Abstract
Buruli ulcer, an infection caused by Mycobacterium ulcerans, is, after tuberculosis and leprosy, the third most common mycobacterial disease. The mode of transmission of M. ulcerans is not exactly known, but since Buruli ulcer often occurs in focalized swampy areas, it is assumed that there is a reservoir of the pathogen in stagnant water. Buruli ulcer usually starts as a painless nodule and can lead to massive destruction of skin, subcutaneous tissue, and eventually muscle and bone. Currently the only recommended treatment is wide surgical excision. In this report we describe the development of a real-time PCR method for the quantification of M. ulcerans DNA (IS2404 TaqMan). The highly specific assay is based on the detection of the M. ulcerans specific insertion sequence IS2404. The IS2404 TaqMan assay turned out to be about 10 times more sensitive than the available conventional PCR-based diagnostic test. It is demonstrated that the IS2404 TaqMan assay is suitable for the quantitative assessment of the dissemination of the mycobacteria in Buruli ulcer lesions. Prototype results obtained with excised tissue from a patient with a late preulcerative Buruli ulcer lesion reconfirmed earlier histopathological findings indicating that tissue damage occurs far beyond the regions in which large numbers of mycobacteria are detectable. The IS2404 TaqMan assay should be a useful tool for both diagnosis and research into the pathology and mode of transmission of this still inadequately investigated mycobacterial disease.
Mycobacterium ulcerans is a slow-growing environmental mycobacterium, which causes a disfiguring condition known as Buruli ulcer. M. ulcerans infections have been found in more than 30 tropical and subtropical countries of Africa, Asia, Latin America, and the Western Pacific but are most common and severe in West Africa (1). Buruli ulcer often occurs in people who live close to rivers and stagnant bodies of water.
The mode of transmission of M. ulcerans is not clear, but it is assumed that the environmental pathogens enter the body through small lesions in the skin (1, 6). Aquatic insects may be involved in some cases (1, 18). After entry, the bacteria proliferate in the subcutaneous tissue. In African patients, early lesions are characterized by coagulative necrosis of lower dermis and subcutaneous fatty tissue associated with some calcification. Production of mycolactone (13), a toxin with an affinity for fatty cells (12), may be important for the pathogenesis of the disease by causing necrosis and thus providing a favorable milieu for the mycobacteria. It is likely, however, that additional bacterial factors are involved in the pathogenesis (10). Even in the most minimal lesions, necrosis of fatty tissue seems to be a primary event (19). In contrast to other pathogenic mycobacteria, M. ulcerans is not a facultative intracellular pathogen but is found primarily as extracellular microcolonies (14, 19).
Depending on its progression, Buruli ulcer has different clinical manifestations. The disease often starts as a painless swelling in the skin, and as it progresses, all elements of the skin become affected. The early preulcerative forms (nodules, papules, plaques, and edemas) are followed by the ulcerative stage. Necrosis of the subcutaneous fatty tissue with vascular occlusion results in sloughing and secondary ulceration of the overlying skin. Acid-fast bacilli (AFB) stained by the Ziehl-Neelsen (ZN) technique in tissue sections seem to be largely confined to the necrotic slough and surrounding necrotic fatty tissue. In the late stages massive areas of skin, subcutaneous tissue, and sometimes muscle and bone are destroyed, leading to gross deformities. If a healing response takes place, fibrosis, scarring, calcification, and contractures with permanent disabilities may result (30). To date, the treatment of choice is surgery, since current antimicrobial therapies appear to be ineffective, but recurrence of the disease after surgical treatment is a common problem that may be due to incomplete removal of the mycobacteria and inadequate excision.
Early treatment of M. ulcerans disease provides a better outcome than treatment of the ulcerative forms, but it is often impaired by the difficulties of diagnosis. The commonly used diagnostic tests are (i) detection of mycobacteria by ZN staining, a technique that lacks sensitivity and specificity; (ii) culture of M. ulcerans, which may take several months; (iii) detection of characteristic histopathological changes in excised tissue; and (iv) detection of M. ulcerans DNA by PCR, representing a rapid, sensitive, and specific diagnostic method (21). IS2404, an insertion element present in multiple copies in the M. ulcerans genome, is commonly used as a target sequence for this purpose (26). IS2404 is specific for M. ulcerans and encodes a 328-amino-acid transposase (27). It has also been found recently in a mycobacterial isolate which may constitute a link between Mycobacterium marinum and M. ulcerans (4).
In this report we describe the development of a PCR method for the quantification of M. ulcerans DNA by monitoring the real-time amplification of IS2404, using the TaqMan system (IS2404 TaqMan). Beyond the possibility of measuring the starting amount of target DNA in clinical specimens and other samples, real-time PCR has several advantages over the conventional endpoint PCR. These include reduction of risk of contamination by eliminating the post-PCR processing and a diminished sensitivity to PCR inhibitors. By applying IS2404 TaqMan PCR to samples from Buruli ulcer patients, it will be possible to correlate the dissemination of M. ulcerans with the progression of the disease. The method may also help to determine the optimal extent of surgical excision in order to reduce recurrence.
MATERIALS AND METHODS
Mycobacterial isolates.
Mycobacterial isolates that have been employed in this study are listed in Table 1. Clinical isolates were identified to the species level by the partial sequencing of the 16S ribosomal DNA gene and by conventional methods (20).
TABLE 1.
List of mycobacterial strains used in this study
| Mycobacterial species | Source | Mediuma | Temp (°C) |
|---|---|---|---|
| M. abscessus | ATCC 19977 | mod. LJ | 35 |
| M. africanum | Swiss NCMb | mod. LJ | 35 |
| M. avium subsp. avium | MAC101 | mod. LJ | 35 |
| M. bohemicum | Clinical isolate | mod. LJ | 35 |
| M. bovis | ATCC 19210 | mod. LJ | 35 |
| M. bovis biovar BCG | ATCC 35734 | mod. LJ | 35 |
| M. bovis subsp. caprae | Clinical isolate | mod. LJ | 35 |
| M. chelonae | DSM 43804 | mod. LJ | 35 |
| M. fortuitum | ATCC 49403 | mod. LJ | 35 |
| M. gordonae | Pasteur 14021.001 | mod. LJ | 35 |
| M. haemophilum | ATCC 29548 | MB 7H10 | 28 |
| M. intracellulare | Clinical isolate | mod. LJ | 35 |
| M. kansasii | NCTC 10268 | mod. LJ | 35 |
| M. lentiflavum | Clinical isolate | mod. LJ | 35 |
| M. malmoense | NCTC 11298 | mod. LJ | 35 |
| M. marinum | ATCC 927 | mod. LJ | 28 |
| M. scrofulaceum | Pasteur 14022.0031 | mod. LJ | 35 |
| M. simiae | Clinical isolate | mod. LJ | 35 |
| M. smegmatis | Pasteur 14133.0001 | mod. LJ | 35 |
| M. terrae | Clinical isolate | mod. LJ | 35 |
| M. tuberculosis H37Rv | Pasteur 14001.0001 | mod. LJ | 35 |
| M. xenopi | Clinical isolate | mod. LJ | 35 |
mod. LJ, modified Löwenstein-Jensen; MB 7H10, Middlebrook agar with 10% oleic acid-albumin-dextrose-catalase enrichment supplement with 0.4% ferrum-ammonium-citrate.
Swiss NCM: Swiss National Centre for Mycobacteria, Zurich, Switzerland.
Clinical specimens.
Samples of about 100 mg were taken from tissue excised from a Buruli ulcer patient (male, 12 years old) in Amasaman, Ghana, with a preulcerative plaque lesion at the ventral side of his left distal forearm. Care was taken to avoid cross-contamination among the different sample areas.
Cotton swabs were used to collect diagnostic samples from the base of undermined margins of lesions of Buruli ulcer patients with ulcerative lesions. The samples were maintained in the cold chain until they were analyzed.
Informed consent.
Informed consent was obtained from the patients or their parents before enrollment in the study.
DNA preparation.
Extraction of DNA from cultivated mycobacteria was done as previously described by Telenti et al. (29). The tips of the cotton swabs were cut and heated for 30 min at 95°C in screw-cap tubes containing 600 μl of extraction buffer (0.2% sodium dodecyl sulfate, 0.05 M NaOH). After a brief centrifugation, 40 μl of a 20-mg/ml proteinase K solution was added to the supernatants. After 30 min of incubation at 60°C, the samples were vortexed for 2 min with 200 μl of 100-μm-diameter glass beads and the DNA in the supernatants was precipitated and washed with ethanol. After another washing with acetone and air drying, the precipitated DNA was resuspended in 60 μl of water and 0.5-μl amounts of the template solutions were used for PCR analysis.
Tissue samples of about 100 mg were heated for 1 h at 95°C in 500 μl of extraction buffer (50 mM Tris-HCl, 25 mM EDTA, 5% monosodium glutamate). One hundred microliters of a 100-mg/ml lysozyme solution was added. After 2 h of incubation at 37°C, 70 μl of proteinase K-10× buffer (100 mM Tris-HCl, 50 mM EDTA, 5% sodium dodecyl sulfate [pH 7.8]) and 10 μl of a 20-mg/ml proteinase K solution was added. After incubation at 45°C overnight, the samples were subjected to treatment with a bead beater (Mikro-Dismembrator; Braun Biotech International) with 300 μl of 0.1-mm-diameter zirconia beads (BioSpec Products) at 3,000 rpm for 7 min. Beads and undigested tissue fragments were removed by brief centrifugation, and the supernatants were transferred to fresh tubes. An equal amount of phenol-chloroform (Fluka) was added, and the DNA contained in the upper phase was precipitated with ethanol and resuspended in 150 μl of water. The DNA yield was measured by use of a spectrophotometer (GeneQuant), and 50 ng of DNA per sample was subjected to amplification by conventional and real-time PCR.
Conventional PCR.
DNA was amplified in a 50-μl reaction mixture containing a 1 μM concentration of each primer (MU1, MU2 [Table 2]), 2.5 U of HotStart DNA polymerase (Qiagen), a 200 μM concentration of each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 1× PCR buffer (Qiagen). PCRs were performed in a Gen Amp PCR System 2400 (Perkin-Elmer) thermal cycler with the following protocol: denaturation at 94°C for 10 min, amplification for 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 7 min. Ten microliters of amplified DNA was subjected to electrophoresis in a 1% agarose gel and detected by ethidium bromide staining and UV transillumination. A 1-kb ladder was used as a size marker.
TABLE 2.
Primer and probe sequences used for the IS2404 TaqMan assay and conventional PCR
| Primer or probe | Sequence (5′-3′) | Reference |
|---|---|---|
| Primers | ||
| F1 | ATTGGTGCCGATCGAGTTG | This study |
| R1 | TCGCTTTGGCGCGTAAA | This study |
| MU1 | GGCAGGCTGCAGATGGCATA | Ross et al. |
| MU2 | GGCAGTTACTTCACTTGCACA | Ross et al. |
| Probe | ||
| P1 | FAM-CACCACGCAGCATTCTTGCCGT-TAMRA | This study |
Real-time PCR.
Primer and probe sequences (Table 2) were selected from a region of the IS2404 sequence with minimal homology with human DNA. The probe was labeled with the fluorescent dyes 5-carboxyfluorescein (FAM) on the 5′ end and N,N,N′,N′-tetramethyl-6-carboxyrhodamine (TAMRA) on the 3′ end. When the dyes are close to each other, the 5′ reporter fluorophore (FAM) transfers laser-induced excitation energy to the 3′ quencher (TAMRA). When the oligoprobe hybridizes to its template, the fluorophores are released by the 5′-3′ exonuclease activity of the Taq polymerase, leading to the separation of the dyes in solution. Once they are separated, the reporter emission is no longer quenched and it is possible to measure the increasing fluorescence, which is proportional to the amount of target DNA produced (16). DNA extracted from M. ulcerans strain Agy98 was used as a standard in all experiments.
Real-time PCR mixtures contained template DNA, 0.3 μM concentrations of each primer, 0.1 μM concentration of the probe, and TaqMan Universal PCR Mastermix (Applied Biosystems) in a total volume of 25 μl. Amplification and detection were performed with the ABI Prism 7700 sequence detection system by using the following profile: 1 cycle of 50°C for 2 min, 1 cycle of 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Analyses were done in triplicates. For quantification, an external standard curve with M. ulcerans Agy98 DNA serially diluted over 6 logs was used. Negative controls were included in each amplification experiment. To analyze the potential inhibitory effect of human DNA on the real-time PCR, increasing amounts of human genomic DNA from 10 to 1,000 ng were added to a fixed amount of 20 fg of M. ulcerans DNA per PCR mix.
RESULTS
Development of an IS2404-based real-time PCR assay.
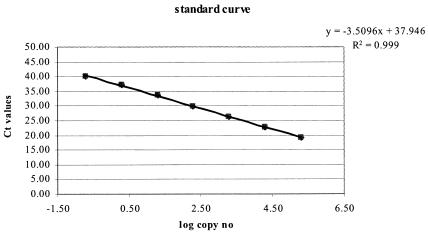
With IS2404, an insertion sequence unique for M. ulcerans, used as the target, an ABI Prism sequence detection system (TaqMan) quantitative real-time PCR method was developed. Primers F1 and R1 comprised residues 406 to 424 and 465 to 449, respectively, in the GenBank IS2404 sequence (no. AF003002) (Table 2). They were used in combination with the probe P1, comprising residues 447 to 426. The standard curve obtained with a serially diluted M. ulcerans genomic DNA preparation was linear over 6 orders of magnitude with a coefficient of correlation of 0.999 and a slope of 3.51, corresponding to a PCR efficiency of >90% (Fig. 1). Assuming that 1 genome copy corresponds to 5 fg of DNA (28), the detection limit was 0.2 genome copies per reaction mixture. Since it is expected that the genome of M. ulcerans isolates contains approximately 50 to 100 copies of IS2404, the detection limit thus corresponded to 10 to 20 copies of IS2404. With amounts of M. ulcerans DNA equivalent to 0.2 to 200,000 genome copies, threshold cycle number (Ct) values ranging from 35 to 16 were obtained with a coefficient of variation of <2 (Table 3).
FIG. 1.
Standard curve generated by the analysis of known amounts of genomic M. ulcerans DNA with the IS2404 TaqMan assay. The regression line calculated for the data points is shown; the coefficient of correlation is more than 0.99.
TABLE 3.
Measurement of replicate standard curves ranging from 0.2 to 200,000 genomes of M. ulcerans per reaction mixturea
| No. of genome copies | Log copy no. | Ct for reaction no.:
|
Mean | SD | CV | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| 0.2 | −0.70 | 35.0 | 35.5 | 34.6 | 35.05 | 0.49 | 1.39 |
| 2 | −0.30 | 31.5 | 32.5 | 31.8 | 31.94 | 0.52 | 1.62 |
| 20 | 1.30 | 29.1 | 29.1 | 28.7 | 28.95 | 0.20 | 0.70 |
| 200 | 2.30 | 26.0 | 25.7 | 26.0 | 25.88 | 0.18 | 0.68 |
| 2,000 | 3.30 | 22.2 | 22.2 | 22.3 | 22.25 | 0.02 | 0.10 |
| 20,000 | 4.30 | 18.8 | 18.9 | 18.8 | 18.85 | 0.06 | 0.29 |
| 200,000 | 5.30 | 15.8 | 15.8 | 16.0 | 15.89 | 0.11 | 0.68 |
Abbreviations: Ct, threshold cycle number; SD, standard deviation; CV, coefficient of variation.
The potential inhibitory effect of human genomic DNA was evaluated by spiking fixed amounts of M. ulcerans DNA with increasing concentrations of human genomic DNA (Table 4). Human DNA on its own yielded no signal, and amounts of up to 500 ng of human DNA per reaction mixture had no inhibitory effect on the amplification of a starting amount of 20 fg of M. ulcerans DNA. Only the addition of 1 μg of human genomic DNA resulted in some inhibition (Table 4). This was compatible with M. ulcerans DNA quantification in clinical specimens, since the amounts of human DNA in extracts from affected tissue were much smaller (50 ng). None of the genomic DNA from the other 22 mycobacterial species tested (Table 1) produced an amplification product, demonstrating the high specificity of the IS2404 TaqMan assay.
TABLE 4.
Evaluation of the potential inhibitory effect of human genomic DNA in quantification of M. ulcerans by IS2404 TaqMan
| Human genomic DNA per PCR (ng) | Ct values |
|---|---|
| 0 | 28.51 |
| 10 | 28.69 |
| 50 | 28.48 |
| 100 | 28.37 |
| 200 | 28.37 |
| 500 | 28.85 |
| 1,000 | 30.20 |
Use of the IS2404 TaqMan assay for the analysis of clinical specimens.
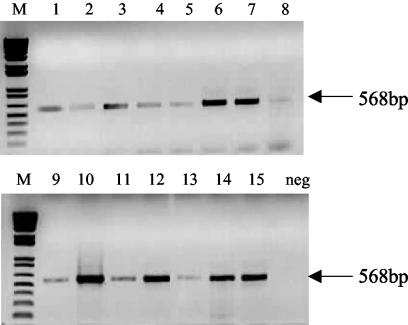
Swab samples taken from the undermined edges of lesions from patients with ulcerative forms of Buruli ulcer were subjected to conventional and quantitative PCR. The conventional PCR allowed the detection of the expected 568-bp band, with some variation in the intensity of the signal among the different samples (Fig. 2). Southern blot hybridization confirmed that IS2404-derived PCR products were obtained (data not shown). The real-time PCR revealed >400-fold differences in the amounts of M. ulcerans DNA, ranging from 13 to 5,267 genome copies/μl of extract (Table 5).
FIG. 2.
Amplification products obtained by conventional diagnostic IS2404 PCR with DNA extracted from swabs used for taking samples from undermined edges of Buruli ulcer lesions (lanes 1 to 15). The lane marked “neg” was loaded with an aliquot from a negative control amplification containing no template DNA.
TABLE 5.
Numbers of genome copies of M. ulcerans in swabs of patients with Buruli ulcer
| Sample | No. of copies/μla | SDb | CVc |
|---|---|---|---|
| 1 | 20 | 1.1 | 10.3 |
| 2 | 106 | 4.0 | 7.5 |
| 3 | 240 | 10.0 | 8.3 |
| 4 | 13 | 0.4 | 5.9 |
| 5 | 20 | 0.9 | 9.4 |
| 6 | 333 | 11.5 | 6.9 |
| 7 | 720 | 28.2 | 7.9 |
| 8 | 16 | 2.5 | 31.4 |
| 9 | 46 | 26.5 | 11.5 |
| 10 | 5,267 | 115.5 | 4.4 |
| 11 | 460 | 200.0 | 8.7 |
| 12 | 105 | 1.5 | 2.9 |
| 13 | 14 | 0.6 | 8.9 |
| 14 | 970 | 7.1 | 1.5 |
| 15 | 1090 | 21.2 | 3.9 |
60 μl of extract was obtained per swab.
SD, standard deviation.
CV, coefficient of variation.
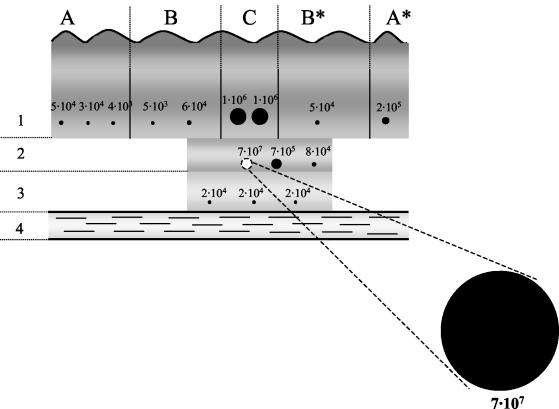
To demonstrate the suitability of the IS2404 TaqMan assay for the quantitative assessment of the dissemination of the mycobacteria in Buruli ulcer lesions, a surgical specimen widely excised from a patient with a nonulcerative plaque on the ventral side of the left forearm was analyzed. In addition to the firm and slightly elevated lesion (B, B*, and C in Fig. 3), the surgeon had excised a clear margin of normal tissue (A and A* in Fig. 3). In the center of the plaque a more elevated region (C in Fig. 3) was detectable, which was presumably the location of a papule or nodule in the earlier stage of the disease. Nine samples of about 100 mg extending from the skin into the subcutaneous fatty tissue were taken at different distances from this center of the lesion in a plane parallel to the skin surface (level 1 in Fig. 3). In the center of the lesion, deep subcutaneous tissue and fascia were also severely affected. The surgeon therefore removed two additional layers into the deeper tissues at this location, with the lowest one (level 3 in Fig. 3) extending deep down to the flexor tendon. Six samples were taken from these tissue specimens (levels 2 and 3 in Fig. 3), which exhibited pathological changes both in color and texture.
FIG. 3.
Schema of the location of tissue samples taken from a surgically removed Buruli ulcer plaque lesion. The dots show the location of the specimens subjected to IS2404 TaqMan assay, and their sizes indicate the amounts of M. ulcerans DNA detected. The values correspond to the mycobacterial genome copies found in 100 mg of tissue sample. C, center of the plaque (diameter, about 1.5 cm); B and B*, firm and slightly elevated area of the plaque as identified by the surgeon (about 4 cm); A and A*, excised margins of apparently healthy tissue (3 and 1 cm, respectively) (A* faces towards the distal part of the forearm); 1, upper layer comprising epidermis and subcutaneous tissue; 2 and 3, deeper layers of subcutaneous tissue; 4, flexor tendon.
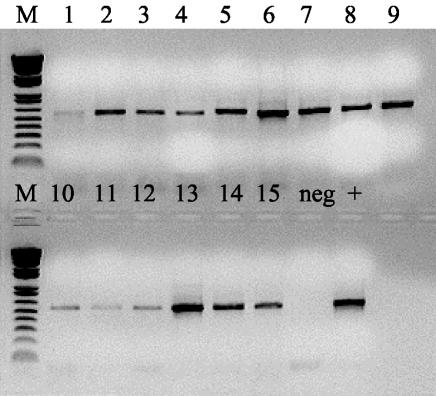
Results of a conventional PCR analysis with the tissue samples demonstrated the presence of M. ulcerans DNA in all the specimens taken and already gave some indication that the amounts of mycobacterial DNA template present in the samples varied (Fig. 4). But only the result of the IS2404 TaqMan assay fully demonstrated the dramatic differences in the burdens of M. ulcerans at different locations of the lesion (Fig. 3). The specimen taken from the middle layer in the very center of the lesion (C2 in Fig. 3) contained about 20 times more mycobacterial DNA than the 14 other samples together. Three neighboring samples from layers 1 and 2 still contained 1 to 2% of the amount of M. ulcerans DNA found in the focus sample. Levels of M. ulcerans DNA in the additional samples from the plaque lesion (from B, B*, and level 3 in Fig. 3) and in the excised margins of apparently healthy tissue (A and A* in Fig. 3) were comparable. None of these samples contained more than 0.3% of the amount of M. ulcerans DNA found in the sample with the highest burden. Table 6 demonstrates that the variation between individual measurements is very low also when tissue lysates instead of DNA purified from cultivated bacteria (Table 3) are used. Values from split tissue samples showed little variation (data not shown), indicating that the yield of M. ulcerans DNA obtained with the optimized DNA extraction method used was quite reproducible. Nevertheless, the absolute values presented may represent underestimates, since the release of mycobacterial DNA may still have been incomplete.
FIG. 4.
Amplification products obtained by conventional diagnostic IS2404 PCR with DNA extracted from tissue samples taken from a surgically removed Buruli ulcer plaque lesion. Lanes 1 to 9, specimens collected from left to right belonging to the layer 1 of Fig. 3; lanes 10 to 12, specimens collected from left to right belonging to the layer 3 of Fig. 3; lanes 13 to 15, specimens collected from left to right belonging to the layer 2 of Fig. 3. The lane marked “neg” was loaded with an aliquot from a negative control amplification containing no template DNA. The lane marked “+” was loaded with an aliquot from a positive control amplification containing M. ulcerans genomic DNA.
TABLE 6.
Measurement of genomic M. ulcerans DNA from tissue samplesa
| Reaction 1e | Reaction 2e | Reaction 3e | Mean | SDb | CVc |
|---|---|---|---|---|---|
| 286 | 308 | 328 | 307 | 21 | 6.75 |
| 245 | 200 | 270 | 238 | 35 | 14.73 |
| 32 | 31 | 30 | 31 | 0.7 | 2.28 |
| 39 | 34 | 36 | 36 | 2 | 6.59 |
| 402 | 391 | 433 | 409 | 22 | 5.42 |
| 8,333 | 8,392 | NDd | 8,362 | 42 | 0.50 |
| 7,782 | 8,340 | 8,388 | 8,170 | 337 | 4.12 |
| 1,273 | 1,483 | 1,550 | 1,435 | 144 | 10.04 |
| 351 | 327 | 325 | 334 | 15 | 4.24 |
| 502,378 | 499,420 | 493,558 | 498,452 | 4,489 | 0.90 |
| 4,585 | 4,674 | 4,794 | 4,684 | 105 | 2.25 |
| 596 | 585 | 489 | 557 | 59 | 10.54 |
| 160 | 161 | ND | 161 | 0.4 | 0.25 |
| 153 | 165 | 161 | 160 | 6 | 4.08 |
| 127 | 119 | 123 | 123 | 4 | 3.22 |
Corresponding to 1 μl of tissue extract (100mg/150μl), the values are ordered referring to tissue samples coming from left to right and from levels 1 to 3 according to the schema of the lesion shown in Fig. 3.
SD, standard deviation.
CV, coefficient of variation.
ND, not detected.
Values are numbers of genome copies.
DISCUSSION
In this paper the development of an IS2404-based real-time PCR assay for the quantification of M. ulcerans DNA is described. IS2404 is also the target of a conventional PCR assay (25) recommended in a slightly modified form (21) by WHO as a test for the confirmation of the clinical diagnosis of M. ulcerans infection. While conventional PCR assays basically provide endpoint measurements suitable for qualitative yes/no results, the real-time IS2404 TaqMan assay quantifies the starting concentration of mycobacterial DNA by monitoring the amount of reaction product during the exponential phase of the amplification cycles. The remarkably broad dynamic range of the IS2404 TaqMan assay allowed us to analyze samples with widely different amounts of M. ulcerans DNA without a prior estimation of template concentrations. When using genomic DNA extracted from cultivated mycobacteria, the IS2404 TaqMan assay exhibited a sensitivity about 10 times higher than that of the conventional IS2404-based PCR, which has been described as having a detection limit of two genome copies. It remains to be demonstrated whether this also converts into a statistically significant higher sensitivity of the IS2404 TaqMan assay when used for diagnostic purposes with clinical specimens. No amplification products were detected when the DNAs of 22 other mycobacteria species were tested with the IS2404 TaqMan assay, which reconfirms specificity data described for the conventional IS2404-based PCR (25).
As expected, IS2404 TaqMan analyses demonstrated that the amounts of M. ulcerans DNA present in extracts from wound swabs as taken routinely for PCR confirmation of clinical diagnosis vary widely. Endpoint determinations by conventional PCR did not fully reflect these differences. The amounts of DNA in the swab extracts were at the lowest end close to the detection limit of the conventional PCR. Already small variations in the reaction conditions may in such cases cause inconsistencies in the results obtained in parallel analyses performed by the same laboratory or by different laboratories. Introduction of the more sensitive IS2404 TaqMan assay will thus improve PCR confirmation of clinical diagnosis of Buruli ulcer and yield fewer false-negative results. Due to their high sensitivity, PCR-based assay systems are prone to yield false-positive results caused by cross-contamination. Real-time PCR reduces this risk considerably by avoiding the post-PCR handling of the samples containing vast amounts of PCR products. Nevertheless, it is advisable, even with the IS2404 TaqMan assay, to strictly adhere to the three-room principle: room 1 for the preparation of the PCR mix, room 2 for the processing of samples and the preparation of template DNA in a biosafety cabinet, and room 3 for PCR amplification.
Apart from the confirmation of clinical diagnosis, real-time PCR tests have a broad range of potential applications, including the quantification of target DNA in clinical specimens and environmental samples. M. ulcerans is an environmental mycobacterium, and it appears that infection with it is related to swampy environments. M. ulcerans has been isolated from aquatic bugs belonging to the genus Naucoris (18) and detected by conventional PCR both in water samples (24) and in water insects (22, 23, 26). The IS2404 TaqMan assay may help in the future to identify the major environmental reservoir(s) of this pathogen.
Currently, surgery is the only proven effective treatment of M. ulcerans disease (3). Lesions in the advanced stages, i.e., late preulcerative and ulcerated forms, may require excisions that include deep fascia and sometimes even muscle. In all forms, excisions must include healthy tissue at the lateral and deep margins. The IS2404 TaqMan assay may help to answer the question of how wide surgical excision has to be to avoid recurrences. Currently the decision on the size of excision is largely left to the experience and judgment of the surgeon. The surgeon has to compromise between the risk of recurrence and an oversized excision, associated with the need for more-extensive skin grafting, increased risk of secondary infections, and longer hospitalization. IS2404 TaqMan analyses will be more suitable for the analysis of the dissemination of mycobacteria in Buruli ulcer lesions than the more labor-intensive and less specific and sensitive enumeration of AFB in ZN-stained tissue sections. Cultivation of M. ulcerans is not a suitable quantification method, since decontamination methods have a detrimental effect on the organism and primary cultivation requires between 6 and 8 weeks or longer (21).
As a typical example, data obtained with excised tissue from a patient with a preulcerative plaque lesion on the forearm are presented in this report. The heaviest mycobacterial burden was found in a sample of subcutaneous tissue located about 0.5 cm below the surface in the center of the lesion. It is likely that this sample is part of the original focus of the infection, which seemed to be still relatively small in this late preulcerative stage, since three other samples taken from the center of the plaque (C in Fig. 3) contained less than 2% of this amount of M. ulcerans DNA. Levels were still lower in all the other samples taken from more-peripheral affected tissues or from macroscopically healthy tissues. In agreement with these findings, immunohistopathological studies of Buruli ulcer lesions have indicated that tissue necrosis extends far beyond the regions in which microcolonies of AFB are detected (5). These results support the hypothesis that diffusible toxins are associated with the pathology of M. ulcerans infection (10, 13). After introduction of the mycobacteria into the dermis or subcutaneous tissue, there is presumably a latent phase during which the slow-growing bacteria proliferate and elaborate sufficient toxin to destroy the surrounding tissue. The subsequent necrosis, especially of fatty tissue, may then provide a favorable milieu for further proliferation (19). Analyses of the spreading of the mycobacteria may contribute to our understanding of the pathogenesis of Buruli ulcer and guide surgical treatment. Lesions appear to progress differently, and it is possible that differences in the dynamics of mycobacterial spreading that require different types of intervention can be distinguished. It is remarkable that the levels of M. ulcerans DNA in affected tissue outside the center of the plaque lesion and in the excised margins of apparently healthy tissue were comparable. During the collection of tissue samples and the preparation of template DNA, extreme care was taken to avoid cross-contamination. Nevertheless, it cannot be completely excluded that at least part of the signals observed with these more peripheral samples are related to contamination from the infection focus during excision. However, based on concentration gradients observed with excised samples from more-advanced Buruli ulcer lesions, it appears likely that the signals observed reflect the spreading of relatively small numbers of mycobacteria. These preliminary analyses also indicate that detection of large quantities of M. ulcerans DNA by real-time PCR correlates with large numbers of AFB in the same region (unpublished results). Removal of the complete burden of M. ulcerans may not be possible even with very wide excision. After surgery, the primed immune system may, however, be able to control small numbers of residual mycobacteria devoid of a protective cloud of necrotic tissues and bacterial toxin(s).
To date, no antibiotic treatment has proven to be consistently effective in the treatment of Buruli ulcer (3). In a mouse footpad model of M. ulcerans infection, treatment with a combination of rifampin and amikacin has yielded promising results (7), and clinical trials with combinations of antibiotics are currently in progress (11). Monitoring of the response to chemotherapy may represent another useful application of the IS2404 TaqMan assay. Real-time PCR has been used to monitor the efficacy of antimalarial (15), antibacterial (2), and antiviral (17, 31) treatment. In the case of an IS6110 TaqMan assay for M. tuberculosis, it has been found that the amounts of DNA quantified in sputum corresponded well with the numbers of AFB counted by microscopy (8). Before initiation of antituberculosis therapy, measures of AFB, M. tuberculosis DNA, and cultivable bacilli were comparable, suggesting that quantification of DNA is a good method for measuring the initial bacillary load. However, the decline in cultivable bacilli in the specimen did not correlate with the rate of disappearance of both AFB and M. tuberculosis DNA. Therefore, these tests were not appropriate for monitoring treatment efficacy (8). In contrast, the rapid disappearance of M. tuberculosis mRNA suggested that it is a good indicator of microbial viability and a useful marker for monitoring the efficacy of chemotherapy (9). The same may hold true for M. ulcerans disease, which may also require the development of a real-time reverse transcription-PCR system. IS2404, encoding a 328-amino-acid transposase, should be a suitable target also for such an assay.
Acknowledgments
We thank Thomas Junghanss for his critical reading of the manuscript and for his help during the sample collection.
This work was in part supported by the Stanley Thomas Johnson Foundation.
REFERENCES
- 1.Asiedu, K., R. Scherpbier, and M. Raviglione. 2000. Buruli ulcer, Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland.
- 2.Bockenstedt, L. K., J. Mao, E. Hodzic, S. W. Barthold, and D. Fish. 2002. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 186:1430-1437. [DOI] [PubMed] [Google Scholar]
- 3.Buntine, J., and K. Crofts (ed.). 2001. Buruli ulcer; management of Mycobacterium ulcerans disease; a manual for health care providers. World Health Organization, Geneva, Switzerland.
- 4.Chemlal, K., G. Huys, F. Laval, V. Vincent, C. Savage, C. Gutierrez, M. A. Laneelle, J. Swings, W. M. Meyers, M. Daffe, and F. Portaels. 2002. Characterization of an unusual Mycobacterium: a possible missing link between Mycobacterium marinum and Mycobacterium ulcerans. J. Clin. Microbiol. 40:2370-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor, D. H., W. M. Meyers, and R. E. Kreig. 1976. Infection by Mycobacterium ulcerans, p. 226-235. In C. H. Binford and D. H. Connor (ed.), Pathology of tropical and extraordinary diseases. Armed Forces Institute of Pathology, Washington, D.C.
- 6.Debacker, M., C. Zinsou, J. Aguiar, W. Meyers, and F. Portaels. 2002. Mycobacterium ulcerans disease (Buruli ulcer) following human bite. Lancet 360:1830. [DOI] [PubMed] [Google Scholar]
- 7.Dega, H., A. Bentoucha, J. Robert, V. Jarlier, and J. Grosset. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 46:3193-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardin, L. E., Y. Chen, M. D. Perkins, L. Teixeira, M. D. Cave, and K. D. Eisenach. 1998. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J. Clin. Microbiol. 36:1964-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desjardin, L. E., M. D. Perkins, K. Wolski, S. Haun, L. Teixeira, Y. Chen, J. L. Johnson, J. J. Ellner, R. Dietze, J. Bates, M. D. Cave, and K. D. Eisenach. 1999. Measurement of sputum Mycobacterium tuberculosis messenger RNA as a surrogate for response to chemotherapy. Am. J. Respir. Crit. Care Med. 160:203-210. [DOI] [PubMed] [Google Scholar]
- 10.Dobos, K. M., P. L. Small, M. Deslauriers, F. D. Quinn, and C. H. King. 2001. Mycobacterium ulcerans cytotoxicity in an adipose cell model. Infect. Immun. 69:7182-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espey, D. K., G. Djomand, I. Diomande, M. Dosso, M. Z. Saki, J. M. Kanga, R. A. Spiegel, B. J. Marston, L. Gorelkin, W. M. Meyers, F. Portaels, M. S. Deming, and C. R. Horsburgh, Jr. 2002. A pilot study of treatment of Buruli ulcer with rifampin and dapsone. Int. J. Infect. Dis. 6:60-65. [DOI] [PubMed] [Google Scholar]
- 12.George, K. M., L. P. Barker, D. M. Welty, and P. L. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 14.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, M. A., C. H. Tan, L. T. Aw, C. S. Tang, M. Singh, S. H. Lee, H. P. Chia, and E. P. Yap. 2002. Real-time fluorescence-based PCR for detection of malaria parasites. J. Clin. Microbiol. 40:4343-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lie, Y. S., and C. J. Petropoulos. 1998. Advances in quantitative PCR technology: 5′ nuclease assays. Curr. Opin. Biotechnol. 9:43-48. [DOI] [PubMed] [Google Scholar]
- 17.Mackay, I. M., K. E. Arden, and A. Nitsche. 2002. Real-time PCR in virology. Nucleic Acids Res. 30:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsollier, L., R. Robert, J. Aubry, J. P. Saint Andre, H. Kouakou, P. Legras, A. L. Manceau, C. Mahaza, and B. Carbonnelle. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers, W. M. 1994. Mycobacterial infections of the skin, p. 291-377. In G. Seifert (ed.), Tropical dermatology. Springer-Verlag, Heidelberg, Germany.
- 20.Palca, A., C. Aebi, R. Weimann, and T. Bodmer. 2002. Mycobacterium bohemicum cervical lymphadenitis. Pediatr. Infect. Dis. J. 21:982-984. [DOI] [PubMed] [Google Scholar]
- 21.Portaels, F., P. Johnson, and W. M. Meyers (ed.). 2001. Buruli ulcer; diagnosis of Mycobacterium ulcerans disease; a manual for health care providers. World Health Organization, Geneva, Switzerland.
- 22.Portaels, F., P. Elsen, A. Guimaraes-Peres, P. A. Fonteyne, and W. M. Meyers. 1999. Insects in the transmission of Mycobacterium ulcerans infection. Lancet 353:986. [DOI] [PubMed] [Google Scholar]
- 23.Roberts, B., and R. Hirst. 1997. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 35:2709-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross, B. C., P. D. Johnson, F. Oppedisano, L. Marino, A. Sievers, T. Stinear, J. A. Hayman, M. G. Veitch, and R. M. Robins-Browne. 1997. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl. Environ. Microbiol. 63:4135-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and P. D. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinear, T., J. K. Davies, G. A. Jenkin, F. Portaels, B. C. Ross, F. Oppedisano, M. Purcell, J. A. Hayman, and P. D. Johnson. 2000. A simple PCR method for rapid genotype analysis of Mycobacterium ulcerans. J. Clin. Microbiol. 38:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stinear, T., B. C. Ross, J. K. Davies, L. Marino, R. M. Robins-Browne, F. Oppedisano, A. Sievers, and P. D. Johnson. 1999. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J. Clin. Microbiol. 37:1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stinear, T. P., G. A. Jenkin, P. D. Johnson, and J. K. Davies. 2000. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J. Bacteriol. 182:6322-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thangaraj, H. S., M. R. Evans, and M. H. Wansbrough-Jones. 1999. Mycobacterium ulcerans disease; Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 93:337-340. [DOI] [PubMed] [Google Scholar]
- 31.Vats, A., R. Shapiro, R. P. Singh, V. Scantlebury, A. Tuzuner, M. Saxena, M. L. Moritz, T. J. Beattie, T. Gonwa, M. D. Green, and D. Ellis. 2003. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation 75:105-112. [DOI] [PubMed] [Google Scholar]