Abstract
Analysis of both the antibiotic resistance and the virulence characteristics of anaerobic human microbial pathogens is important in order to improve our understanding of a number of clinically significant infectious diseases, including Clostridium difficile-associated diarrhea (CDAD). We determined the presence of the clindamycin resistance-associated gene ermB and the ribotype of 33 C. difficile strains isolated from Polish patients suffering from CDAD. While all strains produced cytotoxin B (TcdB), enterotoxin A (TcdA) was produced by a subset of 15 strains only. The results showed that a single ermB-positive, TcdA−B+ C. difficile strain with ribotype A has disseminated widely in the two Warsaw hospitals under investigation. Although different strains with the same phenotype were detected, the genotype A strain appeared to be the only one with a clear epidemic character. Apparently, enhanced local spread of CDAD-causing C. difficile may be restricted to a limited number of bacterial genotypes only.
Clostridium difficile is a gram-positive, spore-forming anaerobic pathogen capable of causing nosocomial C. difficile-associated diarrhea (CDAD) (8). The severity of the infection ranges from the usually self-limiting CDAD to life-threatening pseudomembranous colitis (4). The pathogenicity of this bacterial species is primarily determined by the production of two major toxins, enterotoxin A (TcdA) and cytotoxin B (TcdB). The 308-kDa TcdA protein induces fluid secretion, mucosal edema, and massive inflammation, including neutrophil infiltration (18, 24). The 270-kDa TcdB protein induces profound morphological changes in cultured cells (23). Recently, TcdA-negative, TcdB-positive (TcdA−B+) strains, which produce TcdB only, have been recognized as a cause of CDAD in different countries (1, 2, 11, 16, 17, 22). The TcdB produced by some well-characterized TcdA−B+ C. difficile strains demonstrated an unusually strong cytopathic effect (CPE), the same as the effect caused by the cytotoxin produced by Clostridium sordellii (7). Hospital-wide use of clindamycin was identified as an important risk factor for the development of CDAD (5, 10). Resistance against clindamycin is ignited by a special group of the transferable erm genes encoding methylases that specifically modify the bacterial ribosome (20, 26, 27).
Molecular systems for defining the genotypic characteristics of C. difficile have been developed and validated over the past 10 years. A PCR ribotyping procedure based on polymorphism in the 16S-23S intergenic spacer region is one of the best current methods for epidemiologic typing of C. difficile (25). A recent report from The Netherlands identified a nosocomial outbreak due to a genetically distinct clindamycin-resistant TcdA−B+ C. difficile strain in a university hospital (16). Recently, we reported on the existence of TcdA−B+ C. difficile strains in Polish patients with CDAD (22). The aim of the present study was to determine the distribution of the ermB gene and differentiating ribotypes among CDAD-associated C. difficile strains recently isolated (1999 to 2001) from Polish patients.
MATERIALS AND METHODS
Culture and identification of C. difficile.
C. difficile was identified as described previously (22). Fecal samples were inoculated onto Columbia agar containing 100 mg of cycloserine/liter, 8 mg of cefoxitine/liter, and 2 mg of amphotericin B/liter (CCCA medium, bioMerieux, Marcy-l'Étoile, France). Medium was prereduced by 24-h incubation in an anaerobic atmosphere. During cultivation, plates were incubated anaerobically in a glove box (Forma Scientific) at 37°C for 4 days. Isolates were identified as C. difficile by their characteristic colony morphology, specific horse odor, green-yellow fluorescence under UV light, Gram staining, and biochemical tests (API-20 A, bioMerieux).
Bacterial strains.
We investigated all (n = 33) strains of C. difficile isolated from CDAD patients hospitalized in two different institutions between 1999 and 2001. Patients suffering from CDAD were those individuals who produced more than three liquid stools within 48 h, had an antibiotic therapy in their recent medical history, and had a hospitalization period of more than 5 days. Stool cultures should be negative for Salmonella spp., Shigella spp., pathogenic Escherichia coli, rotaviruses, and intestinal parasites, including Giardia. Twenty-four strains were isolated from adults hospitalized in transplantology (n = 7), general surgery (n = 3), internal medicine (n = 7), and orthopedics wards (n = 7). Nine strains were isolated from children hospitalized in the hematology unit of a separate pediatric hospital. For comparative reasons, we included eight ermB-positive TcdA−B+ C. difficile strains. These strains were also isolated from Polish patients and have been described before (22). A small reference set consisted of a single toxigenic strain (VPI 10463) and a nontoxigenic isolate (NIH BRIGGS 8050) which were included as control strains in the cytotoxin assays and TcdA- and TcdB-specific PCR tests. One additional TcdA−B+ strain (GAI 95601) was used as internal control for detection of repeating sequences in the TcdA gene. One ermB-positive strain (number 630) was included in all of the ermB PCR tests.
DNA isolation.
Prior to DNA isolation, the strains were grown in liquid brain heart infusion medium for 96 h. Cells present in 1 ml of the medium were sedimented by centrifugation and incubated in 200 μl of Tris-EDTA-glucose buffer including 100 μg of lysozyme per ml (Sigma, Roosendaal, The Netherlands). After 1 hour at 37°C, 400 μl of lysis buffer prepared according to the methods of Boom et al. (3) was added. DNA was affinity captured to Celite and purified by several washing steps. DNA was ultimately eluted by incubation in 100 μl of T10E at 56°C. The DNA solution was ready to use for PCR and, when needed, could be stored for more than one-half year at −20°C without apparent loss in quality.
Toxigenicity.
TcdA and TcdB were detected from a single colony that was recultured anaerobically in brain heart infusion medium for 96 h. Supernatants were collected by centrifugation (3,000 × g for 15 min). TcdA was determined by means of the C. difficile toxin A test (Oxoid, Basingstoke, United Kingdom). Briefly, 125 μl of supernatant was put onto the sample window of the test unit. After 30 min, a blue line in the results window indicated a positive result. When this test was negative and corroborated by a negative toxin A PCR (see below), the strain was considered toxin A negative. For detection of either or both of the toxins, the TechLab C. difficile TOX A/B test (TechLab, Inc., Blacksburg, Va.) was employed. One drop of conjugate per microwell was added, followed by 2 drops of culture supernatant. Microwells were incubated at 37°C for 50 min and washed five times. One drop of tetramethylbenzidine substrate was added, followed by 1 drop of hydrogen peroxide in citric acid buffer. After a 10-min incubation at room temperature, reactions were interpreted by visual reading of yellow staining. In vitro, TcdB activity measurements were performed on McCoy cells cultured as described previously (19). Supernatant fluids were collected as described above, and tenfold serial dilutions of culture filtrate were added in duplicate to McCoy cells and incubated for 24 h at 37°C in a 5% CO2 atmosphere. CPE was observed by inverse microscopy. When this CPE could be neutralized by polyclonal goat antiserum against the toxin B, the test was considered positive. Albumin was used as a negative control antigen. For detection of the nonrepeating regions in the TcdA and TcdB genes, PCR was performed with the YT28-YT29 and YT17-YT18 primer pairs, respectively (9, 15). PCR involved 35 cycles of 45 s at 94°C, 30 s at 55°C, and 45 s at 70°C. PCR-mediated amplification aiming at the repeats in the toxin A gene was performed as described previously with primer set NK9-NKV011 (12).
PCR ribotyping.
PCR-mediated ribotyping employed the consensus primers SP1 and SP2 (5′-TTG TAC ACA CAC CGC CCG TCA-3′ and 5′-GGT ACC TTA GAT GTT TCA GTT C-3′ [see reference 14]). Fifty nanograms of DNA was added to a PCR mixture (100 μl) containing 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.5 mM MgCl2, 0.01% gelatin (wt/vol), 0.1% Triton X-100 (vol/vol), 0.2 mM concentrations of the four deoxyribonucleotide triphosphates, 1.2 U of TaqDNA polymerase (Sphaero Q, Leiden, The Netherlands), and 50 pmol of each primer. Amplification was performed in a Biomed model 60 cycler (Biomed, Theres, Germany) with predenaturation at 94°C for 120 s followed by 40 cycles of 60 s at 94°C, 60 s at 55°C, and 60 s at 74°C. Amplicons were analyzed by electrophoresis on a 1% agarose gel for 3 h at 100 V. PCR ribotypes were defined on the basis of single band position differences in the fingerprints. Validation of this guideline has been the subject of previous investigations and consensus has been obtained (14, 25).
Determination of antibiotic susceptibility and ermB PCR.
The antibiotic susceptibility of all strains was investigated by using E-tests (AB-BIOdisc, Solna, Sweden) with clindamycin and erythromycin according to the instructions of the manufacturer. A bacterial suspension with a density of 1 McFarland was streaked to confluence on the surface of brucella agar plates. Plastic strips with antibiotic were applied, and the plates were incubated anaerobically at 37°C for 48 h. The MIC was measured at the intercept of the inhibition ellipses, and high-level resistance was defined when the value was higher than 256. PCR for detection of the ermB gene was performed with primers 2980 and 2981 (10). The cycling condition applied during PCR included 30 cycles of 60 s at 95°C, 120 s at 55°C, and 180 s at 72°C.
RESULTS
Among 33 strains of C. difficile isolated in the period from 1999 to 2001 from patients with CDAD, 15 strains were TcdA+B+ as demonstrated by the C. difficile toxin A test and TcdB-dependent cytotoxicity testing on McCoy cells. The remaining 18 strains were TcdA−B+, and a CPE was observed after cell line challenge. TcdA could not be detected by the commercial toxin A test even when supernatants from 96-h cultures were used. The toxin A/B tests gave positive results for all 33 strains due to the ubiquitous presence of the TcdB gene. Note that the historic control strains (1995 to 1998) are not included in any of the calculations presented in this section. The strains are listed in Table 1, which also summarizes all of the test results obtained for these strains.
TABLE 1.
Distribution of the ermB gene and ribotypes among clinical isolates of C. difficile
| Toxin profile | Isolate no. | Isolation date (mo-yr) | Unit | Patient type | McCoy cytotoxicityf | TcdA gene site (bp)b | PCR ribotypec | PCR result for ermBd | MIC (mg/liter)e
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| CM | EM | |||||||||
| TcdA−B+ | 132 | 1-1999 | Hematology | Child | 10−3 | 700 | A | + | >256 | >256 |
| 202 | 10-1999 | Hematology | Child | 10−1 | 700 | A | + | >256 | >256 | |
| 205 | 11-1999 | Hematology | Child | 10−2 | 700 | A | + | >256 | >256 | |
| 250 | 7-2000 | Hematology | Child | 10−3 | 700 | A | + | >256 | >256 | |
| 254 | 8-2000 | Hematology | Child | 10−2 | 700 | E | + | >256 | >256 | |
| 268 | 11-2000 | Hematology | Child | 10−2 | 700 | A | + | >256 | >256 | |
| 313 | 3-1999 | Internal unit | Child | 10−1 | 700 | A | + | >256 | >256 | |
| 376 | 3-1999 | Internal unit | Child | 10−1 | 700 | A | + | >256 | >256 | |
| 3307 | 12-2000 | Internal unit | Child | 10−2 | 700 | A | + | >256 | >256 | |
| 660 | 5-1999 | Orthopedics | Adult | 10−4 | 700 | A | + | >256 | >256 | |
| 874 | 7-1999 | Orthopedics | Adult | 10−1 | 700 | A | + | >256 | >256 | |
| 1186 | 9-1999 | Orthopedics | Adult | 10−1 | 700 | A | + | >256 | >256 | |
| 1632 | 11-1999 | Orthopedics | Adult | 10−1 | 700 | A | + | >256 | >256 | |
| 1745 | 7-2000 | Orthopedics | Adult | 10−2 | 700 | A | + | >256 | >256 | |
| 3169 | 12-2000 | Orthopedics | Adult | 10−2 | 700 | A | + | >256 | >256 | |
| 114 | 1-2000 | Transplantology | Adult | 10−3 | 700 | A | + | >256 | >256 | |
| 231 | 2-2000 | Transplantology | Adult | 10−1 | 700 | A | + | >256 | >256 | |
| 293 | 2-2000 | Transplantology | Adult | 10−1 | 700 | A | + | >256 | >256 | |
| TcdA+B+ | 2 | 1-1999 | Internal unit | Adult | 10−3 | 2,500 | B | + | >256 | >256 |
| 105 | 1-1999 | Internal unit | Adult | 10−1 | 2,500 | G | + | >256 | >256 | |
| 650 | 5-1999 | Internal unit | Adult | 10−3 | 2,500 | H | + | >256 | >256 | |
| 1405 | 11-1999 | Internal unit | Adult | 10−3 | 2,500 | D | + | >256 | >256 | |
| 711 | 6-1999 | Surgery | Adult | 10−3 | 2,500 | I | + | >256 | >256 | |
| 1166 | 9-1999 | Surgery | Adult | 10−2 | 2,500 | B | + | >256 | >256 | |
| 929 | 8-1999 | Hematology | Child | 10−3 | 2,500 | C | − | 1.5 | 0.125 | |
| 248 | 7-2000 | Hematology | Child | 10−2 | 2,500 | E | − | 0.5 | 0.5 | |
| 277 | 9-2000 | Hematology | Child | 10−3 | 2,500 | F | − | 1.5 | 2.56 | |
| 118 | 1-2000 | Orthopedics | Adult | 10−3 | 2,500 | L | − | 3.0 | 0.094 | |
| 188 | 2-2000 | Surgery | Adult | 10−3 | 2,500 | A | − | 0.016 | >256 | |
| 1140 | 9-1999 | Transplantology | Adult | 10−2 | 2,500 | B | − | 0.023 | 0.023 | |
| 1382 | 10-1999 | Transplantology | Adult | 10−2 | 2,500 | K | − | 0.125 | 0.25 | |
| 28 | 1-2001 | Transplantology | Adult | 10−2 | 2,500 | D | − | 0.5 | 0.5 | |
| 1052 | 11-2000 | Transplantology | Adult | 10−2 | 2,500 | J | − | 2.0 | 2.0 | |
| TcdA−B+a | 2428 | 11-1995 | Surgery | Adult | 10−5 | 700 | A | + | >256 | >256 |
| 2785 | 11-1997 | Internal unit | Child | 10−3 | 700 | A | + | >256 | >256 | |
| 2887 | 12-1997 | Orthopedics | Child | 10−1 | 700 | A | + | >256 | >256 | |
| 399 | 2-1998 | Gastroenterology | Child | 10−5 | 700 | A | + | >256 | >256 | |
| 592 | 2-1998 | Internal unit | Adult | 10−3 | 700 | A | + | >256 | >256 | |
| 1110 | 4-1998 | Hematology | Child | 10−3 | 700 | A | + | >256 | >256 | |
| 2233 | 8-1998 | Neurology | Adult | 10−4 | 700 | A | + | >256 | >256 | |
| 2601 | 9-1998 | Orthopedics | Adult | 10−3 | 700 | A | + | >256 | >256 | |
Clinical strains control group.
PCR with primer pair NK9-VO11 for detection of the variant of the TcdA gene.
PCR ribotyping with primer pair SP1-SP2.
PCR with primer pair 2980-2981 for detection of the ermB gene. The fragment is 688 bp.
CM, clindamycin; EM, erythromycin.
Last dilution of supernatants; demonstrated cytopathic effect.
PCR amplification with YT28-YT29 and YT17-YT18 generated products of 630 and 399 bp for the TcdA and TcdB genes, respectively, for all strains. For the 18 TcdA−B+ strains, PCR with the NK9-NKV01 primer set generated a 700-bp product similar to that obtained for the Japanese GAI 95601 strain. Of 24 C. difficile strains isolated from adults with CDAD, 12 belonged to the TcdA−B+ group. We isolated these strains from stool samples from six orthopedic patients, three transplant patients, and three patients nursed in the internal medicine unit. Among nine strains isolated from children with CDAD hospitalized in the hematology department (aged 4 to 15 years), three strains belonged to the TcdA+B+ group and six strains belonged to the TcdA−B+ cluster. Interestingly, the 18 TcdA−B+ strains were not derived from patients who were clustered in time or space during their hospitalizations (data not shown). Consequently, we can conclude that we are not witnessing a local outbreak but rather persistent and multi-institutional dissemination of a clonal lineage of C. difficile.
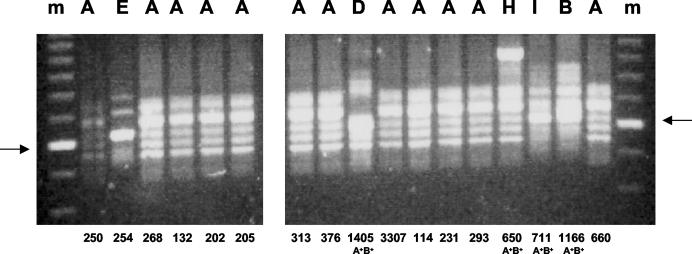
PCR ribotyping identified 12 different ribotypes among the 33 strains (Fig. 1). Eighteen strains belonged to the prevalent ribotype A, three strains belonged to ribotype B, two strains belonged to ribotype D, and two strains belonged to ribotype E. Eight other ribotypes were identified for as many strains. Among the isolates belonging to the TcdA−B+ group, seventeen strains belonged to ribotype A and one strain to ribotype E. Within the group of C. difficile TcdA+B+ strains, we observed more differentiation: 12 different ribotypes were identified. Interestingly, both ribotype A and E were detected in strains with different toxigenicity patterns. Among the 33 strains of C. difficile, 24 (73%) demonstrated high-level resistance to clindamycin and erythromycin. The resistant group included different ribotypes: 17 strains belonged to ribotype A, 2 strains belonged to ribotype B, and 1 strain belonged to each of ribotypes D, G, E, H, and I. All strains which showed high-level resistance to clindamycin possessed the ermB gene, as detected by PCR. All experimental data are summarized in Table 1.
FIG. 1.
PCR ribotyping of C. difficile strains. Examples of the DNA fingerprints are shown, and their interpretation codes are listed above the lanes (see also Table 1). The fingerprints were obtained for the strains identified by number beneath the lanes. All strains were toxin TcdA−B+ except for the four strains for which the toxin A gene could also be detected (A+B+). Molecular size markers are shown in the leftmost and rightmost lanes; the fragment indicated by the arrows is 600 bp in length.
DISCUSSION
We routinely check the susceptibility of C. difficile to clindamycin and erythromycin, and we observed an increase in the number of strains showing elevated levels of resistance. We present here the results of a detailed investigation into a number of consecutive isolates from patients hospitalized in four units of a large university hospital and a separate hematology unit in an independent pediatric hospital. Among these strains, we frequently found high-level resistance against clindamycin and erythromycin associated with ermB gene presence in both TcdA+B+ and TcdA−B+ strains. Seventeen out of the TcdA−B+ strains from symptomatic patients represented the same ribotype. This is in agreement with previous data from our laboratory where we demonstrated the epidemic spread of TcdA−B+ strains isolated from patients hospitalized in two different institutions and at different points in time (22). All TcdA−B+ strains possessed the ermB gene and were highly resistant to clindamycin and erythromycin. Interestingly, among the Polish TcdA−B+ strains isolated from stool samples of CDAD patients, ribotype A seems to predominate. The prevalence of the multiresistant strains was less than 50% percent before 1999, but it expanded significantly during recent years. The fact that there was no obvious overlap in hospitalization periods for most of the patients included suggests that persistence rather than short-term, focused outbreaks of infection was ongoing. This feature was particularly clear for the clindamycin-resistant strain.
Kato et al. investigated isolates of C. difficile from patients with CDAD from three hospitals by three typing methods, including PCR ribotyping. These authors described an epidemic TcdA+B+ strain which was not resistant to clindamycin (13). Similarly, an epidemic ermB-positive C. difficile strain of an unknown toxin status was identified in Sweden (21). Kuijper et al. observed an association between clindamycin resistance and epidemicity of a TcdA−B+ C. difficile strain involved in CDAD (16). This epidemic strain belonged to serogroup F. However, as described by Delmee and Avesani (6), serogroup F strains are usually susceptible to clindamycin and erythromycin. All Polish TcdA−B+ strains, described before (22) and here, possess the ermB gene and share high-level resistance to clindamycin and erythromycin. It is surprising to see that the same ribotype (A or E) is encountered among both the TcdA+B+ and TcdA−B+ C. difficile strains. We suggest that TcdA−B+ C. difficile strains which harbor the ermB gene are significantly associated with CDAD among both adults and children. Whereas the epidemic capacity of some of these strains might be enlarged simultaneously, we propose that determination of macrolide-lincosamide-streptogramin B resistance in TcdA−B+ C. difficile strains is a possible predictor of enhanced CDAD potential.
REFERENCES
- 1.Al-Barrak, A., J. Embil, B. Dyck K. Olekson, D. Nicoll, M. Alfa, and A. Kabani. 1999. An outbreak of toxin A-negative, toxin B-positive Clostridium difficile-associated diarrhoea in a Canadian tertiary-care hospital. Can. Commun. Dis. Rep. 25:65-69. [PubMed] [Google Scholar]
- 2.Alfa, M. J., A. Kabani, D. Lyerly, S. Moncrief, L. M. Neville, A. Al-Barrak, G. K. Harding, B. Dyck, K. Olekson, and J. M. Embil. 2000. Characterization of toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 38:2706-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borriello, S. P. 1998. Pathogenesis of Clostridium difficile infection. J. Antimicrob. Chemother. 41(Suppl. C):13-19. [DOI] [PubMed] [Google Scholar]
- 5.Climo, M. W., D. S. Israel, E. S. Wong, D. Williams, P. Coudron, and S. Markowitz. 1998. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile associated diarrhea and cost. Ann. Intern. Med. 128:989-995. [DOI] [PubMed] [Google Scholar]
- 6.Delmee, M., and V. Avesani. 1988. Correlation between serogroup and susceptibility to chloramphenicol, clindamycin, erythromycin, rifampicin and tetracycline among 308 isolates of Clostridium difficile. J. Antimicrob. Chemother. 22:325-331. [DOI] [PubMed] [Google Scholar]
- 7.Eichel-Streiber, C. V., D. Meyer zu Heringdorf, E. Haberman, and S. Sartingen. 1995. Closing in on the toxic domain through analysis of a variant Clostridium difficile cytotoxin B. Mol. Microbiol. 17:313-321. [DOI] [PubMed] [Google Scholar]
- 8.Gorbach, S. L. 1999. Antibiotics and Clostridium difficile. N. Engl. J. Med. 341:1690-1692. [DOI] [PubMed] [Google Scholar]
- 9.Gumerlock, P. H., Y. J. Tang, J. B. Weiss, and J. Silva. 1993. Specific detection of toxigenic strains of Clostridium difficile in stool specimens. J. Clin. Microbiol. 31:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. I. Rood, P. de Girolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin- resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 11.Johnson S., S. A. Kent, K. J. O'Learly, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135:434-438. [DOI] [PubMed] [Google Scholar]
- 12.Kato, H., N. Kato, S. Katow, T. Muegawa, S. Nakamura, and D. Lyerly. 1999. Deletions in the repeating sequences of the toxin A gene of toxin A-negative, toxin B-positive Clostridium difficile strains. FEMS Microbiol. Lett. 175:197-203. [DOI] [PubMed] [Google Scholar]
- 13.Kato, H., N. Kato, K. Watanabe, T. Yamamoto, K. Suzuki, S. Ishigo, S. Kunihiro, I. Nakamura, G. E. Killgore, and S. Nakamura. 2001. Analysis of Clostridium difficile isolates from nosocomial outbreaks at three hospitals in diverse areas of Japan. J. Clin. Microbiol. 39:1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostman, J. R., T. D Edlind, J. J. LiPuma, and T. L. Stull. 1992. Molecular epidemiology of Pseudomonas cepacia determined by polymerase chain reaction ribotyping. J. Clin. Microbiol. 30:2084-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhl, S. L., Y. J. Tang, P. H. Nawarro, P. H. Gumerlock, and J. Silva. 1993. Diagnosis and monitoring of Clostridium difficile infections with PCR. Clin. Infect. Dis. 16:234-238. [DOI] [PubMed] [Google Scholar]
- 16.Kuijper, E. J., J. de Weerdt, H. Kato, N. Kato, A. P. van Dam, E. R. van der Vorm, J. Weel, C. van Rheenen, and J. Dankert. 2001. Nosocomial outbreak of Clostridium difficile-associated diarrhoea due to a clindamycin-resistant enterotoxin A-negative strain. Eur. J. Clin. Microbiol. Infect. Dis. 20:528-534. [DOI] [PubMed] [Google Scholar]
- 17.Limaye, A. P., D. K. Turgeon, B. D. Cookson, and T. R. Fritsche. 2000. Pseudomembranous colitis caused by a toxin A−B+ strain of Clostridium difficile. J. Clin. Microbiol. 38:1696-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahida, Y. R., A. Galvin, S. Makh, S. Hyde, L. Sanfilippo, S. P. Borriello, and H. F. Sewell. 1998. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 66:5462-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martirosian, G., H. Pituch, P. Obuch-Woszczatynski, G. Rouyan, and F. Meisel-Mikołajczyk. 1999. Evaluation of different methods for detection of Clostridium difficile toxins in Poland. Acta Microbiol. Polon. 48:349-353. [PubMed] [Google Scholar]
- 20.Mullany, P., M. Wilks, and S. Tabaqchali. 1995. Transfer of macrolide-lincosamide-streptogramin B (MLS) resistance in Clostridium difficile is linked to a gene homologous with toxin A and is mediated by a conjugative transposon Tn 5398. J. Antimicrob. Chemother. 35:305-315. [DOI] [PubMed] [Google Scholar]
- 21.Noren, T., Y. J. Tang-Feldman, S. H. Cohen, J. Silva, and P. Olcen. 2002. Clindamycin resistant strains of Clostridium difficile isolated from cases of C. difficile associated diarrhea (CDAD) in a hospital in Sweden. Diagn. Microbiol. Infect. Dis. 42:149-151. [DOI] [PubMed] [Google Scholar]
- 22.Pituch, H., N. van den Braak, W. van Leeuwen, A. van Belkum, G. Martirosian, P. Obuch-Woszczatynski, M. Luczak, and F. Meisel-Mikołajczyk. 2001. Clonal dissemination of a toxin-A-negative/toxin-B-positive Clostridium difficile strain from patients with antibiotic-associated diarrhea in Poland. Clin. Microbiol. Infect. 7:442-446. [DOI] [PubMed] [Google Scholar]
- 23.Riegler, M., R. Sedivy, C. Pothoulakis, G. Hamilton, J. Zacherl, G. Bischof, E. Cosenti, W. Feil, R. Sciesel, J. T. La Mont, and E. Wenzl. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vivo. J. Clin. Investig. 95:2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha, M. F., A. M. Soares, C. A. Flores, T. S. Steiner, D. M. Lyerly, R. L. Guerrant, R. A. Ribeiro, and A. A. Lima. 1998. Intestinal secretory factor released by macrophages stimulated with Clostridium difficile toxin A: role of interleukin 1β. Infect. Immun. 66:4910-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubbs, S. L. J., J. S. Brazier, G. L. O'Neill, and B. I. Duerden. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of the library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wust, J., and U. Hardegger. 1983. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob. Agents Chemother. 23:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]