Abstract
Respiratory syncytial virus (RSV) accounts for the majority of respiratory virus infections, producing high mortality rates in immunocompromised patients with hematologic malignancies. The available methods for the rapid detection of RSV by antigen detection or PCR either lack sensitivity, require complex laboratory manipulation, or have not been evaluated in this patient population. To assess the applicability of a TaqMan-based real-time PCR technique for the detection of RSV A and B in immunocompromised adults, we developed a rapid, sensitive detection method that simultaneously detects RSV A and B and can be applied in routine diagnostics. The specificity of the assay was assessed using a panel of reference strains of other respiratory viruses and RSV. Electron microscopy-counted stocks of RSV A and B were used to develop a quantitative PCR format. Eleven copies of viral RNA could be detected for RSV A strain Long, and 14 copies could be detected for RSV B strain 9320, corresponding to 50% tissue culture infective doses of 0.86 and 0.34, respectively. The assay was evaluated on 411 combined nose and throat swabs derived from immunocompromised adults with or without signs of respiratory tract infection. The diagnostic efficacy of the TaqMan PCR determined on the clinical samples showed that this real-time PCR technique was substantially more sensitive than the combination of conventional viral culture and shell vial culture. None of the clinical specimens derived from patients without signs of respiratory illness were found to be positive for RSV by real-time TaqMan PCR.
Respiratory syncytial virus (RSV) has long been recognized as a major cause of respiratory tract infection in infants and young children (14). More recent studies have shown that those at risk for developing serious disease following RSV infection also include the elderly, adults with underlying cardiopulmonary disease, and the severely immunocompromised (3, 4, 7, 17, 21, 25, 26). Compared with pneumonias caused by other respiratory viruses, RSV pneumonias are associated with the highest mortality rates in bone marrow transplant recipients and leukemia patients (25, 27).
Children are known to shed RSV in high titers for up to several weeks, whereas shedding of virus in adults and the elderly is presumed to be of relatively low titer and short duration (8). Consequently, laboratory techniques such as conventional cell culture and antigen detection assays that are suitable for diagnosis in young children are hampered by lack of sensitivity in older patients (6). Even serologic analysis may not always be reliable in certain patient groups because of their impaired immune response.
To overcome this lack of sensitivity and to obtain more rapid diagnostic results, a number of different PCR techniques have been developed (16, 22). Recently, van Elden et al. described a substantial increase in the detection of respiratory viruses involved in pneumonia in patients with hematologic malignancies by use of nested PCR methods (20). Reverse transcription-PCR (RT-PCR) has also been proven to be more sensitive than viral culture in adults with or without cardiopulmonary disease with respiratory illness and is a well-considered alternative for the rapid diagnosis of RSV infection (5).
However, RT-PCR and nested PCR are difficult to implement in a routine diagnostic setting because they need time-consuming sample handling and post-PCR analysis, often requiring specific detection methods and posing serious hazards for amplification product carryover. The current development of real-time PCR methods would seem to overcome these problems (19, 24). A recent study used real-time RT-PCR and immunofluorescence with nasopharyngeal aspirates derived from children that contained moderate to low levels of RSV and showed that the sensitivities of the two methods were more or less equal, indicating that the advantage of real-time PCR in children consists primarily of the automated analysis of results and the possibility of direct virus quantification (10).
RSV infection can have devastating consequences in patients treated for leukemia (26). Since specimens derived from children are known to contain high viral loads and the recovery rates for RSV are generally lower in adult patients, we were interested to see whether the increased sensitivity of real-time PCR would also provide more clinical benefit in immunocompromised adults.
The purpose of our study was to assess the applicability of a TaqMan-based real-time PCR technique for the detection of RSV A and B in immunocompromised adults. Therefore, we compared real-time PCR with conventional cell culture, shell vial culture, and our in-house nested PCR. To determine the clinical value of the assay, specimens were taken from symptomatic as well as asymptomatic patients.
MATERIALS AND METHODS
Virus stocks.
RSV A strain Long was propagated on human embryonic lung fibroblast cells, and RSV B strain 9320 was propagated on HEp-2 cells at 35°C in Eagle's minimal essential medium supplemented with 0.01 M HEPES, 0.084% bicarbonate, 100 U of penicillin and streptomycin/ml, 0.625 μg of amphotericin B (Fungizone)/ml, and 0.2 M glutamine (Foundation for the Advancement of Public Health and Environment, Bilthoven, The Netherlands). After development of a cytopathic effect, the supernatant was harvested and the virus particle count of each stock was determined by quantitative electron microscopy (EM) (Advanced Biotechnologies Inc., Columbia, Md.). As previously described, a well-defined panel of various respiratory viruses provided by the Laboratory for Virology, National Institute for Public Health and the Environment (Bilthoven, The Netherlands), was used to determine the specificity of the real-time quantitative PCR (19). In addition, 16 strains of wild-type RSV obtained from successive seasons were tested.
Clinical specimens.
From October 1999 through November 2002, a prospective surveillance study was carried out with a group of patients (n = 73) who underwent autologous or allogeneic stem cell transplantation. Combined nose and throat (NT) swabs were collected at set time points and during episodes of upper or lower respiratory tract symptoms. The majority of the samples were obtained from patients who participated in this study. The other clinical samples were collected from patients (n = 17) known to have hematologic malignancies who had signs of respiratory tract infection. In addition, 30 combined NT swabs from healthy, asymptomatic volunteers were collected. All NT samples were placed on ice immediately after collection and transported in 5 ml of virus transport medium to the laboratory within 2 h. There, the samples were vortexed for 10 s and centrifuged at 2,000 × g for 15 min. One milliliter of the supernatant was used directly for virus culturing. The remaining material was stored at −70°C until RNA extraction.
Diagnostic methods for the routine detection of RSV.
For shell vial cultures, 100 μl of clinical specimen was inoculated on tertiary rhesus monkey kidney cells grown in flat-bottom tubes, centrifuged for 1 h at 2,000 × g, and incubated for 2 days at 33°C. Then, usually before a cytopathic effect could be noticed, cells were fixed and stained with virus-specific monoclonal antibodies (Dako Imagen). Immunofluorescence microscopy was used to detect RSV. For routine viral cultures, 100 μl of each clinical specimen was inoculated on HEp-2 cells, R-HELA cells, and tertiary rhesus monkey kidney cells and incubated at 33°C for a maximum of 10 days with 100 μl of each clinical sample. In cultures showing a cytopathic effect, virus was identified by immunofluorescence with commercial monoclonal antibodies (Dako Imagen) for RSV.
Viral RNA isolation and cDNA synthesis.
RNA extraction was performed by using a MagnaPure LC total nucleic acid kit (Roche Diagnostics, Mannheim, Germany). Briefly, 10 to 100 μl of clinical specimen or EM-counted virus stock was mixed with lysis buffer and proteinase K and subsequently incubated with magnetic particles to allow binding of the nucleic acids. Unbound material was removed by several washing steps. The RNA was then eluted either in 100 μl of 40-ng/μl poly(A) RNA before one-tube RT-PCR was performed (13) or in 100 μl of elution buffer and directly used for cDNA synthesis and real-time TaqMan PCR.
The isolated viral RNA was reverse transcribed using MultiScribe reverse transcriptase and random hexamers (TaqMan reverse transcription reagents; ABI). Each 50-μl reaction mixture contained 10 μl of eluted RNA, 5 μl of 10× RT buffer, 5.5 mM MgCl2, 500 μM concentrations of each of the deoxynucleoside triphosphates, 2.5 μM random hexamer, and 20 U of RNase inhibitor (all from Applied Biosystems International). The cDNA synthesis was performed as described previously (19), and the cDNA was stored at −70°C until real-time TaqMan PCR.
In-house nested PCR.
A nested PCR was performed for RSV A and B. One-tube RT-PCR was followed by a second (nested) amplification. First-round amplification primers and nested primers were defined in the N gene (first-round primer set RS-1 [5′-GGATTGTTTATGAATGCCTATGGT-3′] and RS-2 [5′-TTCTTCTGCTGTYAAGTCTARTACAC-3′] and second-round primer set RS-3 [5′-GGATTCTACCATATATTGAACAA-3′] and RS-4 [5′-CTRTACTCTCCCATTATGCCTAG-3′]). The RT-PCR and nested PCR conditions were applied as described by M. Nijhuis et al. (13) by using a PE 9600 thermocycler (Perkin-Elmer). PCR products were visualized on an ethidium bromide-stained agarose gel by using UV illumination. A 100-bp marker was used to control fragment lengths.
Real-time TaqMan PCR.
Primers and probes for both RSV A and B were selected using primer express software (PE Applied Biosystems) and were based on the highly conserved genomic regions of the N gene. To provide subgroup analysis, type-specific primers and probes were chosen for RSV A and B. The forward and reverse primers (RSA-1, RSA-2, RSB-1, and RSB-2) and probes (RSA probe and RSB probe) used are listed in Table 1. Primers and probes were tested for possible interactions to make sure that they could be used together in a multiplex assay. After optimization of primer and probe concentrations, samples were assayed in duplicate in a 25-μl reaction mixture containing 5 μl of cDNA, 12.5 μl of 2× TaqMan universal PCR master mix (PE Applied Biosystems), 900 nM concentrations of each forward primer, 900 nM concentrations of the reverse primers, and 200 nM concentrations of each of the probes. The fluorogenic probes that can be labeled with different fluorogenic dyes were both labeled with the 5′ reporter dye 6-carboxy-fluorescein (FAM) and the 3′ quencher dye 6-carboxy-tetramethyl-rhodamine (TAMRA). Amplification and detection were performed with an ABI Prism 7700 sequence detection system under the following conditions: 2 min at 50°C to attain optimal AmpErase uracil-N-glycosylase activity, 10 min at 95°C to activate the AmpliTaq Gold DNA polymerase, and 45 cycles of 15 s at 95°C and 1 min at 60°C.
TABLE 1.
Selected primers and probes for the TaqMan amplification of viral RNA from RSV A and B
| RSV type (target) | Primer or probe | Sequence | Nucleotide positiona |
|---|---|---|---|
| A (N gene) | RSA-1 | 5′ AGATCAACTTCTGTCATCCAGCAA | 1137 |
| RSA-2 | 5′ ATTGATACTCCTAATTATGATGTGC | 1192 | |
| RSA probe | 5′ CACCATCCAACGGAGCACAGGAGAT | 1164 | |
| B (N gene) | RSB-1 | 5′ AAGATGCAAATCATAAATTCACAGGA | 1248 |
| RSB-2 | 5′ CACTATAAAGATACTTAAAGATGCTGGATATCA | 1318 | |
| RSB probe | 5′ AGGTATGTTATATGCTATGTCCAGGTTAGGAAGGGAA | 1279 |
During amplification, the ABI Prism sequence detector monitored real-time PCR amplification by quantitatively analyzing the fluorescence emissions. The reporter dye (FAM) signal was measured relative to the internal reference dye (ROX) to normalize for non-PCR-related fluorescence fluctuations occurring from well to well. The threshold cycle number represented the refractional cycle number at which a positive amplification reaction was measured and was set at 10 times the standard deviation of the mean baseline emission calculation for PCR cycles 3 to 15.
RESULTS
Specificity and sensitivity.
The specificity of the real-time TaqMan PCR was assessed by testing a variety of other respiratory viruses (rhinoviruses 1A and 14, coronaviruses OC43 and 229E, parainfluenza viruses 1 to 4, influenza viruses B/Lee/40 and A/PR/8/34, and enteroviruses CVA9 and CVA11) and a panel of 16 wild-type RSV strains circulating at consecutive seasons. All of the RSV strains but none of the other respiratory viruses were found to be positive by real-time TaqMan PCR. No fluorescent signal was observed in 30 specimens collected from healthy adults without symptoms of respiratory infection.
The sensitivity of the assay was determined by comparing real-time PCR results with results from (i) EM particle counting and (ii) a virus infectivity assay. RSV A Long and RSV B 9320 were first counted by EM and subsequently titrated by serial dilution. The 50% tissue culture infective doses (TCID50s), calculated by the Kärber formula, were 1.0 × 106 and 3.2 × 105/ml, respectively, corresponding to 1.28 × 107 and 1.30 × 107 viral particles, respectively.
A 10-fold dilution series of the two strains was then amplified using the real-time PCR assay, indicating detection limits of 11 particles of RSV A Long and of 14 particles of RSV B 9320. This level of sensitivity correlated with TCID50s of 0.86 for RSV A Long and 0.34 for RSV B 9320.
Detection of viruses in clinical samples.
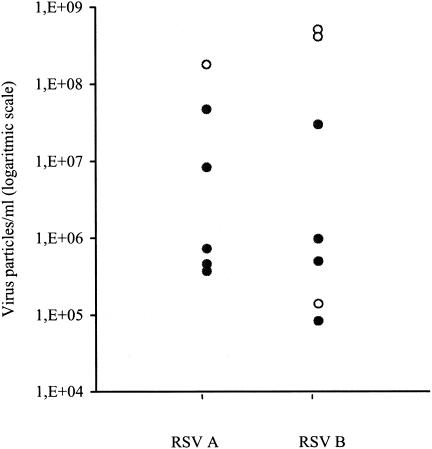
TaqMan-based real-time RT-PCR has been shown to be a rapid, sensitive, and specific assay for the detection of RSV A and B in children. To assess the applicability of a real-time PCR assay in immunocompromised adults, the assay was evaluated on combined NT swabs collected from patients with hematologic malignancies. A total of 411 NT samples from 90 patients were tested by real-time TaqMan PCR, an in-house nested PCR, conventional culture, and shell vial culture. Of the 411 samples, 168 (41%) were obtained during an episode of suspected upper or lower respiratory tract infection and 243 (59%) were taken during an asymptomatic period. Overall, RSV was identified in a total of 13 (3.2%) specimens (Table 2). Of these RSV-positive specimens, only four (31%) were detected by cell culture and none were detected by shell vial culture. All samples positive by real-time PCR were confirmed by the in-house nested PCR (100%). All four culture-positive samples were found to be positive by the TaqMan-based real-time PCR assay. Interestingly, none of the 243 samples taken during a symptom-free period were found to be positive for RSV by any of the applied methods. The TaqMan-based real-time PCR was found to be about 70% more sensitive than culture in this specific patient population. The number of viral RNA copies in the clinical samples was determined by extrapolation to a standard curve generated upon amplification of serial dilutions of the EM-counted virus stocks. Results of the quantification of the clinical specimens are shown in Fig. 1. Samples with a viral load above 108 viral particles/ml (threshold cycle below 30) could also be detected by virus culture. Surprisingly, one sample with a viral load of 1.4 × 105 viral particles/ml could be detected by culture as well.
TABLE 2.
Comparison of conventional culturing, shell vial culturing, in-house nested PCR, and real-time TaqMan PCR for the detection of RSV in 411 clinical specimens
| Method | No. of RSV-positive samples
|
|
|---|---|---|
| During period of symptoms (n = 168) | During symptom-free period (n = 243) | |
| Conventional culture | 4 | 0 |
| Shell vial culture | 0 | 0 |
| In-house nested PCR | 13 | 0 |
| Real-time TaqMan PCR | 13 | 0 |
FIG. 1.
Amount of virus particles of RSV A and B that could be detected in the clinical specimens (n = 13). The filled circles represent the clinical samples detected by real-time TaqMan PCR only, whereas the open circles represent the clinical samples detected by both virus culture and real-time TaqMan PCR.
DISCUSSION
Our study shows that the TaqMan real-time PCR can be used as a rapid and sensitive diagnostic tool for the detection of RSV in immunocompromised adults. The study confirms the lack of sensitivity of viral culture for RSV in the adult population. Moreover, our data indicate that the detection of RSV by nested PCR and TaqMan real-time PCR is superior to that by shell vial culture as well.
The group which we studied, consisting mainly of immunocompromised patients who recently underwent a stem cell transplantation, was relatively small. We consequently acquired only a small proportion of RSV-positive samples. The proportional contribution of RSV infection in our study does not differ, however, from that in other studies. In two large epidemiological studies, the estimated frequencies of different respiratory viruses causing respiratory infection in the immunocompromised host showed that the contribution of RSV varies between 1.5 and 15% (12, 27).
Both shell vial culture and conventional virus culture are well-established standard techniques that are used routinely in laboratories for the detection of respiratory viruses in adults (11, 15). Rapid laboratory methods such as direct antigen detection are often used for point-of-care diagnosis of RSV infection in infants and children (18, 23). In adults, these methods have been shown to be unreliable, partly because of the sampling methods and partly because of the common belief that adults tend to shed less virus (2). Our study shows poor results for the conventional virus culture and the shell vial culture, although every effort was made to optimize sample handling, such as transporting samples on ice and processing samples within 2 h. It has been reported before that the use of RT-PCR in adults with respiratory illness can double the number of RSV infections detected compared to viral culture (5). The type of specimen collection may be another explanation for the poor results with virus culture. In accordance with general experience, we found that the majority of sometimes very ill, immunocompromised patients did not consent to the collection of nasal wash specimens. Therefore, we decided to only evaluate combined NT swabs, which are generally inferior to nasal wash specimens for the detection of RSV by viral culture. Our results indicate that conventional culture as well as shell vial culture might not be suitable for the identification of low viral loads. The majority of culture-positive samples contained a viral load above 108 viral particles/ml (threshold cycle below 30), whereas viral loads in the lower ranges were mainly detected by the TaqMan real-time PCR assay.
RT-PCR has proven to be a sensitive method for the detection of RSV infection in adults with respiratory illness (5). Because PCR-based diagnostics offer excellent potential for rapid diagnosis, with substantial consequences such as more rapid clinical intervention through therapy and infection control measures, their use has gained interest over the last couple of years. Yet although immunocompromised patients and children are known to shed virus for a long period of time and RT-PCR methods have been found to be extremely sensitive, the clinical interpretation of a positive result is considered to be difficult (1, 9). To our knowledge, none of the published reports have analyzed control specimens by RT-PCR to exclude false-positive results and evaluate viral RNA detection shedding in immunocompromised adult patients during symptom-free episodes. We did not find any clinically false-positive result for RSV in the specimens taken at set symptom-free moments.
In conclusion, we have shown that TaqMan real-time PCR is a sensible and sensitive method for the rapid diagnosis of RSV infection in immunocompromised adults that can be easily implemented in a routine diagnostic setting. It represents a significant improvement over existing virus detection methods for this patient group at risk for serious RSV infection.
REFERENCES
- 1.Bowden, R. A. 1997. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am. J. Med. 102:27-30. [DOI] [PubMed] [Google Scholar]
- 2.Englund, J. A., P. A. Piedra, A. Jewell, K. Patel, B. B. Baxter, and E. Whimbey. 1996. Rapid diagnosis of respiratory syncytial virus infections in immunocompromised adults. J. Clin. Microbiol. 34:1649-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englund, J. A., C. J. Sullivan, M. C. Jordan, L. P. Dehner, G. M. Vercellotti, and H. H. Balfour, Jr. 1988. Respiratory syncytial virus infection in immunocompromised adults. Ann. Intern. Med. 109:203-208. [DOI] [PubMed] [Google Scholar]
- 4.Falsey, A. R., C. K. Cunningham, W. H. Barker, R. W. Kouides, J. B. Yuen, M. Menegus, L. B. Weiner, C. A. Bonville, and R. F. Betts. 1995. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J. Infect. Dis. 172:389-394. [DOI] [PubMed] [Google Scholar]
- 5.Falsey, A. R., M. A. Formica, and E. E. Walsh. 2002. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J. Clin. Microbiol. 40:817-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey, A. R., R. M. McCann, W. J. Hall, and M. M. Criddle. 1996. Evaluation of four methods for the diagnosis of respiratory syncytial virus infection in older adults. J. Am. Geriatr. Soc. 44:71-73. [DOI] [PubMed] [Google Scholar]
- 7.Falsey, A. R., J. J. Treanor, R. F. Betts, and E. E. Walsh. 1992. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J. Am. Geriatr. Soc. 40:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, C. B., R. G. Douglas, Jr., and J. M. Geiman. 1976. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. J. Pediatr. 89:11-15. [DOI] [PubMed] [Google Scholar]
- 9.Hall, C. B., K. R. Powell, N. E. MacDonald, C. L. Gala, M. E. Menegus, S. C. Suffin, and H. J. Cohen. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315:77-81. [DOI] [PubMed] [Google Scholar]
- 10.Hu, A., M. Colella, J. S. Tam, R. Rappaport, and S. M. Cheng. 2003. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J. Clin. Microbiol. 41:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston, S. L., and C. S. Seigel. 1991. A comparison of direct immunofluorescence, shell vial culture, and conventional cell culture for the rapid detection of influenza A and B. Diagn. Microbiol. Infect. Dis. 14:131-134. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman, P. 1997. Respiratory virus infections in bone marrow transplant recipients: the European perspective. Am. J. Med. 102:44-47. [DOI] [PubMed] [Google Scholar]
- 13.Nijhuis, M., C. A. Boucher, and R. Schuurman. 1995. Sensitive procedure for the amplification of HIV-1 RNA using a combined reverse-transcription and amplification reaction. BioTechniques 19:178-180, 182. [PubMed] [Google Scholar]
- 14.Parrott, R. H., H. W. Kim, J. O. Arrobio, D. S. Hodes, B. R. Murphy, C. D. Brandt, E. Camargo, and R. M. Chanock. 1973. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am. J. Epidemiol. 98:289-300. [DOI] [PubMed] [Google Scholar]
- 15.Smith, M. C., C. Creutz, and Y. T. Huang. 1991. Detection of respiratory syncytial virus in nasopharyngeal secretions by shell vial technique. J. Clin. Microbiol. 29:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockton, J., J. S. Ellis, M. Saville, J. P. Clewley, and M. C. Zambon. 1998. Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. J. Clin. Microbiol. 36:2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teichtahl, H., N. Buckmaster, and E. Pertnikovs. 1997. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest 112:591-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas, E. E., and L. E. Book. 1991. Comparison of two rapid methods for detection of respiratory syncytial virus (RSV) (TestPack RSV and Ortho RSV ELISA) with direct immunofluorescence and virus isolation for the diagnosis of pediatric RSV infection. J. Clin. Microbiol. 29:632-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Elden, L. J., M. Nijhuis, P. Schipper, R. Schuurman, and A. M. van Loon. 2001. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 39:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Elden, L. J., M. G. van Kraaij, M. Nijhuis, K. A. Hendriksen, A. W. Dekker, M. Rozenberg-Arska, and A. M. van Loon. 2002. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin. Infect. Dis. 34:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh, E. E., A. R. Falsey, and P. A. Hennessey. 1999. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am. J. Respir. Crit. Care Med. 160:791-795. [DOI] [PubMed] [Google Scholar]
- 22.Walsh, E. E., A. R. Falsey, I. A. Swinburne, and M. A. Formica. 2001. Reverse transcription polymerase chain reaction (RT-PCR) for diagnosis of respiratory syncytial virus infection in adults: use of a single-tube “hanging droplet” nested PCR. J. Med. Virol. 63:259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waner, J. L., N. J. Whitehurst, S. J. Todd, H. Shalaby, and L. V. Wall. 1990. Comparison of Directigen RSV with viral isolation and direct immunofluorescence for the identification of respiratory syncytial virus. J. Clin. Microbiol. 28:480-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiley, D. M., M. W. Syrmis, I. M. Mackay, and T. P. Sloots. 2002. Detection of human respiratory syncytial virus in respiratory samples by LightCycler reverse transcriptase PCR. J. Clin. Microbiol. 40:4418-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whimbey, E., R. E. Champlin, R. B. Couch, J. A. Englund, J. M. Goodrich, I. Raad, D. Przepiorka, V. A. Lewis, N. Mirza, H. Yousuf, J. J. Tarrand, and G. P. Bodey. 1996. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin. Infect. Dis. 22:778-782. [DOI] [PubMed] [Google Scholar]
- 26.Whimbey, E., R. B. Couch, J. A. Englund, M. Andreeff, J. M. Goodrich, I. I. Raad, V. Lewis, N. Mirza, M. A. Luna, B. Baxter, J. J. Tarrand, and G. P. Bodey. 1995. Respiratory syncytial virus pneumonia in hospitalized adult patients with leukemia. Clin. Infect. Dis. 21:376-379. [DOI] [PubMed] [Google Scholar]
- 27.Whimbey, E., J. A. Englund, and R. B. Couch. 1997. Community respiratory virus infections in immunocompromised patients with cancer. Am. J. Med. 102:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]