Abstract
Blood donors are often used as proxies for the general population in studies of Helicobacter pylori epidemiology. Our aim was to test if the age-specific seroprevalence rates among blood donors match with the corresponding rates in a random population sample. This descriptive study was based on sera obtained from 3,502 blood donors representing all Swedish counties and cities. An age-stratified random population sample of 1,030 from Stockholm County served as comparison. Sera were analyzed by an in-house enzyme-linked immunosorbent assay for H. pylori immunoglobulin G antibodies. In the population sample, we found the expected increase with age in the seroprevalence of H. pylori infection. This was true also among young blood donors, while the prevalence-by-age curve showed a deflection downward among blood donors who are ≥ 50 years of age. In this age group, the probability of being seropositive was reduced by 73% (95% confidence interval [CI], 63 to 81%) relative to the population sample. Overall, the adjusted odds ratio for H. pylori seropositivity among blood donors was decreased by 43% (95% CI, 28 to 55%). Thus, it appears that blood donors who are H. pylori seropositive selectively disappear from the blood donor cohort. We speculate that H. pylori-seropositive blood donors may tolerate repeated bleedings less well than do noninfected individuals and/or that the general well-being among those who are infected may be somewhat impaired. Our unexpected observation indicates that blood donors may be less suitable as proxies for the general population in analytic studies of H. pylori infection and that the underlying cause needs further study.
Helicobacter pylori is accepted as a principal cause of nonautoimmune chronic gastritis (10). Moreover, eradication of H. pylori prevents recurrence of peptic ulcers (14), and evidence for a causal role in gastric cancer is accumulating (11, 17, 30).
H. pylori infections are mainly acquired in early childhood (13). In developing countries, the seroprevalence of the infection is higher than in developed countries, where it rises gradually with age (12, 23). This latter phenomenon is considered to reflect a birth cohort effect. Hence, the higher infection prevalence in older individuals represents higher childhood infection rates in these birth cohorts rather than acquisition during adult life (1, 9).
In European epidemiological studies, blood donors are often used to represent the general population since all European countries have unpaid donors (34, 36). The appropriateness of this assumption has not been rigorously tested for H. pylori seroprevalence.
We took advantage of a large nationwide sample of blood donors, drawn for the purpose of investigating the seroprevalence of Borrelia burgdorferi infection in different parts of Sweden (28), to study also the age-specific seroprevalence of H. pylori infection, presuming that the blood donors were representative for the general population. We then compared the seroprevalence pattern among blood donors with that in a population control series consisting of sera from a representative sample of the adult population of Stockholm County.
MATERIALS AND METHODS
Subjects. (i) Blood donors.
Sera were collected from unpaid blood donors from all 25 counties and metropolitan districts of Sweden. Representativity was achieved by including all consecutive blood donors from day 1 until all the predetermined sample size had been attained. All samples were collected during the same period, i.e., from September to December of 1995. The sample size was predetermined at 30:100,000 inhabitants or at least 100 samples from each county or district. A total of 3,502 samples were obtained. Each sample represented 10 to 12 ml of blood or 4 to 5 ml of serum. Each sample was accompanied by information on the county of donation and on the age and gender of the donor. No further information on the donors was available, and the identity of the donors was erased from the records sent to the laboratory.
(ii) General population.
A stratified random sample of 1,863 individual was drawn from the computerized and continuously updated population register of Stockholm County. The primary purpose was to measure the effect of a diphtheria vaccination campaign. In total, 1,087 of the selected subjects (58.3%) volunteered for the ensuing blood sampling in 1998 to 1999 (8). Participation rates were 60% among women and 49% among men. Among subjects older than 45 years, the participation rates were 70 and 62% for women and men, respectively. Selected subjects were sent two letters of invitation to participate, and a follow-up telephone interview with some of the nonparticipants indicated that the main reasons for nonparticipation (in order of decreasing importance) were as follows: (i) unwillingness to participate, (ii) unavailability, in some cases due to traveling abroad, (iii) disease or impediment that prohibited participation, and (iv) death between selection and blood sample. No further information on the control population was available.
ELISA for immunoglobulin G antibodies.
This in-house enzyme-linked immunosorbent assay (ELISA) used sonicated H. pylori antigen, based on culture of seven clinical isolates and the NCTC 11438 strain (3). The wells of the 96-well microplates were coated at a concentration of 7 μg/ml. Sera were diluted in 1:1,000 and were tested in duplicate. Serum dilutions were made in phosphate-buffered saline (PBS), first 1:100 in PBS only and then 1:10 in PBS containing 70 mg of sonicated Campylobacter jejuni antigen/ml (five clinical isolates) to remove cross-reacting antibodies. Alkaline-phosphate conjugated anti-human immunoglobulin G (Euro Diagnostica, Malmö, Sweden) was used to detect bound antibodies. An optical density at 405 nm of 0.36 was used as a cutoff and had been established in a clinical material of 83 H. pylori culture-positive patients and 45 patients negative by microscopy, culture, and rapid urease test (3). The ELISA was found to have a sensitivity of 100% (83 of 83) and a specificity of 96% (43 of 45), established in the aforementioned 83 H. pylori culture-positive Swedes and 45 seronegative individuals (3).
Statistical methods.
First, the seroprevalence rates among blood donors and population controls were compared in 10-year age strata by using Mantel-Haenszel pooled estimates. Since blood donors and population controls represented only partly overlapping geographical areas (blood donors came from all over Sweden, while population controls came exclusively from Stockholm), we examined the importance of county and health care region in multivariate logistic regression models restricted to blood donors only. In these models, we adjusted for age (in 10-year age categories) and gender. We then fitted multivariate logistic regression models where the effect of blood donor status was estimated while controlling for age, gender, and place of residence (large cities versus other parts of Sweden). In order to avoid confounding by geographical area of residence, a further round of analyses were restricted to blood donors and population controls from Stockholm. Effect modification by age was evaluated through stratified analyses (17 to 49 and >50 years) and by introducing a term for interaction (blood donor status times age category) in the full model. We used the Logistic procedure in the SAS 6.12 statistical package (the SAS Institute, Cary, N.C.).
RESULTS
A total of 4,532 blood samples were analyzed among 3,502 blood donors (mean age ± standard deviation, 50.5 ± 12.9 years) and 1,030 population control subjects (mean age, 49.9 ± 16.7 years). Characteristics of the two study groups are given in Table 1. The mean (± standard deviation) anti-H. pylori antibody optical densities among blood donors and population controls were 0.30 (±0.40) and 0.36 (±0.45), respectively. The corresponding median values were 0.14 and 0.13.
TABLE 1.
Characteristics of blood donors and stockholm population sample
| Characteristic | No. of blood donors (% of total) | No. of individuals in Stockholm population sample (% of total) |
|---|---|---|
| n | 3,502 | 1,030 |
| Gender | ||
| Men | 2,194 (63) | 418 (41) |
| Women | 1,299 (37) | 608 (59) |
| Age (yr) | ||
| 17-19 | 32 (1) | 0 (0) |
| 20-29 | 197 (6) | 143 (14) |
| 30-39 | 473 (13) | 168 (17) |
| 40-49 | 905 (26) | 190 (19) |
| 50-59 | 964 (28) | 206 (20) |
| 60-69 | 689 (20) | 152 (15) |
| 70-79 | 231 (7) | 121 (12) |
| ≥80 | 0 (0) | 33 (3) |
| Health care region | ||
| South | 605 (17) | 0 (0) |
| West | 664 (19) | 0 (0) |
| Southeast | 352 (10) | 0 (0) |
| Stockholm/Gotland | 659 (19) | 1,030 (100) |
| Central Sweden | 773 (22) | 0 (0) |
| North | 449 (13) | 0 (0) |
| H. pylori status | ||
| Positive | 620 (18) | 261 (25) |
| Negative | 2,882 (82) | 767 (75) |
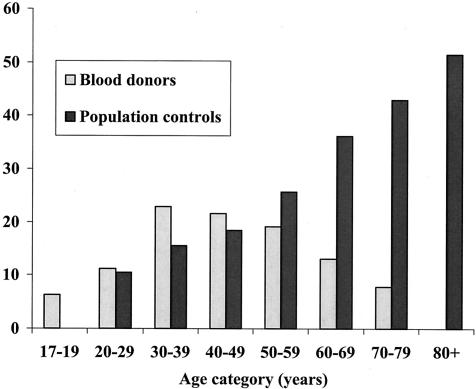
Bar charts of seroprevalence rates by age category revealed two different patterns for blood donors and population controls (Fig. 1). While the seroprevalence increased with age in the youngest age groups both among blood donors and in the population sample (albeit the rates were somewhat higher among the former), the curve for the blood donors deflected after ages 40 to 49. After that age, the seroprevalence fell with age. In the population sample, on the other hand, the seroprevalence increased monotonically with age throughout the whole age range. This difference was highly significant; the Mantel-Haenszel pooled-odds ratio for being seropositive among blood donors was 0.65 (95% confidence interval, 0.55 to 0.77), relative to the population sample (P = 0.001).
FIG. 1.
Prevalence (%) of antibodies to H. pylori by age among blood donors and in a population sample.
Since blood donors and population controls represented populations that overlapped only in Stockholm, we examined the importance of geographical area of residence in a multivariate logistic regression model with adjustment for age and gender. This analysis was restricted to blood donors only. Table 2 shows that the area of residence was of minor importance for the risk of being seropositive. Although the odds ratio for seropositivity tended to be somewhat lower in central Sweden and somewhat higher in northern Sweden, none of the estimates attained statistical significance. We also observed an almost statistically significant gender difference in H. pylori seroprevalence. The odds ratio for being seropositive was 0.85 (95% confidence interval [CI], 0.70 to 1.02) among women relative to men. A similar difference was seen also in the population sample. There, the odds ratio among women was 0.77 (95% CI, 0.57 to 1.04).
TABLE 2.
Multivariate analysis of the effects of gender, age, and geographical area of residence on the likelihood of being H. pylori seropositiveb
| Determinant | No. of positive donors/total (%) | Odds ratioa | 95% CI |
|---|---|---|---|
| Gender | |||
| Men | 412/2,192 (19) | 1 | Reference |
| Women | 207/1,298 (16) | 0.85 | 0.70-1.02 |
| Age (yr) | |||
| 17-19 | 2/32 (6) | 0.24 | 0.06-1.02 |
| 20-29 | 22/197 (11) | 0.48 | 0.30-0.77 |
| 30-39 | 108/473 (23) | 1.08 | 0.82-1.41 |
| 40-49 | 195/905 (22) | 1 | Reference |
| 50-59 | 184/964 (19) | 0.86 | 0.69-1.08 |
| 60-69 | 90/689 (13) | 0.55 | 0.42-0.72 |
| 70-79 | 18/231 (8) | 0.31 | 0.19-0.51 |
| Health care region | |||
| South | 117/605 (19) | 1 | Reference |
| West | 114/664 (17) | 0.91 | 0.68-1.22 |
| Southeast | 63/352 (18) | 0.91 | 0.65-1.28 |
| Stockholm/Gotland | 111/659 (17) | 0.93 | 0.69-1.24 |
| Central Sweden | 120/773 (16) | 0.78 | 0.59-1.04 |
| North | 95/449 (21) | 1.22 | 0.90-1.67 |
Mutually adjusted for other variables in table.
The analysis is restricted to blood donors only. All estimates are mutually adjusted.
Finally, we estimated the odds ratio for H. pylori seropositivity associated with blood donor status, relative to the population sample. In these multivariate analyses, performed on the entire material (both blood donors and population controls), the estimates were adjusted for gender, age (in 10-year classes), and place of residence (cities of >500,000 inhabitants versus the rest of Sweden). Overall, the adjusted odds ratio for H. pylori seropositivity among blood donors was decreased by 43% (95% CI, 28 to 55%), relative to population controls (Table 3, first section, left column). In stratified analyses, it was revealed that the effect of blood donor status was modified by age (Table 3, first section, columns 2 and 3). In the age stratum of 17 to 49 years, blood donor status was associated with a 48% (95% CI, 2 to 113%) increased risk of H. pylori seropositivity, although this increase was only of borderline significance. Among subjects who were ≥ 50 years of age, the risk of being seropositive for blood donors was reduced by 73% (95% CI, 63 to 81%), relative to the risk in the population sample. This latter interaction was highly statistically significant (P = 0.0001). In order to ensure against confounding by place of residence, we repeated the analyses in the subset of 513 blood donors and 1,030 population controls living in Stockholm. The results were almost identical to those obtained in the entire material (Table 3, second section).
TABLE 3.
Adjusted odds ratios for being H. pylori seropositive, overall and in analyses stratified by age
| Study group | Data for:
|
|||||
|---|---|---|---|---|---|---|
| Donors of all ages
|
Donors aged 17-49 yr
|
Donors aged ≥50 yr
|
||||
| ORa | 95% CI | OR | 95% CI | OR | 95% CI | |
| Entire country | ||||||
| Population control | 1 | Reference | 1 | Reference | 1 | Reference |
| Blood donors | 0.57 | 0.45-0.72 | 1.48 | 1.02-2.13 | 0.27 | 0.19-0.37 |
| Stockholmers | ||||||
| Population control | 1 | Reference | 1 | Reference | 1 | Reference |
| Blood donors | 0.57 | 0.44-0.76 | 1.47 | 0.98-2.20 | 0.26 | 0.18-0.39 |
Odds ratio adjusted for gender, age (in 10-year classes), and place of residence (big cities versus rest of Sweden).
DISCUSSION
The most salient finding in our cross-sectional data was a paradoxical drop in H. pylori seroprevalence with age among Swedish blood donors over the age of 50 years, who thus markedly differ from the general population with the same age and gender. The seroprevalence among younger blood donors, on the other hand, was close to, or even somewhat higher than, the age-specific seroprevalence in the general population.
Other studies conducted among blood donors in developed countries have usually not revealed a decline in H. pylori seroprevalence similar to the one observed by us (5, 7, 15, 20, 26, 33-36). A closer inspection of the previous data, however, unveils lower-than-expected seroprevalence rates above the age of 50 in several studies, (5, 34, 36), but few of them included subjects who were older than 60. In the ages below 60, the deviant pattern was less clear also in our study. An Italian study (31) that included older blood donors found the same seroprevalence pattern as in our study.
The most simplistic explanation for our findings is that blood donations on a regular basis favor disappearance of the infection. This appears biologically implausible, although empirical data are lacking. Removal of antibodies in blood donors could be another possibility but has not been found for other infections. The sample showed no corresponding decrease in the prevalence of antibodies to B. burgdorferi (28).
A biased distribution in socioeconomic background would result in a biased H. pylori prevalence but not in a decline with age (12, 23). A successive shift over time in the socioeconomic status of newly recruited blood donors, from low to high, would theoretically lead to lower prevalence rates with increasing age of the donors. However, the prevalence gradient across successive birth cohorts is likely to be steeper than that across socioeconomic strata. Thus, a shift in the socioeconomic status distribution, if any, would probably not have more than marginal impact on the birth cohort effect, the latter resulting in increasing prevalence rates with age. This argument will also counter another possible variant of selection bias, namely, that only socioeconomically privileged blood donors will remain active after age 50. We therefore tentatively refute this explanation, too.
A third alternative explanation would be that subjects who remain active blood donors for the longest time are those with rare and coveted blood groups and that some of these blood groups may be associated with a lower risk of H. pylori colonization. Published data, however, consistently refute any important associations between blood group and H. pylori prevalence (16, 19, 21, 22, 24, 27). Therefore, this explanation is unlikely.
The fourth, and in our view most probable, explanation is that infected individuals are preferentially removed from the blood donor cohort as they get older. There could be several possible reasons for this selective removal: one would remove them if they are likelier than noninfected individuals to develop laboratory test abnormalities that result in active exclusion. H. pylori could potentially affect iron metabolism by causing ulcer-related occult bleeding or impaired absorption of nonheme iron or by scavenging heme iron or ferritin (2, 6). Low serum iron and/or ferritin values occur more frequently in infected than in noninfected adults (4, 25, 29). It is conceivable that the H. pylori-induced interference with normal iron metabolism will become clinically overt more readily in blood donors than in people without similar blood losses. H. pylori also seems to be a causative agent in the development of adult vitamin B12 deficiency (18). However, this reason does not hold up, since few blood donors develop persistent anemia and few are excluded for that reason (J. Lundahl, Department of Clinical Immunology and Transfusion Medicine, Karolinska Hospital, personal communication).
The most common reason for exclusion among older Swedish blood donors is that they themselves decide to stop giving blood. The reason why is not fully understood, but we hypothesize that H. pylori-infected subjects may feel less well after donations than do noninfected blood donors and that the recovery time may be prolonged. Moreover, if H. pylori infection is causally or noncausally linked to other severe diseases such as coronary heart disease, then these diseases will also contribute to a selective disappearance of infected individuals from the blood donor category.
Our study confirmed the previously reported gender difference (32) with a tendency towards higher H. pylori prevalence in men than in women, both among blood donors and in the population sample. Although not quite reaching statistical significance, women had an approximately 20% lower probability of being infected than did men. A recent meta-analysis (32) showed that only in two studies out of six was the H. pylori seroprevalence higher in women than in men and that the summary odds ratio for seropositivity among men, relative to women, was 1.2 (95% CI, 1.02 to 1.4). Interestingly, the gender difference has not been seen in children, an observation also confirmed in our recent study of 10- to 12-year-old Swedish schoolchildren (13). The reasons behind the gender difference among adults is unlikely to be due to a preferential eradication treatment in men since screening and eradication, at the time of the drawing of study samples, were little practiced in Sweden. The gender difference may account in part for the increased incidence of H. pylori-related diseases (peptic ulcer and stomach cancer) among men in later decades of life (32).
Strengths of our study include representativity of our sample vis-à-vis Swedish blood donors. As an indication of this representativity, the sample showed the expected geographical variation as well as age- and sex-dependent variability in B. burgdorferi seroprevalence (28). Another strength is our truly population-based comparison sample. The low participation rate may raise concerns about possible selection bias. The control population did, however, show exactly the expected cohort effect described in numerous studies from developed countries (12, 23). Also, in the age groups where our important findings were made (>45 years), the participation rate (66%) was acceptable.
In conclusion, our data, supported by findings in other studies, are consistent with a falling prevalence of H. pylori infection with age among elderly blood donors. The reasons for this paradoxical decrease remain conjectural, but the most reasonable explanation is that H. pylori-positive subjects are preferentially removed from the blood donor cohort. Since active exclusions due to persistent anemia are uncommon, our results imply that elderly individuals infected with H. pylori may tolerate repeated bleedings less well than do noninfected individuals and/or that the general well-being among those who are infected may be somewhat impaired. This observation ought to prompt more studies of the role of H. pylori in the general health status of elderly people. And our findings emphasize the dangers of using blood donor data in lieu of epidemiological sound, population-based observations.
Acknowledgments
This work was supported by the Swedish Society of Medicine and by the Richard and Ruth Juhlins Foundation.
REFERENCES
- 1.Banatvala, N., K. Mayo, F. Megraud, R. Jennings, J. J. Deeks, and R. A. Feldman. 1993. The cohort effect and Helicobacter pylori. J. Infect. Dis. 168:219-221. [DOI] [PubMed] [Google Scholar]
- 2.Barabino, A. 2002. Helicobacter pylori-related iron deficiency anemia: a review. Helicobacter 7:71-75. [DOI] [PubMed] [Google Scholar]
- 3.Befrits, R., M. Granström, M. Rylander, and C. Rubio. 1993. Helicobacter pylori in 205 consecutive endoscopy patients. Scand. J. Infect. Dis. 25:185-191. [DOI] [PubMed] [Google Scholar]
- 4.Berg, G., G. Bode, M. Blettner, H. Boeing, and H. Brenner. 2001. Helicobacter pylori infection and serum ferritin: a population-based study among 1806 adults in Germany. Am. J. Gastroenterol. 96:1014-1018. [DOI] [PubMed] [Google Scholar]
- 5.Bergenzaun, P., K. G. Kristinsson, B. Thjodleifsson, E. Sigvaldadottir, S. Molstad, M. Held, and T. Wadström. 1996. Seroprevalence of Helicobacter pylori in south Sweden and Iceland. Scand J. Gastroenterol. 31:1157-1161. [DOI] [PubMed] [Google Scholar]
- 6.Bini, E. J. 2001. Helicobacter pylori and iron deficiency anemia: guilty as charged? Am. J. Med. 111:495-497. [DOI] [PubMed] [Google Scholar]
- 7.Breuer, T., T. Sudhop, J. Hoch, T. Sauerbruch, and P. Malfertheiner. 1996. Prevalence of and risk factors for Helicobacter pylori infection in the western part of Germany. Eur. J. Gastroenterol. Hepatol. 8:47-52. [DOI] [PubMed] [Google Scholar]
- 8.Christenson, B., U. Hellström, S. P. Sylvan, L. Henriksson, and M. Granström. 2000. Impact of a vaccination campaign on adult immunity to diphtheria. Vaccine 19:1133-1140. [DOI] [PubMed] [Google Scholar]
- 9.Cullen, D. J., B. J. Collins, K. J. Christiansen, J. Epis, J. R. Warren, I. Surveyor, and K. J. Cullen. 1993. When is Helicobacter pylori infection acquired? Gut 34:1681-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon, M. F., and G. M. Sobala. 1992. Gastritis and duodenitis: histopathological spectrum. Eur. J. Gastroenterol. Hepatol. 4(Suppl. 2):17-23. [Google Scholar]
- 11.Ekström, A. M., M. Held, L. E. Hansson, L. Engstrand, and O. Nyrén. 2001. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology 121:784-791. [DOI] [PubMed] [Google Scholar]
- 12.The EUROGAST Study Group. 1993. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut 34:1672-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granström, M., Y. Tindberg, and M. Blennow. 1997. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J. Clin. Microbiol. 35:468-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentschel, E., G. Brandstätter, B. Dragosics, A. M. Hirschl, H. Nemec, K. Schutze, M. Taufer, and H. Wurzer. 1993. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and recurrence of duodenal ulcer. N. Engl. J. Med. 328:308-312. [DOI] [PubMed] [Google Scholar]
- 15.Holtmann, G., N. J. Talley, H. Mitchell, and S. Hazell. 1998. Antibody response to specific H. pylori antigens in functional dyspepsia, duodenal ulcer disease, and health. Am. J. Gastroenterol. 93:1222-1227. [DOI] [PubMed] [Google Scholar]
- 16.Hook-Nikanne, J., P. Sistonen, and T. U. Kosunen. 1990. Effect of ABO blood group and secretor status on the frequency of Helicobacter pylori antibodies. Scand. J. Gastroenterol. 25:815-818. [DOI] [PubMed] [Google Scholar]
- 17.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1994. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 61:1-241. [PMC free article] [PubMed] [Google Scholar]
- 18.Kaptan, K., C. Beyan, A. U. Ural, T. Cetin, F. Avcu, M. Gulen, R. Finci, and A. Yalcin. 2000. Helicobacter pylori—is it a novel causative agent in Vitamin B12 deficiency? Arch. Intern. Med. 160:1349-1353. [DOI] [PubMed] [Google Scholar]
- 19.Kopanski, Z., E. Golec, B. Witkowska, E. Slowakiewicz, A. Migas-Nirska, and A. Cienciala. 1996. The relationship between the frequency of the appearance of IgG against Helicobacter pylori and the main blood groups among patients with ulcer sickness and stomach cancer. Eur. J. Med. Res. 1:280-282. [PubMed] [Google Scholar]
- 20.Kosunen, T. U., J. Hook, H. I. Rautelin, and G. Myllyla. 1989. Age-dependent increase of Campylobacter pylori antibodies in blood donors. Scand. J. Gastroenterol. 24:110-114. [DOI] [PubMed] [Google Scholar]
- 21.Martin de Argila, C., D. Boixeda, S. Valdezate, N. Mir, R. Barcena, J. P. Gisbert, A. Garcia Plaza, and R. Canton. 1998. ABO blood groups, rhesus factor and Helicobacter pylori. Rev. Esp. Enferm. Dig. 90:263-268. [PubMed] [Google Scholar]
- 22.McKeown, I., P. Orr, S. Macdonald, A. Kabani, R. Brown, G. Coghlan, M. Dawood, J. Embil, M. Sargent, G. Smart, and C. N. Bernstein. 1999. Helicobacter pylori in the Canadian arctic: seroprevalence and detection in community water samples. Am. J. Gastroenterol. 94:1823-1829. [DOI] [PubMed] [Google Scholar]
- 23.Megraud, F., M. P. Brassens-Rabbe, F. Denis, A. Belbouri, and D. Q. Hoa. 1989. Seroepidemiology of Campylobacter pylori infection in various populations. J. Clin. Microbiol. 27:1870-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentis, A., C. C. Blackwell, D. M. Weir, C. Spiliadis, A. Dailianas, and N. Skandalis. 1991. ABO blood group, secretor status and detection of Helicobacter pylori among patients with gastric or duodenal ulcers. Epidemiol. Infect. 106:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milman, N., S. Rosenstock, L. Andersen, T. Jorgensen, and O. Bonnevie. 1998. Serum ferritin, hemoglobin, and Helicobacter pylori infection: a seroepidemiologic survey comprising 2794 Danish adults. Gastroenterology 115:268-274. [DOI] [PubMed] [Google Scholar]
- 26.Nandurkar, S., N. J. Talley, H. Xia, H. Mitchell, S. Hazel, and M. Jones. 1998. Dyspepsia in the community is linked to smoking and aspirin use but not to Helicobacter pylori infection. Arch. Intern. Med. 158:1427-1433. [DOI] [PubMed] [Google Scholar]
- 27.Oberhuber, G., A. Kranz, C. Dejaco, B. Dragosics, I. Mosberger, W. Mayr, and T. Radaszkiewicz. 1997. Blood groups Lewis(b) and ABH expression in gastric mucosa: lack of inter-relation with Helicobacter pylori colonisation and occurrence of gastric MALT lymphoma. Gut 41:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell, S., M. Granström, J. S. Gray, and G. Stanek. 1998. Epidemiology of European Lyme borreliosis. Zentbl. Bakteriol. 287:229-240. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson, A. J., B. D. Gold, L. Bulkow, R. B. Wainwright, B. Swaminathan, B. Khanna, K. M. Petersen, and M. A. Fitzgerald. 2000. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin. Diagn. Lab. Immunol. 7:885-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreic, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 31.Pellicano, R., M. G. Mazzarello, S. Morelloni, M. Allegri, V. Arena, M. Ferrari, M. Rizzetto, and A. Ponzetto. 1999. Acute myocardial infarction and Helicobacter pylori seropositivity. Int. J. Clin. Lab. Res. 29:141-144. [DOI] [PubMed] [Google Scholar]
- 32.Replogle, M. L., S. L. Glaser, R. A. Hiatt, and J. Parsonnet. 1995. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am. J. Epidemiol. 142:856-863. [DOI] [PubMed] [Google Scholar]
- 33.Russo, A., M. Eboli, P. Pizzetti, G. Di Felice, F. Ravagnani, P. Spinelli, A. M. Hotz, P. Notti, G. Maconi, S. Franceschi, D. Ferrari, and L. Bertario. 1999. Determinants of Helicobacter pylori seroprevalence among Italian blood donors. Eur. J. Gastroenterol. Hepatol. 11:867-873. [DOI] [PubMed] [Google Scholar]
- 34.Vaira, D., M. Miglioli, P. Mule, J. Holton, M. Menegatti, M. Vergura, G. Biasco, R. Conte, R. P. Logan, and L. Barbara. 1994. Prevalence of peptic ulcer in Helicobacter pylori positive blood donors. Gut 35:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagtmans, M. J., A. M. Witte, D. R. Taylor, I. Biemond, R. A. Veenendaal, H. W. Verspaget, C. B. Lamers, and R. A. van Hogezand. 1997. Low seroprevalence of Helicobacter pylori antibodies in historical sera of patients with Crohn's disease. Scand. J. Gastroenterol. 32:712-718. [DOI] [PubMed] [Google Scholar]
- 36.Wilhoite, S. L., D. A. Ferguson, Jr., D. R. Soike, J. H. Kalbfleisch, and E. Thomas. 1993. Increased prevalence of Helicobacter pylori antibodies among nurses. Arch. Intern. Med. 153:708-712. [PubMed] [Google Scholar]