In 1994, the U.S. Cystic Fibrosis Foundation sponsored a consensus conference (33) on microbiology and infectious disease in patients with cystic fibrosis (CF), a homozygous recessive disease in individuals of Caucasian descent. The organization of that conference followed the recognition that the discovery of the CF gene in 1989 was not going to yield a quick cure for CF and that chronic pulmonary infection was the primary culprit in the early mortality associated with this disease. The resulting document made recommendations for the detection, isolation, identification, and susceptibility testing of organisms recognized as significant or potentially significant in the lung diseases of this patient population. The microbial species that are clearly associated with lung disease in CF patients (CF lung disease) are relatively few, including Staphylococcus aureus, Pseudomonas aeruginosa, and Burkholderia cepacia complex (Table 1). Organisms having a secondary role in CF lung disease include respiratory viruses, such as respiratory syncytial virus and influenza virus; Haemophilus influenzae; and Aspergillus fumigatus. Mycobacterium spp. but not Mycobacterium tuberculosis, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans are being seen with increasing frequencies in CF patients, in part because of the increasing life spans of CF patients and the relentless use of antimicrobial agents in this patient population. However, the role of the latter organisms in CF lung disease has not been clearly determined. In addition, organisms that phenotypically resemble B. cepacia complex organisms (i.e., Burkholderia gladioli, Ralstonia spp., and Pandoraea spp.) are also being seen with increasing frequencies due to the use of selective media that improve the rates of recovery of B. cepacia complex isolates. These media also improve the rates of recovery of these other species. Although there are no data that suggest that these non-B. cepacia complex organisms play a role in CF lung disease, these organisms are difficult to differentiate phenotypically from B. cepacia complex. As we will review, misidentification of these seemingly harmless saprophytes as B. cepacia complex organisms may have profound consequences for the patient. This minireview discusses the state of the art in the laboratory diagnosis of the infectious agents associated with CF lung disease.
TABLE 1.
Potential pathogens and related organisms that can be detected in the respiratory tracts of CF patients
| Organism | Frequency (%) of isolation | % of laboratories that culturea | CF population | Role in CF lung disease |
|---|---|---|---|---|
| P. aeruginosa | 59b | 95 | All | Proven |
| S. aureus | 48 | 100 | Predominantly children and adolescents | Proven |
| B. cepacia complex | 3 | 98 | Predominantly adolescents and adults | Proven |
| S. maltophilia | 8.40 | 97 | Predominantly adolescents and adults | Unknown |
| A. xylosoxidans | unknown | NRc | Predominantly adolescents and adults | Unknown |
| B. gladioli | <1 | NR | Predominantly adolescents and adults | Unlikely |
| Ralstonia spp. | <1 | NR | Predominantly adolescents and adults | Unlikely |
| Pandoraea spp. | <1 | NR | Predominantly adolescents and adults | Unlikely |
| Mycobacterium spp. but not M. tuberculosis | 13d | NR | Predominantly adolescents and adults | Provend |
| H. influenzae | 15b | 100 | Children | Likely |
| A. fumigatus | 9e | 100 | All | Provenf |
| Respiratory syncyntial virus | unknown | NR | Children | Proven |
| Influenza virus | unknown | NR | All | Proven |
Percentage of laboratories that culture specimens from CF patients for the specific organism either routinely or by request. Data are from reference 34.
The data are from reference 11.
NR, not reported.
Requires high organism loads, as evidenced by three positive cultures or two positive cultures with a positive smear (30).
The datum is from reference 10.
Allergic bronchopulmonary aspergillosis.
STAPHYLOCOCCUS AUREUS
S. aureus was the first pulmonary pathogen recognized in patients with CF. During the preantibiotic era, the organism was frequently found in the lungs of young children at autopsy. With the development of antistaphylococcal penicillins, the rates of morbidity and particularly the rates of mortality due to S. aureus declined (14). S. aureus continues to be a frequently encountered pathogen, recovered from approximately 50% of CF patients (11). Laboratorians should be familiar with two issues concerning S. aureus in this patient population: the decreased reliability of S. aureus recovery and the frequency of oxacillin-resistant isolates.
It has been recognized for close to 20 years that auxotrophic forms of S. aureus are recovered with increased frequencies from the respiratory tracts of CF patients (15). Individuals harboring these auxotrophic isolates generally have received long-term antistaphylococcal therapy, typically trimethoprim-sulfamethoxazole. In the earlier CF literature, these organisms were described as thymidine-dependent isolates. More recent literature describes these auxotrophic organisms as small-colony variants (22). Kahl and colleagues (22) reported that small-colony variants could be recovered from up to 50% of CF patients harboring S. aureus. These organisms yield small, nonhemolytic, nonpigmented, slowly growing colonies on enriched media such as sheep blood or chocolate agars, thus making these isolates difficult to recognize as S. aureus. The selective medium mannitol salts agar supports the growth of S. aureus auxotrophs and prevents the overgrowth of S. aureus isolates by gram-negative rods such as P. aeruginosa and B. cepacia complex. Thus, mannitol salts agar should be used for the recovery of S. aureus in cultures of all respiratory tract specimens from CF patients.
Oxacillin-resistant strains are being seen with increasing frequency in CF patients. Approximately 6% of S. aureus isolates recovered from CF patients nationally are oxacillin resistant, with some centers (including our own) reporting that ∼20% of isolates are resistant (11). Molecular epidemiology studies show that CF patients harboring oxacillin-resistant S. aureus isolates frequently acquire the isolate while they are hospitalized. Some patients become chronically infected with these organisms, while others may be only transiently infected (16).
PSEUDOMONAS AERUGINOSA
P. aeruginosa is the most important pathogen of CF lung disease, infecting approximately 60% of the entire CF population and close to 80% of adolescents and adults (26). Studies have shown that P. aeruginosa infections in CF patients can be seen as early as infancy (4). The initial strains of P. aeruginosa that infect CF patients are described as “rough” or “planktonic” strains. These strains tend to be sensitive to a variety of antimicrobials, are motile and prototrophic, and have smooth lipopolysaccharide. It is at this stage that some investigators believe that aggressive antimicrobial therapy can eradicate this organism (13). However, most patients develop chronic infection with an unusual phenotype of P. aeruginosa referred to as “mucoid” (Fig. 1). Mucoid isolates are nonmotile, are frequently auxotrophic, have rough lipopolysaccharide, and are frequently resistant to a wide variety of antimicrobial agents (37). Mucoid strains of P. aeruginosa grow as biofilms in the airways of CF patients. Examination of sputa from patients chronically infected with mucoid strains of P. aeruginosa reveal gram-negative rods in small clusters surrounded by amorphous material that stains Gram negative. This material is a polysaccharide polymer referred to as alginate, which forms the biofilm matrix and renders the embedded Pseudomonas organisms refractory to clearance by the immune system (14). The presence of mucoid strains of P. aeruginosa signals the beginning of the chronic phase of infection (26). The chronic phase of infection due to P. aeruginosa is characterized by pulmonary exacerbations (fever, elevated white blood cell count, increased sputum production, and decreased pulmonary function) that require antimicrobial therapy. CF exacerbations are typically interspersed with intervening periods of relative quiescence, with each phase lasting various lengths of time. However, lung function continuously declines, the infecting strains become increasingly resistant, and inevitably, the patient succumbs to cardiopulmonary failure. There is a growing consensus that the lung pathology that occurs during chronic P. aeruginosa infection is due to a large extent to the immune response directed against pseudomonal biofilms. High levels of cytokines and leukocyte-derived proteases can be detected in airway fluid from CF patients and are believed to be responsible for much of the lung damage that occurs in this patient population (26).
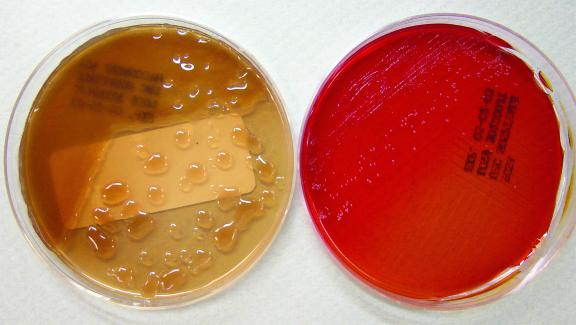
FIG. 1.
Use of BCSA improves recovery of B. cepacia complex. (Left) MacConkey agar demonstrating overgrowth by a mucoid strain of P. aeruginosa; (right) BCSA showing the growth of B. cenocepacia (genomovar III) that cannot be visualized on the corresponding MacConkey agar.
Isolation of P. aeruginosa from the respiratory secretions of CF patients is easily accomplished, with both rough and mucoid isolates being readily recovered on agar selective for gram-negative organisms, such as MacConkey and eosin-methylene blue agars. Presumptive identification is relatively easily accomplished on the basis of a positive oxidase test, pigment production, growth at 42°C, and, in some cases, mucoid colony morphology. However, as chronic infection progresses, some strains lose the ability to produce pigments as well as other phenotypic characteristics. The loss of these phenotypes is most likely an evolutionary process in which the corresponding genes are either down-regulated or lost in a nutrient-rich environment. As a result of this evolutionary change, it may become difficult to identify these strains of P. aeruginosa with commercial identification systems. As many as 15% of P. aeruginosa strains recovered from CF patients could not be accurately identified with the MicroScan Autoscan system (Dade International Inc., West Sacramento, Calif.), even after extended (48-h) incubation (31). Interestingly, in that study many isolates were misidentified as Alcaligenes spp., an organism that can be recovered from the sputum of CF patients but whose clinical significance has not been determined (31). These data suggest that the use of commercial systems should be applied cautiously to the identification of suspected P. aeruginosa isolates.
Susceptibility testing of P. aeruginosa isolates recovered from CF patients is an area of some controversy. Multiple morphotypes may be recovered from patient sputum. Studies that compared the performance of susceptibility testing of a mixture of different morphotypes versus the performance of testing of each morphotype individually suggest that testing of mixed morphotypes may underestimate resistance (29). It is unclear how widespread the practice of testing individual morphotypes is in laboratories that culture specimens from CF patients. Recent investigations suggest that a variety of commercial susceptibility testing systems, E-test, and disk diffusion work well compared to a reference MIC method for determination of the susceptibilities of P. aeruginosa isolates recovered from CF patients (5, 6).
The impacts of two areas of investigation on P. aeruginosa susceptibility testing and interpretation have not been resolved. The first is a study that suggests that P. aeruginosa grows anaerobically within the airways of CF patients (41). If that is true, aminoglycosides should not be active against those organisms, regardless of their in vitro activities. This directly conflicts with numerous data in the CF literature that both aerosolized and intravenously administered aminoglycosides have a positive impact on the lung functions and life expectancies of CF patients (7). However, anaerobically growing P. aeruginosa isolates may contribute to this organism's ability to persist in the lungs of CF patients in the face of high concentrations of aminoglycosides. The second area of investigation has been determination of the microbial form of P. aeruginosa that should be used for susceptibility testing. Recently, equipment called the “Calgary device” has been developed that allows susceptibility testing to be performed with mucoid P. aeruginosa isolates growing as biofilms. The susceptibilities of mucoid P. aeruginosa isolates growing as biofilm have been compared to those of strains growing planktonically, which are used in clinical laboratory susceptibility testing. Biofilm strains were significantly more resistant to antipseudomonal drugs. The number of isolates tested was quite small, and no clinical correlations were presented. However, these data suggest that susceptibility results obtained by testing planktonically growing isolates may underestimate the drug resistance of mucoid P. aeruginosa (1).
BURKHOLDERIA CEPACIA COMPLEX AND RELATED ORGANISMS
B. cepacia was first described as a significant pathogen among CF patients in 1984 (21). Subsequent studies have shown that B. cepacia includes at least nine genomovars, or genomic species, collectively referred to as B. cepacia complex. Although only ∼3% of American CF patients and ∼15% of the Canadian CF population are colonized with B. cepacia complex, the repercussions of infection are extensive. In CF patients, infection with B. cepacia complex is associated with increased rates of morbidity and mortality. Roughly 20% of CF patients colonized with B. cepacia complex develop the “cepacia syndrome” (36). These patients experience a rapid decline in pulmonary function, frequent bacteremia, and, ultimately, death due to lung failure. Of additional concern is the person-to-person transmission of B. cepacia complex among CF patients. Person-to-person spread has been documented within CF centers, regionally, and intercontinentally (9). Infection with B. cepacia complex can have devastating consequences for CF patients, as colonized patients are excluded from social events and scientific conferences for CF patients and are rejected as potential lung transplant recipients at many CF centers due to potentially poor outcomes (8). Therefore, it is crucial that laboratory identification of B. cepacia complex from specimens from CF patients be performed with utmost accuracy.
Unfortunately, the diversity of B. cepacia complex organisms has made precise isolation and identification difficult. Genomovars are organisms that are genetically distinct but that are difficult to distinguish phenotypically and therefore may be differentiated only by molecular testing. However, eight of the nine genomovars have been given a species designation on the basis of phenotypic or genomic characterization. A summary of the nine genomovars, including their species designations and potential roles in CF disease, is provided in Table 2 (38). Although genomovars I to VIII have each been isolated from the lungs of CF patients, it is interesting that only Burkholderia multivorans (genomovar II) and Burkholderia cenocepacia (genomovar III) have a distinct correlation with CF lung disease. Together, B. multivorans and B. cenocepacia constitute over 85% of B. cepacia complex isolates recovered from CF patients, with B. cenocepacia representing ∼50% (18). In addition, a B. cenocepacia clone expressing a rare adhesin called cable pilin is associated with increased transmission and was the clone identified in intercontinental spread (35). A poor prognosis posttransplantation is also associated with B. cenocepacia infection (2). These data suggest that only patients harboring B. cenocepacia (genomovar III) should be excluded from lung transplantation.
TABLE 2.
B. cepacia complex
| Genomovar | Species designation | Role in CF lung disease |
|---|---|---|
| I | B. cepacia | Unlikely |
| II | B. multivorans | Proven |
| III | B. cenocepacia | Proven |
| IV | B. stabilis | Unlikely |
| V | B. vietnamiensis | Unlikely |
| VI | Unnamed | Unlikely |
| VII | B. ambifaria | Unlikely |
| VIII | B. anthina | Unlikely |
| IX | B. pyrrocinia | Unknown |
Isolation of B. cepacia complex from respiratory secretions from CF patients has improved with the use of selective media. Three media, Pseudomonas cepacia (PC) agar, oxidative-fermentative base, polymyxin B, bacitracin, and lactose (OFPBL) agar, and B. cepacia selective agar (BCSA), are used to recover B. cepacia complex isolates from respiratory specimens from CF patients. These media inhibit the growth of other potential pathogens of CF patients, such as P. aeruginosa, which often grows more rapidly and in larger quantities than members of B. cepacia complex and therefore may mask its presence (Fig. 1). A multicenter analysis of the three selective media demonstrated increased sensitivity and specificity of BCSA over both PC agar and OFPBL agar in the recovery of B. cepacia complex. Therefore, BCSA is the preferred medium for isolation of B. cepacia complex (19).
Once isolated, B. cepacia complex poses the problem of accurate identification. In addition to the challenge of distinguishing between B. cepacia genomovars, differentiation must be made between B. cepacia complex and other phenotypically similar organisms, such as B. gladioli, Ralstonia spp., and Pandoraea spp. Although these B. cepacia-like organisms have been isolated in cultures of respiratory specimens from CF patients (28), at present, no data that support their role as pulmonary pathogens in this patient population.
Typical identification schemes for B. cepacia complex include the use of selective media along with conventional biochemical analysis and/or commercial bacterial identification systems. Growth on BCSA should not be used to definitively identify B. cepacia complex, as B. gladioli and Ralstonia species can also grow on BCSA. However, the use of BCSA provides a first screen for the presence of B. cepacia-like organisms in cultures of respiratory specimens from CF patients, and further characterization of these isolates should follow. B. gladioli, Ralstonia spp., and Pandoraea spp. can be differentiated from B. cepacia by biochemical analysis, but phenotypic variation within each species may cause identification difficulties. Conventional biochemical tests can also differentiate several B. cepacia genomovars, but similarly, phenotypic variation within each genomovar makes biochemical analysis unreliable as a sole identification scheme. Although genetically distinct, genomovars I and III cannot be separated phenotypically, and genomovars II and VI are phenotypically indistinguishable. Commercial identification systems are inadequate for differentiation among B. cepacia complex genomovars and often cannot distinguish B. cepacia complex members from B. cepacia-like organisms. Commercial test system identification of B. cepacia should be corroborated with growth on selective media and a supplemental biochemical battery.
Definitive identification of B. cepacia genomovars relies on molecular analysis. Differentiation of B. cepacia complex from other similar organisms can be accomplished by whole-cell protein analysis, whole-cell fatty acid analysis, and/or 16S rRNA gene sequencing. To identify B. cepacia to the genomovar level molecularly, techniques such as amplified fragment length polymorphism fingerprinting or recA restriction fragment length polymorphism analysis must be applied. Of interest, recent research has demonstrated progress toward direct detection of B. cepacia genomovars in the sputum of CF patients by PCR (12, 27). Although these techniques are often not suitable for use in the clinical laboratory, molecular identification of presumed B. cepacia isolates is available through the Cystic Fibrosis Foundation B. cepacia Research Laboratory and Repository (J. J. LiPuma, University of Michigan Medical School).
B. cepacia complex is commonly panresistant to antimicrobial agents, including cephalosporins and aminoglycosides, therefore making treatment challenging. High-level resistance to multiple antibiotics by B. cepacia complex is achieved through a combination of mechanisms, including selective cell wall permeability, cellular target alteration, enzymatic inactivation of antibiotics, and drug efflux pumps. B. cepacia strains that have not been challenged with antibiotics are typically susceptible only to piperacillin, piperacillin-tazobactam, cefoperazone, ceftazidime, chloramphenicol, and trimethoprim-sulfamethoxazole, with variable susceptibilities to imipenem and meropenem. Remarkably, strains isolated after multiple antimicrobial treatments are often resistant to all known antibiotics. Thus, eradication of B. cepacia complex infections is difficult, if not impossible, making prevention of infection via aggressive infection control practices critical. For example, CF clinics often schedule appointments for patients colonized with B. cepacia complex either at the end of the day or on different clinic days or at different locales, therefore limiting contact with other outpatients. It is also recommended that CF patients not share inpatient rooms and that B. cepacia-infected CF inpatients not have direct contact with non-B. cepacia-infected CF inpatients, especially in settings such as playrooms and hospital lounges. Patient segregation and infection control have been proven to prevent the transmission of B. cepacia complex among CF patients. However, of critical importance is the correct laboratory identification of B. cepacia complex infection in the CF population. A false-positive identification has unnecessary ramifications both psychologically and socially on the CF patient and his or her family, and a false-negative identification could profoundly affect the entire CF community.
STENOTROPHOMONAS AND ALCALIGENES
S. maltophilia and Alcaligenes spp. are both being seen with increasing frequencies, primarily in the adult CF population (17, 32). However, the role of either of these agents in CF lung disease has not been determined by case-control studies, as has been done with B. cepacia complex. Identification of S. maltophilia and Alcaligenes spp. isolated from CF respiratory specimens can be problematic. Several studies have demonstrated that both S. maltophilia and Alcaligenes spp. can be misidentified by clinical laboratories as B. cepacia complex, and vice versa (28). Identification is partially complicated by the growth of some S. maltophilia and Alcaligenes spp. strains on B. cepacia selective media such as OFPBL and PC agars. Similar to B. cepacia complex, both S. maltophilia and Alcaligenes spp. are often resistant to a wide variety of antimicrobial agents. Susceptibility testing of S. maltophilia should be performed by an MIC method, since very major errors may be seen when disk diffusion is performed by using P. aeruginosa breakpoints (i.e., reporting as susceptible an isolate that might be resistant) (3, 25). Clinicians caring for CF patients are interested in knowing if these organisms are present, and in some clinical situations, physicians target antimicrobial therapy to eradicate these bacteria.
HAEMOPHILUS INFLUENZAE
H. influenzae is the third most commonly recovered bacterium from the respiratory tracts of CF patients (∼15% of patients) (11). This organism is typically recovered from children with CF and is infrequently seen in adults with CF. The inability to detect H. influenzae in adults with CF could be explained by the organism being obscured by mucoid P. aeruginosa. Three strategies for growing H. influenzae from respiratory specimens from CF patients have been proposed: the use of chocolate agar supplemented with bacitracin, incubation of plates anaerobically to suppress the growth of P. aeruginosa, or performance of quantitative cultures (33, 40). Bacitracin-supplemented chocolate agar plates allow the growth of P. aeruginosa, and their use is of limited value for patients chronically infected with P. aeruginosa. Anecdotal experience at the Clinical Microbiology-Immunology Laboratories of University of North Carolina Hospitals is that anaerobic incubation of chocolate agar plates with specimens from CF patients infected with P. aeruginosa did not enhance the recovery of H. influenzae. Our experience also indicates that H. influenzae can be recovered from quantitatively cultured, bronchoscopically obtained specimens from children with CF, but this organism is rarely recovered from adults with CF. Despite its relatively frequent recovery from children with CF, there are few data to support a primary role for this organism in CF lung disease (26).
ASPERGILLUS SPP. AND OTHER FUNGI
Filamentous fungi are frequently recovered from respiratory specimens from CF patients, especially with the increased use of B. cepacia selective agars, which support fungal growth. Only Aspergillus spp. have been recognized as being associated with pulmonary symptoms in CF patients. Organism of this genus are associated with an entity called allergic bronchopulmonary aspergillosis. The diagnosis of this entity is based on clinical criteria rather than the recovery of the organism. Patients may harbor Aspergillus spp. without having this syndrome (24). Therefore, documentation of the presence of filamentous fungi in this patient population is of limited value.
MYCOBACTERIUM SPP.
M. tuberculosis is rarely recovered from CF patients, and this patient population does not appear to be at increased risk for infection by this organism. However, a case series in the early 1990s, along with several case reports, suggests that Mycobacterium avium complex and rapidly growing mycobacterial species are recovered from as many as 20% of CF patients (23). In order to ensure the recovery of nontuberculous mycobacteria from CF patients, stringent decontamination conditions are needed due to P. aeruginosa contamination of mycobacterial cultures. Sequential treatment of respiratory specimens with standard N-acetyl cysteine-NaOH followed by oxalic acid treatment has been shown to significantly increase the rate of recovery of nontuberculous mycobacteria by reducing P. aeruginosa overgrowth (39). By using this culture technique, a multicenter prevalence study showed that 13% of CF patients were culture positive for nontuberculous mycobacteria. Patients with higher organism burdens, indicated by positive smears or multiple positive cultures, were more likely to have changes on high-resolution chest computed tomography but did not have significant declines in lung function. Nevertheless, these patients may benefit from antimycobacterial therapy (30).
VIRAL INFECTIONS
Both influenza and respiratory syncytial viruses have been reported to be associated with pulmonary exacerbations in CF patients (20). Studies of viral disease in CF patients have depended on either serology or viral culture. There is a heavy reliance on serologic data since viral cultures tend to be positive less frequently for CF patients than for non-CF patient controls (20). If newer antiviral agents active against influenza virus are to be used in a cost-effective manner in this patient population, antigen detection or molecular amplification tests will need to be used. The reliability of either of these approaches has not yet been systematically studied for this patient population.
PRACTICAL APPROACHES TO MICROBIOLOGY FOR CF PATIENTS
With the requirement that at least two selective media (mannitol salts agar for S. aureus and BCSA for B. cepacia complex) be used, in addition to the standard battery of isolation media, and the need to identify and perform susceptibility testing with multiple isolates, culture of respiratory tract specimens from CF patients is one of the most labor-intensive and expensive culture procedures performed in the clinical microbiology laboratory. Over the past 10 years, in collaboration with our physicians caring for CF patients, we have developed a strategy to try to limit our costs while at the same time delivering high-quality microbiology services for this patient population.
The first part of our strategy is to eliminate routine Gram stains from cultures of all sputum specimens from CF patients, other than those who have recently received lung transplants. We found that few specimens were rejected (a major reason for doing a sputum Gram stain), and the organisms seen by Gram staining were highly predictable.
The second part of our strategy is to limit the workup of cultures respiratory specimens from both CF outpatients and CF inpatients. An extensive culture workup (i.e., complete identification and susceptibility testing of significant isolates [Table 1]) is done every 3 months for outpatients. Cultures of outpatient specimens (sputum and deep pharyngeal specimens) submitted less than 3 months after an extensive workup are sight read and reported, with identification and sensitivity testing done only by physician request. The same strategy is also used for CF inpatients; the initial sputum and deep pharyngeal specimens receive an extensive workup, but subsequent sputum and deep pharyngeal specimens are sight read. Bronchoscopy specimens are obtained at some expense and danger to the patient and are considered “gold standard” specimens. Therefore, cultures of sputum and deep pharyngeal specimens are sight read, as described above, for patients for whom culture of a bronchoscopy specimen had been performed within the previous 3 months for outpatients and during a hospital stay for inpatients. In addition, identification of molds recovered from CF patients is limited to once per calendar year. Many of these patients have long-term colonization with Aspergillus spp., and repeated identification has no clinical relevance. Physicians always have the option, after consultation with the laboratorian, to request further workup for any specimen submitted to the laboratory; but in our experience, this is a relatively uncommon occurrence. It should be noted that strategies for performing cultures with specimens from CF patients who have received lung transplants are very different, and discussion of those strategies is beyond the scope of this review.
Since susceptibility testing also results in significant expense, this is the focus of our third strategy. Complete S. aureus susceptibility testing is performed once per calendar year. Otherwise, isolates are screened for resistance by using an oxacillin screening plate. The first time that a patient's S. aureus isolate is oxacillin resistant, as detected by screening, complete susceptibility testing is performed. As described above, susceptibility testing of P. aeruginosa isolates from CF patients is challenging. Similar to our identification scheme, we perform susceptibility testing by disk diffusion for each morphotype of P. aeruginosa once every 3 months for outpatients or at admission for inpatients. The same strategy is used for A. xylosoxidans, B. cepacia complex, and related organisms, although the caveat “nonstandardized susceptibility” is added to alert the physician that very major errors are possible. As mentioned above, S. maltophilia susceptibility testing must be performed by MIC methodologies. Using P. aeruginosa susceptibility breakpoints, we use E-tests and test only three drugs, ceftazidime, trimethoprim-sulfamethoxazole, and ticarcillin-clavulanic acid. Because both B. cepacia complex and P. aeruginosa isolates may be resistant to multiple antibiotics, physicians often request synergy testing for these isolates to determine the very best antimicrobial combination with which to treat these patients. To that end, the U.S. Cystic Fibrosis Foundation has established a reference laboratory where this testing is performed without charge. For more information on this service, visit the U.S. Cystic Fibrosis Foundation website at www.cff.org.
In summary, the microbiology of pulmonary disease in CF patients has multiple layers of complexity. In addition to the number of different bacterial species isolated from respiratory specimens from this patient population, the intra- and interspecies interactions are also numerous. The formation of biofilms and anaerobic growth conditions in the lungs of CF patients threaten to discount the in vitro susceptibility data generated for bacterial isolates from CF patients. As we have addressed, there are also problems associated with the isolation and identification of these organisms in the face of evolutionary change. However, it should be noted that diligent research toward the understanding of the microbiology and physiology of CF patients not only has led to an extended life span for many CF patients but also has contributed to improving the quality of life for this population. The clinical laboratory can also contribute to the well-being of the CF population by striving to provide high-quality, cost-effective management of these ever-challenging cultures.
REFERENCES
- 1.Aaron, S. D., W. Ferris, K. Ramotar, K. Vandemheen, F. Chan, and R. Saginur. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J. Clin. Microbiol. 40:4172-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aris, R. M., J. C. Routh, J. J. LiPuma, D. G. Heath, and P. H. Gilligan. 2001. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am. J. Respir. Crit. Care Med. 164:2102-2106. [DOI] [PubMed] [Google Scholar]
- 3.Arpi, M., M. A. Victor, I. Mortensen, A. Gottschau, and B. Bruun. 1996. In vitro susceptibility of 124 Xanthomonas maltophilia (Stenotrophomonas maltophilia) isolates: comparison of the agar dilution method with the E-test and two agar diffusion methods. APMIS 104:108-114. [PubMed] [Google Scholar]
- 4.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. L., L. Saiman, S. Whittier, J. Krzewinski, Z. Liu, D. Larone, S. A. Marshall, and R. N. Jones. 2001. Comparison of two commercial systems (Vitek and MicroScan-WalkAway) for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 39:257-260. [DOI] [PubMed] [Google Scholar]
- 6.Burns, J. L., L. Saiman, S. Whittier, D. Larone, J. Krzewinski, Z. Liu, S. A. Marshall, and R. N. Jones. 2000. Comparison of agar diffusion methodologies for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 38:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, J. L., J. M. Van Dalfsen, R. M. Shawar, K. L. Otto, R. L. Garber, J. M. Quan, A. B. Montgomery, G. M. Albers, B. W. Ramsey, and A. L. Smith. 1999. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J. Infect. Dis. 179:1190-1196. [DOI] [PubMed] [Google Scholar]
- 8.Chaparro, C., J. Maurer, C. Gutierrez, M. Krajden, C. Chan, T. Winton, S. Keshavjee, M. Scavuzzo, E. Tullis, M. Hutcheon, and S. Kesten. 2001. Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. Am. J. Respir. Crit. Care Med. 163:43-48. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. S., K. A. Witzmann, T. Spilker, R. J. Fink, and J. J. LiPuma. 2001. Endemicity and inter-city spread of Burkholderia cepacia genomovar III in cystic fibrosis. J. Pediatr. 139:643-649. [DOI] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation. 1998. Patient registry 1997 annual report. Cystic Fibrosis Foundation, Washington, D.C.
- 11.Cystic Fibrosis Foundation. 2002. Patient registry 2001 annual report. Cystic Fibrosis Foundation, Washington, D.C.
- 12.Drevinek, P., H. Hrbackova, O. Cinek, J. Bartosova, O. Nyc, A. Nemec, and P. Pohunek. 2002. Direct PCR detection of Burkholderia cepacia complex and identification of its genomovars by using sputum as source of DNA. J. Clin. Microbiol. 40:3485-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederiksen, B., C. Koch, and N. Hoiby. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23:330-335. [DOI] [PubMed] [Google Scholar]
- 14.Gilligan, P. H. 1991. Microbiology of airway disease in patients with cystic fibrosis. Clin. Microbiol. Rev. 4:35-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilligan, P. H., P. A. Gage, D. F. Welch, M. J. Muszynski, and K. R. Wait. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 25:1258-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givney, R., A. Vickery, A. Holliday, M. Pegler, and R. Benn. 1997. Methicillin-resistant Staphylococcus aureus in a cystic fibrosis unit. J. Hosp. Infect. 35:27-36. [DOI] [PubMed] [Google Scholar]
- 17.Graff, G. R., and J. L. Burns. 2002. Factors affecting the incidence of Stenotrophomonas maltophilia isolation in cystic fibrosis. Chest 121:1754-1760. [DOI] [PubMed] [Google Scholar]
- 18.Heath, D. G., K. Hohneker, C. Carriker, K. Smith, J. Routh, J. J. LiPuma, R. M. Aris, D. Weber, and P. H. Gilligan. 2002. Six-year molecular analysis of Burkholderia cepacia complex isolates among cystic fibrosis patients at a referral center for lung transplantation. J. Clin. Microbiol. 40:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry, D., M. Campbell, C. McGimpsey, A. Clarke, L. Louden, J. L. Burns, M. H. Roe, P. Vandamme, and D. Speert. 1999. Comparison of isolation media for recovery of Burkholderia cepacia complex from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 37:1004-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiatt, P. W., S. C. Grace, C. A. Kozinetz, S. H. Raboudi, D. G. Treece, L. H. Taber, and P. A. Piedra. 1999. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 103:619-626. [DOI] [PubMed] [Google Scholar]
- 21.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 22.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 23.Kilby, J. M., P. H. Gilligan, J. R. Yankaskas, W. E. Highsmith, Jr., L. J. Edwards, and M. R. Knowles. 1992. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest 102:70-75. [DOI] [PubMed] [Google Scholar]
- 24.Knowles, M. R., P. H. Gilligan, and R. C. Boucher. 2000. Cystic fibrosis, p. 767-772. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practices of infectious diseases, 5th ed. Churchill Livingstone, New York, N.Y.
- 25.Krueger, T. S., E. A. Clark, and D. E. Nix. 2001. In vitro susceptibility of Stenotrophomonas maltophilia to various antimicrobial combinations. Diagn. Microbiol. Infect. Dis. 41:71-78. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDowell, A., E. Mahenthiralingam, J. E. Moore, K. E. Dunbar, A. K. Webb, M. E. Dodd, S. L. Martin, B. C. Millar, C. J. Scott, M. Crowe, and J. S. Elborn. 2001. PCR-based detection and identification of Burkholderia cepacia complex pathogens in sputum from cystic fibrosis patients. J. Clin. Microbiol. 39:4247-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 117:1661-1665. [DOI] [PubMed] [Google Scholar]
- 29.Morlin, G. L., D. L. Hedges, A. L. Smith, and J. L. Burns. 1994. Accuracy and cost of antibiotic susceptibility testing of mixed morphotypes of Pseudomonas aeruginosa. J. Clin. Microbiol. 32:1027-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivier, K. N., D. J. Weber, J. H. Lee, A. Handler, G. Tudor, P. L. Molina, J. Tomashefski, and M. R. Knowles. 2003. Nontuberculous mycobacteria. II. Nested-cohort study of impact on cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 167:835-840. [DOI] [PubMed] [Google Scholar]
- 31.Saiman, L., J. L. Burns, D. Larone, Y. Chen, E. Garber, and S. Whittier. 2003. Evaluation of MicroScan Autoscan for identification of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 41:492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saiman, L., Y. Chen, S. Tabibi, P. San Gabriel, J. Zhou, Z. Liu, L. Lai, and S. Whittier. 2001. Identification and antimicrobial susceptibility of Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. J. Clin. Microbiol. 39:3942-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saiman, L., D. Schidlow, and A. Smith (ed.). 1994. Concepts in care: microbiology and infectious disease in cystic fibrosis, vol. V. Cystic Fibrosis Foundation, Washington, D.C.
- 34.Shreve, M. R., S. Butler, H. J. Kaplowitz, H. R. Rabin, D. Stokes, M. Light, and W. E. Regelmann. 1999. Impact of microbiology practice on cumulative prevalence of respiratory tract bacteria in patients with cystic fibrosis. J. Clin. Microbiol. 37:753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, L., R. Z. Jiang, S. Steinbach, A. Holmes, C. Campanelli, J. Forstner, U. Sajjan, Y. Tan, M. Riley, and R. Goldstein. 1995. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat. Med. 1:661-666. [DOI] [PubMed] [Google Scholar]
- 36.Tablan, O. C., T. L. Chorba, D. V. Schidlow, J. W. White, K. A. Hardy, P. H. Gilligan, W. M. Morgan, L. A. Carson, W. J. Martone, J. M. Jason, and W. R. Jarvis. 1985. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J. Pediatr. 107:382-387. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, S. R., A. Ray, M. E. Hodson, and T. L. Pitt. 2000. Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 55:795-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandamme, P., B. Holmes, T. Coenye, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. Govan. 2003. Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res. Microbiol. 154:91-96. [DOI] [PubMed] [Google Scholar]
- 39.Whittier, S., R. L. Hopfer, M. R. Knowles, and P. H. Gilligan. 1993. Improved recovery of mycobacteria from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 31:861-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong, K., M. C. Roberts, L. Owens, M. Fife, and A. L. Smith. 1984. Selective media for the quantitation of bacteria in cystic fibrosis sputum. J. Med. Microbiol. 17:113-119. [DOI] [PubMed] [Google Scholar]
- 41.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]