeensis on days 13, 15, 20, 47, 68, and 110 p.i. and positive for E. ewingii on days 47 and 68 p.i. PCR assays of blood from WTD76 and WTD81 did not detect E. ewingii on day 82 p.i. and detected E. chaffeensis only once thereafter (60). A. marginale was not detected in any of the deer at any time.
The WTD agent in tick cell cultures. (i) Light microscopy.
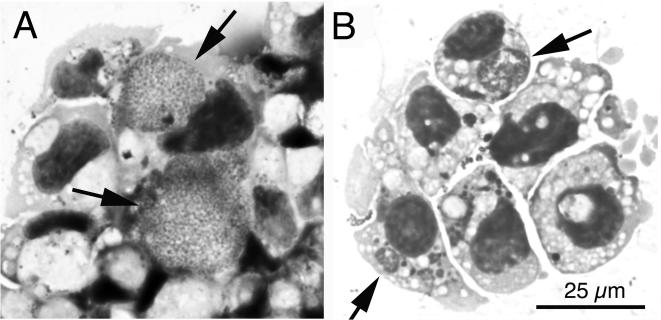
Inoculation of tick cell cultures with the buffy coat harvested from each blood sample did not cause detectable cytopathic effect. Due to the weak adherence of tick cells to the substrate, only a portion of the blood cell inoculum was removed during the twice-weekly medium changes. The first rickettsial inclusions were seen in Giemsa-stained ISE6 cell spreads prepared 8 days p.i. with WTD76 blood. The first passage to fresh ISE6 wells was made after 5 weeks of culture, when the WTD76 culture was about 70% infected and the WTD81 culture was about 90% infected. By light microscopy, large inclusions were filled with numerous small, uniform organisms (Fig. 1A), although morulae presenting larger, pleomorphic bacteria were also common (Fig. 1B).
FIG. 1.
Light microscopic appearance of the WTD-Anaplasma isolate in Giemsa-stained cytocentrifuge preparations of tick cell culture. (A) WTD-Anaplasma isolate from WDT76 in its eighth passage in tick cells. Arrows indicate large intracytoplasmic inclusions filled with numerous bacteria. (B) WTD-Anaplasma reisolated from WTD86. Arrows point to intracytoplasmic morulae. The scale bar in panel B gives the magnification for both panels.
The first two passages were made by transferring 0.5 ml (1/10th) of an infected tick cell suspension to a new, confluent ISE6 culture when the parent cultures were about 90% infected. With continued culture, the organisms replicated more quickly, and subsequent passages were done with inoculum diluted 100-fold every 2 weeks.
(ii) Electron microscopy.
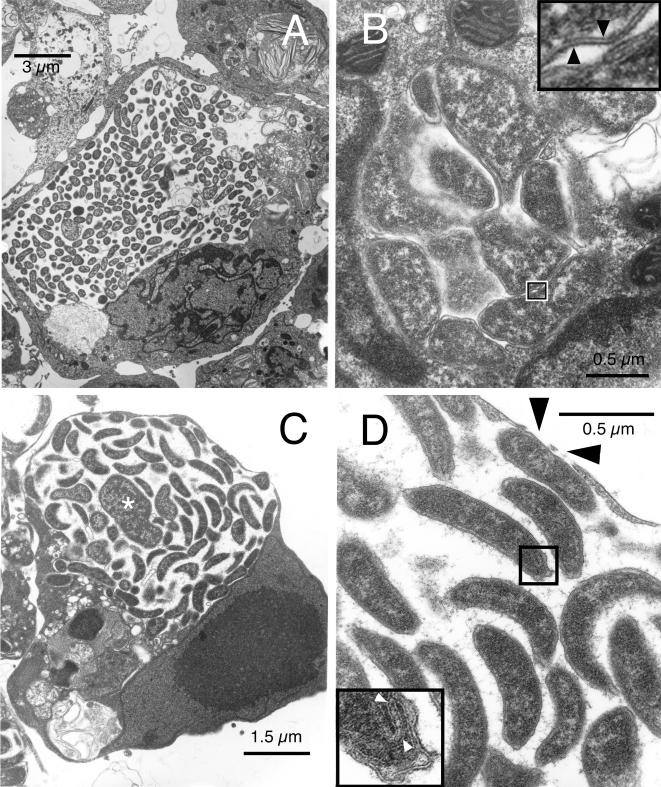
An examination of thin sections of tick cell cultures infected with the WTD agent showed three morphological types of inclusions. Large inclusions were filled with numerous, rod-shaped or round to ovoid, reticulate forms that did not demonstrate much association with the endosomal membrane (Fig. 2A). There were also small morulae containing a few highly irregularly shaped organisms tightly packed together and closely abutting the endosomal membrane (Fig. 2B). Other large endosomes (Fig. 2C) contained condensed, distinctly crescent-shaped bacteria of rather uniform size, approximately 0.2 μm in width by 1.5 to 2 μm in length, interspersed with a few much larger and odd-shaped bacteria (asterisk). Rod-shaped organisms lined the inner membrane, which seemed stretched thin to the point of rupture. Thin, wispy strands of flocculent material radiated from their outer membrane, tethering individual bacteria together. Black arrowheads in Fig. 2 point to gaps in the endosomal and cell membranes (Fig. 2D). Individual bacteria were enveloped by a bilayer cell wall in which the leaflets were separated by a clear space of equal thickness (Fig. 2B, inset). The cell wall enclosed a thin periplasmic space which contained patches of dense amounts of material separating it from the periplasmic membrane. Some crescent-shaped bacteria showed infolding of portions of the cell wall at their tips (Fig. 2D, inset).
FIG. 2.
Ultrastructure of the WTD-Anaplasma in tick cell culture. (A) Inclusion that appears to occupy most of its host cell. Note the lack of association of the bacteria with the endosomal membrane. (B) Image of a small morula with pleomorphic bacteria that tightly abut each other and the endosomal membrane. Inset, high magnification of the boxed-in area, showing the double-layered profile of the cell wall (arrowheads). (C) Third type of inclusion containing primarily slightly curved rods but also larger and more irregular organisms (asterisk). Note the intimate association of Anaplasma with the endosomal membrane. (D) Higher magnification of panel C. Black arrowheads indicate gaps in the endosomal membrane; white arrowheads within the inset point to folded membrane structures at one end of a curved rod, enlarged from the boxed-in area.
PCR and DNA sequencing of WTD76 and WTD81 in tick cells.
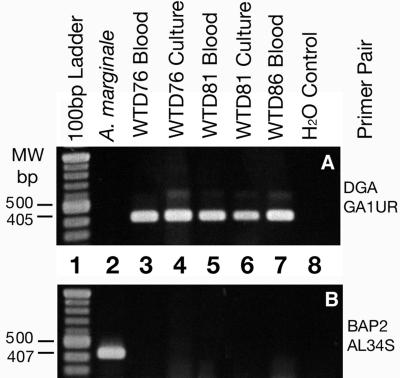
DNA extracted from WTD76 and WTD81 grown in ISE6 tick cell cultures when amplified with the WTD-Anaplasma-specific primers DGA and GA1UR yielded the expected product of 405 bp (Fig. 3A), as did DNA from blood from fawns 76, 81, and 86. DNA from a culture of A. marginale was not amplified with these primers but yielded the correct product when the A. marginale-specific primers BAP2 and AL34S were used, which did not amplify the WTD-Anaplasma DNA (Fig. 3B). All other species-specific primer pair combinations (Table 1) also failed to amplify WTD76 and WTD81 culture isolate DNA but amplified positive-control DNA from the respective target organism as expected (data not shown). Nucleotide sequencing of the WTD76 isolate further confirmed the identity of the organisms cultured in tick cells. From results of a BLAST search and alignment with a 379-bp segment of DNA obtained from the fifth tick cell passage of WTD76 (Fig. 4, WTD76), five sequences of “ehrlichiae” detected in WTD in Georgia and Oklahoma were selected (GenBank accession numbers U27104, U52514, U27103, U27102, and U27101) and aligned with each other to resolve unidentified nucleotides, and a consensus sequence was derived (Fig. 4, WTD sequences). Likewise, five sequences from A. platys (GenBank accession numbers AF303467, AY077619, AY040853, U54806, and AF318023) were randomly chosen, as well as five sequences from A. phagocytophilum (GenBank accession numbers AY055469, AF481855, AF189153, U02521, and AF482761) and a further five sequences from A. marginale (GenBank accession numbers AF309868, AF309867, AF309866, AF414877, and AF414873), and consensus sequences were derived for each species (A. platys, A. phagocytophilum, and A. marginale, Fig. 4). There was no difference among the 379-bp sequences within each species. Nucleotides were numbered with respect to nucleotide positions of the complete 16S sequence available for A. phagocytophilum (GenBank accession numbers AY055469, AF189153, and U02521). Alignment of all sequences revealed 100% identity of WTD76 with the WTD sequences. The WTD76 sequence differed from A. platys in the first three nucleotides of readable sequence and additionally at positions 120, 131, 169, and 196 (Fig. 4, boldface letters and asterisks). There was only one difference, at position 83, with respect to A. phagocytophilum sequences, whereas the changes at nucleotides 120, 131, 169, and 196 were the same as those seen in A. platys. Further differences between the WTD76 and the A. phagocytophilum sequences occurred at positions 251 and 252 (inversion of AG to GA) and at nucleotides 416 and 437. Alignment of the sequences with those from A. marginale showed the same changes at positions 120, 131, 169, 196, 251, 416, and 437 as did some of the other species examined, while nucleotides 82, 83, 84, and 252 were identical to those in WTD76. However, the A. marginale sequence deviated from all others at positions 212, 229, 236, 259, and 300.
FIG. 3.
Agarose gel of PCR products amplified by use of primers DGA and GA1UR, specific for a 405-bp portion of the 16S rDNA of WTD-Anaplasma (A), and primers BAP2 and AL34S, specific for a 407-bp portion of the msp1β gene of A. marginale (B).
FIG. 4.
Alignment of the partial 16S rDNA sequence from the WDT-Anaplasma (WTD76) isolate with 16S rDNA sequences available in GenBank. Bases are numbered with reference to sequence AY055469 representing complete codons for the 16S rDNA of A. phagocytophilum. Five sequences from “Ehrlichia” sp. strains found in WTD (accession numbers U27104, U52514, U27103, U27102, and U27101) were used to arrive at the consensus sequence designated “WTD sequences.” Five sequences from A. platys (AF303467, AY077619, AY040853, U54806, and AF318023) were aligned to arrive at the sequence labeled A. platys, five A. phagocytophilum sequences (AY055469, AF481855, AF189153, U02521, and AF482761) were aligned and are represented as “A. phagocyto,” and a further five A. marginale sequences (AF309868, AF309867, AF309866, AF414877, and AF414873) were likewise aligned and are shown in the line labeled A. marginale. With the exception of eight unidentifiable bases in the five sequences from Ehrlichia sp. strains in WTD, there was no difference in the sequences from the respective sets within the gene segments aligned, and the WTD76 sequence was identical to the consensus WTD sequence. Differences between the WTD76 sequences and the other sequences are indicated by boldface and asterisks.
Infection of naive WTD.
WTD86 became positive by nRT-PCR for WTD-Anaplasma by using the WTD-Anaplasma-specific primer pair DGA-GA1UR on days 12, 17, 27, and 33 and on every sample date up to day 214 p.i. (when the animal was euthanized) following inoculation with WTD76-infected ISE6 tick cells. Two milliliters of EDTA blood from WTD86 was sent to Minnesota on day 33 p.i. and processed for culture as outlined above. Nineteen days later, 80% of ISE6 cells contained inclusions of WTD-Anaplasma (Fig. 1B, arrows).
DISCUSSION
We utilized ISE6 tick cell cultures to isolate and morphologically characterize the WTD agent, which was then identified as an undescribed Anaplasma sp. strain, most closely related to A. platys, based on 16S rDNA sequencing. Each of three culture attempts in ISE6 cells resulted in successful isolation of this Anaplasma sp. strain from the blood of WTD. Further, productively infected ISE6 cell cultures were used to infect a naive WTD fawn with this Anaplasma sp. strain, thus proving the ability of the isolate to infect WTD. Comparison of 16S rDNA sequences obtained from the Anaplasma sp. strain in ISE6 culture disclosed identity with those reported for the WTD agent, which has been detected by PCR from numerous WTD populations in 13 southern states (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Maryland, Missouri, North Carolina, Oklahoma, South Carolina, Tennessee, and Virginia) (7, 14, 38, 39). A 16S rDNA sequence identical to that in the Anaplasma sp. strain also was found in Lone Star ticks from Georgia (40), and parasitism by Lone Star ticks has been associated with the presence of this organism among WTD populations (7). The type species of the genus, A. marginale, has been isolated from many wild ruminants including WTD but does not cause disease in any, except giraffes (35). Nevertheless, WTD probably do not play a role in the maintenance of this cattle pathogen in nature. Although WTD in the southwestern United States and Mexico have been reported to react serologically, they could be infected experimentally only with difficulty, and parasitemias remained extremely low (31, 42, 44). We did not find evidence of infection with A. marginale in the deer used in this study by use of Giemsa-stained blood films and PCR with primers specific for the msp1β gene (53). Moreover, the primer pair DGA-GA1UR targeting 16S rDNA amplified only WTD-Anaplasma DNA, due to a 7-nucleotide mismatch for A. marginale in the forward primer sequence. By using these two primer sets, either of these pathogens can reliably be identified in blood or culture samples.
The Anaplasma sp. isolates from WTD now available in ISE6 cultures will provide a new means to investigate and characterize this agent under controlled conditions in the laboratory and provide a system to develop additional diagnostic and immunologic research tools. This study reaffirms the value of tick cell lines for research and in particular cell lines ISE6 and IDE8 from I. scapularis, which have proven useful for cultivation of multiple bacteria in the genera Anaplasma and Ehrlichia (5, 46, 47, 58; Ewing et al., Proc. 76th Conf. Res. Workers Anim. Dis.). Advantages of using these cell lines are that they are broadly supportive of tick-borne pathogens; can be maintained at high cell densities (>106 cells/ml) for weeks without deteriorating, which may enhance the ability of intracellular organisms to spread between cells and replicate; and do not appear to lose their susceptibility to Anaplasma with extended passage number (24).
Morphologically, the isolate from WTD76 displays the characteristics of anaplasmas and ehrlichiae, in particular demonstrating significant pleomorphism even among organisms within one endosome. Nevertheless, the Anaplasma sp. strain from WTD76 comprised slender, crescent-shaped bacteria that appeared more uniform than variably shaped organisms in other inclusions in the same culture. Because inoculation of the experimental deer with whole blood from wild deer had produced infection with E. chaffeensis, E. ewingii, and Theileria cervi in addition to the undescribed Anaplasma sp. strain (60; Tate et al., unpublished), one might argue that the morphological variability of organisms in ISE6 culture could reflect the presence of a mixed population of two or more species of Anaplasma or Ehrlichia. However, our PCR results clearly rule out this possibility, because only the primer pair DGA and GA1UR amplified DNA from cultures, whereas primers specific for some of the other organisms yielded PCR products from infected blood, as well as positive controls, indicating that the assay was working properly. Notably, A. marginale was never amplified from any samples. Furthermore, experimental WTD76 and WTD81 were PCR negative for these Ehrlichia spp. on day 82, when blood was first collected for culture, and they were PCR negative for Ehrlichia spp. on all but one day thereafter (60; Tate et al., unpublished).
It was important to use captive fawns with a known history of infection as a source of blood for the culture inoculum. In particular, an absence of or low burden of infection with Trypanosoma cervi (37) contributed to the success of the cultures. Previous ISE6 culture attempts, using blood from wild WTD infected with T. cervi, resulted in cultures that became overgrown with flagellates which eventually caused the destruction of the tick cells (U. G. Munderloh, unpublished data), and similar T. cervi problems have occurred in DH82 cell cultures (40, 41).
We were surprised to find that blood cultures from both WTD76 and WTD81 yielded only isolates of Anaplasma sp. and none of the Ehrlichia spp. infecting the animals. This is despite the fact that E. chaffeensis can readily be grown in I. scapularis cells (Jauron et al., Proc. 80th Conf. Res. Workers Anim. Dis.). De la Fuente et al. (16) recently described interference of different genotypes of A. marginale with each other that prevented the establishment of one genotype in tick cell cultures already infected with a different genotype. This and our findings are reminiscent of the interference described between spotted fever group rickettsiae (3, 10), which prevents acquisition or transmission of these organisms by arthropod vectors that are already infected with another one. The molecular mechanisms responsible for this blockage are unknown but might be related to alterations of host cell surface receptors induced through prior infection with a rickettsial pathogen, so that subsequent events of receptor-mediated binding and internalization are prevented. Such receptor recycling and modulation are known to occur in human immunodeficiency virus infections (50). Alternatively, anaplasma infection of the tick cells could induce production of antimicrobials such as defensins, the genes for which have been identified in I. scapularis (57) as well as in Dermacentor variabilis (29). Our observation could also be related to the phenomenon of “superinfection exclusion” described for members of the Alphaviridae, in which a protease destroys an enzyme needed for the replication of the minus strand of viral DNA (30).
Many emerging disease agents of the temperate zone are vector borne and have wild animal reservoirs. These include the human monocytic ehrlichiosis (HME) agent, E. chaffeensis, and the human granulocytic anaplasmosis (formerly ehrlichiosis) agent, A. phagocytophilum (17). In the southern United States, WTD are often heavily infested with Lone Star ticks, A. americanum, the vector of E. chaffeensis (13). Examination of wild WTD and subsequent laboratory inoculation and experimental tick transmission (18) strongly suggested their role as mammalian reservoirs and that of Lone Star ticks as principal vectors of E. chaffeensis (12, 40, 41). Other tick species also readily parasitize WTD both in the south and further north. Among those, the black-legged tick, I. scapularis, is known to transmit A. phagocytophilum (36, 54), which infects a variety of feral animals, including WTD (6), as well as humans (4, 11). Because they are long-lived, inhabit areas near human dwellings, and are susceptible to concurrent infection by multiple tick-borne pathogens (7, 39, 60), WTD may be reservoir hosts for zoonotic pathogens other than the HME agent. Not all of them have not been isolated in culture but have been detected through immunologic and molecular means. One of these, i.e., E. ewingii, also infects dogs and/or humans (1, 8, 9, 22, 25, 27, 43, 49, 59), but a second one, termed WTD agent (7, 14, 38), which is closely related to, but not identical with, A. platys (27, 43), has thus far been detected only in cervids (WTD as well as possibly mule deer and black-tailed deer [21]). The public or veterinary health importance of this agent has not been determined.
Acknowledgments
This work was supported primarily by the National Institutes of Allergy and Infectious Diseases (5R01 AI044235 to W.R.D., and R01 AI42792 to U.G.M.). Additional support was provided by the Federal Aid to Wildlife Restoration Act (50 Stat. 917) and through sponsorship of SCWDS by the state fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kansas, Kentucky, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Puerto Rico, South Carolina, Tennessee, Virginia, and West Virginia.
We thank M. Page Luttrell, Vivien G. Dugan, and Michael J. Yabsley for laboratory assistance, and these and other staff at the Southeastern Cooperative Wildlife Disease Study for assistance with sampling experimental animals.
REFERENCES
- 1.Anderson, B. E., C. E. Greene, D. C. Jones, and J. E. Dawson. 1992. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int. J. Syst. Bacteriol. 42:299-302. [DOI] [PubMed] [Google Scholar]
- 2.Anziani, O. S., S. A. Ewing, and R. W. Barker. 1990. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 51:929-931. [PubMed] [Google Scholar]
- 3.Azad, A. F., and C. B. Beard. 1998. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 4:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 5.Bell-Sakyi, L., E. A. Paxton, U. G. Munderloh, and K. J. Sumption. 2000. Growth of Cowdria ruminantium, the causative agent of heartwater, in a tick cell line. J. Clin. Microbiol. 38:1238-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandsma, A. R., S. E. Little, J. M. Lockhart, W. R. Davidson, D. E. Stallknecht, and J. E. Dawson. 1999. Novel Ehrlichia organism (Rickettsiales: Ehrlichieae) in white-tailed deer associated with Lone Star tick (Acari: Ixodidae) parasitism. J. Med. Entomol. 36:190-194. [DOI] [PubMed] [Google Scholar]
- 8.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buller, R. S., M. Arens, S. P. Hmiel, C. D. Paddock, J. W. Sumner, Y Rikhisa, A. Unver, M. Gaudreault-Keener, F. A. Manian, A. M. Liddell, N. Schmulewitz, and G. A. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148-155. [DOI] [PubMed] [Google Scholar]
- 10.Burgdorfer, W., and P. L. Brinton. 1975. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann. N. Y. Acad. Sci. 266:61-72. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson, W. R., J. M. Lockhart, D. E. Stallknecht, E. W. Howerth, J. E. Dawson, and Y. Rechav. 2001. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 37:538-546. [DOI] [PubMed] [Google Scholar]
- 13.Dawson, J. E., D. E. Stallknecht, E. W. Howerth, C. Warner, K. Biggie, W. R. Davidson, J. M. Lockhart, V. F. Nettles, J. G. Olson, and J. E. Childs. 1994. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J. Clin. Microbiol. 32:2725-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olson. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 15.de La Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, S. D. Rodriguez, M. A. Garcia, and K. M. Kocan. 2001. Evolution and function of tandem repeats in the major surface protein 1a of the ehrlichial pathogen Anaplasma marginale. Anim. Health Res. Rev. 2:163-173. [PubMed] [Google Scholar]
- 16.De La Fuente, J., J. C. Garcia-Garcia, E. F. Blouin, J. T. Saliki, and K. M. Kocan. 2002. Infection of tick cells and bovine erythrocytes with one genotype of the intracellular ehrlichia Anaplasma marginale excludes infection with other genotypes. Clin. Diagn. Lab. Immunol. 9:658-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ′HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 18.Ewing, S. A., J. E. Dawson, A. A. Kocan, R. W. Barker, C. K. Warner, R. J. Panciera, J. C. Fox, K. M. Kocan, and E. F. Blouin. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32:368-374. [DOI] [PubMed] [Google Scholar]
- 19.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2001. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol. 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fingerle, V., U. G. Munderloh, G. Liegl, and B. Wilske. 1999. Coexistence of ehrlichiae of the Phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from Southern Germany. Med. Microbiol. Immunol. 188:145-149. [DOI] [PubMed] [Google Scholar]
- 21.Foley, J. E., J. E. Barlough, R. B. Kimsey, J. E. Madigan, E. DeRock, and A. Poland. 1998. Ehrlichia spp. in cervids from California. J. Wildl. Dis. 34:731-737. [DOI] [PubMed] [Google Scholar]
- 22.French, T. W., and J. W. Harvey. 1983. Serologic diagnosis of infectious cyclic thrombocytopenia in dogs using an indirect fluorescent antibody test. Am. J. Vet. Res. 44:2407-2411. [PubMed] [Google Scholar]
- 23.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 24.Goodman, J. L., C. M. Nelson, M. B. Klein, S. F. Hayes, and B. W. Weston. 1999. Leukocyte infection by the granulocytic ehrlichiosis agent is linked to expression of a selectin ligand. J. Clin. Investig. 103:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey, J. W., C. F. Simpson, and J. M. Gaskin. 1978. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis. 137:182-188. [DOI] [PubMed] [Google Scholar]
- 26.IJdo, J. W., W. Sun, Y. Zhang, L. A. Magnarelli, and F. Fikrig. 1998. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect. Immun. 66:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inokuma, H., K. Fujii, K. Matsumoto, M. Okuda, K. Nakagome, R. Kosugi, M. Hirakawa, and T. Onishi. 2002. Demonstration of Anaplasma (Ehrlichia) platys inclusions in peripheral blood platelets of a dog in Japan. Vet. Parasitol. 110:145-152. [DOI] [PubMed] [Google Scholar]
- 28.Jauron, S. D., C. M. Nelson, V. Fingerle, M. D. Ravyn, J. L. Goodman, R. C. Johnson, R. Lobentanzer, B. Wilske, and U. G. Munderloh. 2001. Host cell-specific expression of a p44 epitope by the human granulocytic ehrlichiosis agent. J. Infect. Dis. 184:1445-1450. [DOI] [PubMed] [Google Scholar]
- 29.Johns, R., D. E. Sonenshine, and W. L. Hynes. 2001. Identification of a defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect Biochem. Mol. Biol. 31:857-865. [DOI] [PubMed] [Google Scholar]
- 30.Karpf, A. R., E. Lenches, E. G. Strauss, J. H. Strauss, and D. T. Brown. 1997. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 71:7119-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keel, M. K., W. L. Goff, and W. R. Davidson. 1995. An assessment of the role of white tailed deer in the epizootiology of anaplasmosis in the southeastern United States. J. Wildl. Dis. 31:378-385. [DOI] [PubMed] [Google Scholar]
- 32.Kocan, K. M., J. A. Hair, S. A. Ewing, and L. G. Stratton. 1981. Transmission of Anaplasma marginale Theiler by Dermacentor andersoni Stiles and Dermacentor variabilis (Say). Am. J. Vet. Res. 42:15-18. [PubMed] [Google Scholar]
- 33.Kocan, K. M., E. F. Blouin, and A. F. Barbet. 2000. Anaplasmosis control: past, present and future. Ann. N. Y. Acad. Sci. 916:501-509. [DOI] [PubMed] [Google Scholar]
- 34.Kurtti, T. J., U. G. Munderloh, S. F. Hayes, D. E. Krueger, and G. G. Ahlstrand. 1994. Ultrastructural analysis of the invasion of tick cells by Lyme disease spirochetes (Borrelia burgdorferi) in vitro. Can. J. Zool. 72:977-994. [Google Scholar]
- 35.Kuttler, K. L. 1984. Anaplasma infections in wild and domestic ruminants: a review. J. Wildl. Dis. 20:12-20. [DOI] [PubMed] [Google Scholar]
- 36.Levin, M. L., and D. Fish. 2000. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect. Immun. 68:2183-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine, S., D. Fish, L. A. Magnarelli, and J. F. Anderson. 1987. Choroid plexitis in white-tailed deer (Odocoileus virginianus) in southern New York State. Vet. Pathol. 24:207-210. [DOI] [PubMed] [Google Scholar]
- 38.Little, S. E., J. E. Dawson, J. M. Lockhart, D. E. Stallknecht, C. K. Warner, and W. R. Davidson. 1997. Development and use of specific polymerase reaction for the detection of an organism resembling Ehrlichia sp. in white-tailed deer. J. Wildl. Dis. 33:246-253. [DOI] [PubMed] [Google Scholar]
- 39.Little, S. E., D. E. Stallknecht, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 1998. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. J. Parasitol. 84:897-901. [PubMed] [Google Scholar]
- 40.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and S. E. Little. 1997. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the piedmont physiographic province of Georgia. J. Parasitol. 83:887-894. [PubMed] [Google Scholar]
- 41.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and E. W. Howerth. 1997. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 35:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez, A., A. Salinas, F. Martinez, A. Cantu, and D. K. Miller. 1999. Serosurvey for selected disease agents in white-tailed deer from Mexico. J. Wildl. Dis. 35:799-803. [DOI] [PubMed] [Google Scholar]
- 43.Mathew, J. S., S. S. Ewing, G. L. Murphy, K. M. Kocan, R. E. Corstvet, and J. C. Fox. 1997. Characterization of a new isolate of Ehrlichia platys (order Rickettsiales) using electron microscopy and polymerase chain reaction. Vet. Parasitol. 68:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Morley, R. S., and M. E. Hugh-Jones. 1989. Seroepidemiology of Anaplasma marginale in white-tailed deer (Odocoileus virginianus) from Louisiana. J. Wildl. Dis. 25:342-346. [DOI] [PubMed] [Google Scholar]
- 45.Munderloh, U. G., Y. Liu, M. Wang, C. Chen, and T. J. Kurtti. 1994. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80:533-543. [PubMed] [Google Scholar]
- 46.Munderloh, U. G., E. F. Blouin, K. M. Kocan, N. L. Ge, W. L. Edwards, and T. J. Kurtti. 1996. Establishment of the tick (Acari:Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales:Anaplasmataceae) in tick cell culture. J. Med. Entomol. 33:656-664. [DOI] [PubMed] [Google Scholar]
- 47.Munderloh, U. G., J. E. Madigan, J. S. Dumler, J. L. Goodman, S. F. Hayes, J. E. Barlough, C. M. Nelson, and T. J. Kurtti. 1996. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J. Clin. Microbiol. 34:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munderloh, U. G., S. D. Jauron, V. Fingerle, L. Leitritz, S. F. Hayes, J. M. Hautman, C. M. Nelson, B. Huberty, T. J. Kurtti, G. G. Ahlstrand, B. Greig, M. A. Mellencamp, and J. L. Goodman. 1999. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 37:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paddock, C. D., S. M. Folk, G. M. Shore, L. J. Machado, M. M. Huycke, J. N. Slater, A. M. Liddell, R. S. Bulle, G. A. Storch, T. P. Monson, D. Rimland, J. W. Sumner, J. Singleton, K. C. Bloch, Y. W. Tang, S. M. Standaert, and J. E. Childs. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586-1594. [DOI] [PubMed] [Google Scholar]
- 50.Pelchen-Matthews, A., N. Signoret, P. J. Klasse, A. Fraile-Ramos, and M. Marsh. 1999. Chemokine receptor trafficking and viral replication. Immunol. Rev. 168:33-149. [DOI] [PubMed] [Google Scholar]
- 51.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spielman, A., J. F. Levine, and M. L. Wilson. 1984. Vectorial capacity of North American Ixodes ticks. Yale J. Biol. Med. 57:507-513. [PMC free article] [PubMed] [Google Scholar]
- 53.Stich, R. W., J. R. Sauer, J. A. Bantle, and K. M. Kocan. 1993. Detection of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in secretagogue-induced oral secretions of Dermacentor andersoni (Acari: Ixodidae) with the polymerase chain reaction. J. Med. Entomol. 30:789-794. [DOI] [PubMed] [Google Scholar]
- 54.Telford, S. R., III, J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 11:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telford, S. R., III, P. M. Armstrong, P. Katavolos, I. Foppa, A. S. Garcia, M. L. Wilson, and A. Spielman. 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg. Infect. Dis. 3:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theiler, A. 1910. Anaplasma marginale (gen. and spec. nov.). The marginal points in the blood of cattle suffering from a specific disease. Report to the Government, Transvaal, South Africa. Veterinary Bacteriology, Department of Agriculture, 1908-1909, p. 7-64.
- 57.Valenzuela, J. G., I. M. Francischetti, V. M. Pham, M. K. Garfield, T. N. Mather, and J. M. Ribeiro. 2002. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 205:2843-2864. [DOI] [PubMed] [Google Scholar]
- 58.Woldehiwet, Z., B. K. Horrocks, H. Scaife, G. Ross, U. G. Munderloh, K. Bown, S. W. Edwards, and C. A. Hart. 2002. Cultivation of an ovine strain of Ehrlichia phagocytophila in tick cell cultures. J. Comp. Pathol. 127:142-149. [DOI] [PubMed] [Google Scholar]
- 59.Woody, B. J., and J. D. Hoskins. 1991. Ehrlichial diseases of dogs. Vet. Clin. N. Am. Small Anim. Pract. 21:75-98. [DOI] [PubMed] [Google Scholar]
- 60.Yabsley, M. J., A. S. Varela, C. M. Tate, V. G. Dugan, D. E. Stallknecht, S. E. Little, and W. R. Davidson. 2002. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg. Infect. Dis. 8:668-671. [DOI] [PMC free article] [PubMed] [Google Scholar]