Abstract
Acute tick-borne encephalitis is diagnosed by detection of IgM antibodies to tick-borne encephalitis virus (TBEV) (genus Flavivirus) in patient serum. TBEV membrane (M) and envelope (E) proteins have previously been shown to form virus-like particles when expressed in mammalian cells. We expressed the prM/M and E proteins in insect cells with a recombinant baculovirus system and obtained antigenic protein secreted into the cell culture medium, as evidenced by detection by a panel of five monoclonal antibodies to TBEV E protein. According to the sedimentation pattern in sucrose gradient centrifugation, the proteins were most likely secreted as virus-like particles. A μ-capture immunoglobulin M-enzyme immunoassay (IgM-EIA) test was developed and compared to a commercially available TBEV-IgM test (Progen) based on inactivated purified TBEVs. With a panel of 100 TBEV-IgM-negative, 50 TBEV-IgM-positive, and seven dengue virus-IgM-positive serum samples from our diagnostic laboratory, a sensitivity of 100% and specificity of 99% were obtained, and the correlation coefficient of EIA absorbances with the reference test was 0.93. The antigen was also suitable for IgG antibody detection in an immunofluorescent assay format. This is the first time that secreted, fully antigenic E protein has been produced in insect cells for this arthropod-borne flavivirus.
Tick-borne encephalitis virus (TBEV) is a member of the genus Flavivirus of the family Flaviviridae. Other important flaviviruses pathogenic to humans include yellow fever, Japanese encephalitis, and dengue viruses (13). TBEV has three structural proteins: C (core), M (membrane), and E (envelope). The M and E proteins together with the cell-derived lipid bilayer form the envelope of TBEV. The E protein is the dominant antigen in TBEV (12).
There are three main genetic lineages of TBEV, the European, Siberian, and Far Eastern subtypes (5, 7, 13), formerly known as the Central European, West Siberian, and Russian spring-summer encephalitis viruses, respectively. The variation at the amino acid level of different virus strains within a genetic lineage is 2.2% and between different subtypes not more than 5.6% (5).
Tick-borne encephalitis is a zoonotic arbovirus infection. The European subtype is transmitted by Ixodes ricinus, and the Siberian and Far Eastern subtypes are transmitted by Ixodes persulcatus ticks, of which the latter subtype may cause a more severe disease (5, 8). However, e.g., in Latvia, all three subtypes are circulating with no clear evidence of differences in severity (25). The course of tick-borne encephalitis is biphasic: during the first phase, approximately 7 days after a tick bite, the patient has general symptoms such as fever, headache, and malaise; the patient is viremic, and TBEV can be isolated. After an asymptomatic interval of approximately 8 days, the the disease progresses to the second phase in 20 to 30% of patients. In this second phase, central nervous system manifestations (meningitis, encephalitis, and meningoencephalitis) occur (4, 20). About one-third of patients who experience the second phase have sequelae (4, 9, 10, 19). IgM antibodies are detectable in the serum during the second stage of the illness, but reverse transcription-PCR is almost always negative (31). The reported case fatality rates vary between 0 and 3% in Europe (19, 36) to up to 30% in Asian parts of Russia, which may reflect differences in the virulence of TBEV strains or differences in clinical alert or hospitalization rates in western European countries and in Asia (5).
The methods used to detect TBEV antibodies in serum include hemagglutination inhibition, neutralization, immunofluorescence, complement fixation, and enzyme immunoassay (EIA). The serology is complicated by the wide cross-reactivity between different flaviviruses (32). There are some commercially available immunoglobulin M (IgM) and IgG tests (30) mainly based on purified and inactivated TBEV antigen. Although the use of commercially available EIA tests in a diagnostic laboratory does not require any special safety precautions, the production and purification of TBEV antigen requires biosafety level 3 facilities and specially trained staff.
The TBEV M and E proteins are translocated to the endoplasmic reticulum and acquire their mature conformations in the secretory pathway, where prM is cleaved by furin into its mature form, M (3, 24, 35). These proteins have also been expressed in recombinant expression systems in mammalian cells, where they have been shown to form secreted virus-like particles (1, 14, 33). The structure of the particles has been characterized (6). They are about 30 nm in diameter, compared to about 50 nm for virions. Virus-like particles are apparently also formed normally during flavivirus infection (32).
The aims of this study were, first, to express optimally folded, secreted, and antigenic TBEV M and E proteins or virus-like particles in insect cells, not reported before for this arthropod-borne biohazardous virus, and subsequently to assess the applicability of the antigen in diagnostic tests for tick-borne encephalitis.
MATERIALS AND METHODS
Construction of recombinant baculoviruses.
RNA was isolated from mouse brains infected with TBEV strain Kumlinge A52 (2) with TriPure reagent (Roche Molecular Biochemicals, Basel, Switzerland). The segment coding for the TBEV prM and E proteins was reverse transcribed to cDNA with forward primer 5′-TTTTCTAGATCTAATGGTTGGCTTGCAAAAACG and reverse primer 5′-TTTCTGCAGCTAGTCATACCATTCTGAGACCTC. The reverse transcription reaction was performed with the Moloney murine leukemia virus reverse transcriptase enzyme (Fermentas, Vilnius, Lithuania) at 37°C for 1.5 h. The resulting cDNA was amplified by PCR with the same primers as in the reverse transcription reaction and the enzymes Taq DNA polymerase (Fermentas) and Taq extender (Stratagene, La Jolla, Calif.) with six cycles of 95°C for 50 s, 50°C for 50 s, and 72°C for 4 min, followed by 31 cycles of 95°C for 50 s, 55°C for 50 s, and 72°C for 3 min.
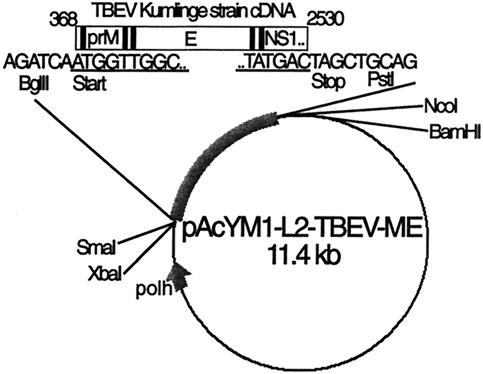
The amplified DNA was then extracted from the 1% agarose gel with the QIAquick gel extraction kit protocol (Qiagen, Hilden, Germany) and cloned into the pGEM-T vector (Promega, Madison, Wis.) according to the manufacturer's instructions. From the plasmid vector, the insert was cut with BglII and PstI and subcloned into the baculovirus expression vector pAcYML2 (kindly provided by Johan Peränen, Institute of Biotechnology, University of Helsinki), modified at the polylinker site from pAcYM1 (28). pAcYML2-TBEV-prME (Fig. 1) was purified with the Qiagen plasmid maxi kit (Qiagen) and cotransfected with BaculoGold virus DNA (PharMingen, San Diego, Calif.) into Sf9 (Spodoptera frugiperda) cells with FuGene reagent (Roche Molecular Biochemicals).
FIG. 1.
Construction of pACYML2-TBEV-prME. Black bars represent hydrophobic signal and transmembrane sequences. polh, polyhedrin promoter; underlining, TBEV Kumlinge strain-specific sequence.
Expression of recombinant prM-E.
Sf9 American Type Culture Collection (ATCC) and, for comparison, High Five (Invitrogen, Carlsbad, Calif.) cells infected with recombinant baculovirus were grown as monolayers in Sf-900 medium (Invitrogen) supplemented with 10% fetal calf serum, fungizone, glutamine, penicillin, and streptomycin for 4 to 6 days at 27°C. The cells were pelleted in an Eppendorf centrifuge 5403 at 1,500 rpm (equals 400 × g) for 5 min at 4°C and washed twice with phosphate-buffered saline (PBS). The pellets were used as the antigen in an immunofluorescence assay (IFA) or stored at −20°C and used in immunoblotting. The supernatant was stored at 4°C and used as an antigen for the enzyme immunoassay (EIA).
Antigenic properties of recombinant TBEV proteins determined with MAbs.
The production and characterization of the monoclonal antibodies directed against TBEV E protein have been described earlier in detail (29). Briefly, the monoclonal antibodies MAbs were raised against TBEV antigen which consisted of formaldehyde-inactivated purified TBEV virus (strain K23) by immunizing mice with 100 μg of the antigen. The recognition of recombinant E protein by five MAbs, 1493, 171, 1718, 1786, and 1418, was detected by immunoblotting and immunofluorescence. In immunoblotting, the samples were reduced with 2-mercaptoethanol, separated on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. MAbs (3.3 μg/ml) in TEN-T (0.05% Tween 20 in 50 mM Tris-HCl, 5 mM EDTA, and 150 mM NaCl) with 3% skim milk powder were incubated on filters for 1 h at room temperature. Peroxidase-conjugated anti-mouse immunoglobulin (Dako, Glostrup, Denmark), diluted 1:1,000 in TEN-T with 3% skim milk, was incubated for 1 h. Excess MAbs and conjugate were washed with TEN-T. The enzyme reaction was detected with electrochemiluminescence.
In the immunofluorescence assay, recombinant baculovirus-infected and uninfected High Five cells were fixed to microscope slides with acetone. MAbs (5 μg/ml) were incubated for 30 min at 37°C. Fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (Dako, Glostrup, Denmark), diluted 1:30 in PBS, was incubated for 30 min at 37°C. Excess antibody and conjugate were washed away with PBS and deionized distilled water. The slides were covered with mounting medium (50% glycerol) and coverslips and examined with a fluorescence microscope.
Purification of antigens by ultracentrifugation.
The insect cell supernatant was first centrifuged at 11,500 rpm in a Sorvall SS-34 rotor (equals 15,900 × g) for 30 min at 4°C. The supernatant from this centrifugation was ultracentrifuged in a Beckman SW41 Ti rotor at 38,000 rpm (equals 240,000 × g) for 2 h at 4°C. The pellet was resuspended in 50 μl of TEN (50 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl). The sucrose gradient ultracentrifugation of this pellet was done in a 10 to 60% (wt/vol) sucrose gradient in a Beckman SW50.1 Ti rotor at 43,000 rpm (equals 230,000 × g) for 23 h at 4°C. Fractions of 250 μl were collected by upward displacement and assayed by EIA. EIA for each fraction diluted 1:16 was performed with a μ-capture IgM format, as described below, with a positive control human serum.
Serum panels.
We studied a panel of 150 serum samples. The serum samples were sent to our diagnostic laboratory (Helsinki University Central Hospital Laboratory Diagnostics, Department of Virology, Zoonosis unit) because of suspicion of tick-borne encephalitis. Fifty serum samples were previously found positive and 100 controls were previously found negative in a commercial TBEV IgM test (Progen Biotechnik GmbH, Heidelberg, Germany). Two of these IgM-positive serum samples were positive for rheumatoid factor. To detect the possible cross-reactions with antibodies to other flaviviruses, we also included seven serum samples found to be positive for dengue virus IgM antibodies in a commercial dengue virus IgM test (Focus Technologies, Cypress, Calif.) in our diagnostic laboratory.
Reference tests.
As an IgM reference test, we used the Immunozym FSME IgM test (Progen Biotechnik GmbH, Heidelberg, Germany) according to the manufacturer's instructions. This is a two-step EIA with rheumatoid factor absorbent and based on EIA wells coated with inactivated, purified TBEV. Briefly, diluted serum samples and subsequently the anti-human IgM conjugate were incubated in the wells, and the bound conjugate was detected by incubation with a substrate; the reaction was stopped with sulfuric acid, and the optical density was measured at a wavelength of 450 nm. The cutoff values were provided by the manufacturer in the test kits and had some variation (0.320 to 0.675) depending on the particular lot used.
Total antibodies to TBEV from serum were also determined by an in-house hemagglutination inhibition test. Inactivated TBEV-infected cell culture supernatant was used as the antigen. Diluted serum samples were incubated with kaolin and goose erythrocytes to adsorb nonspecific agglutinating factors. Dilution series of preadsorbed serum samples were incubated overnight at 4°C in microtiter wells with TBEV antigen. On the next day, a 0.2% dilution of goose erythrocytes (pH 6.4) was incubated in each well at room temperature for 1 h, after which the result was read (38).
The dengue fever virus IgM capture ELISA test (Focus Technologies, Cypress, Calif.) was used as a reference test for the diagnosis of dengue virus IgM antibodies from serum samples according to the manufacturer's instructions. Briefly, it is a μ-capture IgM test. EIA wells were coated with anti-human IgM antibody. First, diluted serum samples and then the dengue virus antigen, containing equal proportions of inactivated dengue virus serotypes 1 to 4, were incubated in the wells, and then the peroxidase-conjugated mouse anti-dengue virus antibody was added and incubated. Excess reagents were washed away after each incubation. The enzyme reaction was detected by incubation with a substrate, the reaction was stopped by sulfuric acid, and the optical density was measured at a wavelength of 450 nm.
TBEV IgM μ-capture assay (EIA).
Goat anti-human IgM serum (Cappel, West Chester, Pa.), diluted 1:500 in 0.05 M carbonate buffer (pH 9.6), was incubated in microtiter wells at room temperature overnight and stored at 4°C until use. Patient serum samples diluted 1:200 in EIA buffer (PBS with 0.5% bovine serum albumin and 0.05% Tween 20) were incubated for 1 h at 37°C in duplicate wells. Recombinant baculovirus-expressed TBEV prME antigen (1:5 dilution of unprocessed insect cell supernatant in EIA buffer) was incubated for 1 h at 37°C. Peroxidase-conjugated anti-TBEV MAb 1786 (24) was used at a concentration of 6 μg/ml and incubated for 1 h at 37°C. Unbound excess antibodies, antigen, and conjugate were washed away after each incubation with PBS-0.05% Tween 20. Specific antibody binding was detected with tetramethylbenzidine substrate (Sigma, St. Louis, Mo.). After incubation for 15 min at room temperature, the enzyme reaction was stopped with 0.5 M H2SO4, and the absorbance at 450 nm was measured. To control the interplate variation, an acute-phase tick-borne encephalitis patient serum was used as an internal standard in all plates. The mean of duplicate standard absorbances was given a value of 2,000, and the means of the sample absorbances were adjusted to this after reducing the mean absorbance value with the background mean absorbance value (obtained with diluent only) (37).
EIA of the ultracentrifugation fractions was performed with a μ-capture IgM format in a similar way. A positive-control human serum was diluted 1:200, ultracentrifugation fractions 1:16, and peroxidase-conjugated MAb 1786 1:400. All the incubations and washing steps were done in the same way as for the patient samples as described above.
TBEV IgG IFA.
Infected and uninfected High Five cells were fixed onto microscope slides with acetone for 5 min and stored at 4°C until use. Patient serum samples diluted 1:10, 1:40, 1:160, 1:640, and 1:2,560 in PBS were incubated for 30 min at 37°C. Fluorescein isothiocyanate-conjugated anti-human IgG (Jackson ImmunoResearch, West Grove, Pa.), diluted 1:50 in PBS, was incubated for 30 min at 37°C. Unbound antibodies and anti-human IgG were washed away with PBS and distilled water. The slides were covered with mounting medium and coverslips and examined with a fluorescence microscope.
Nucleotide sequence accession number.
The coding sequence for the genes coding for the prM and E proteins of TBEV strain Kumlinge A52 (2,163 nucleotides) was submitted to GenBank and given accession number AY268437.
RESULTS
Expression of TBEV prM and E.
Sf9 and High Five insect cells were infected with recombinant baculovirus containing the coding sequences for the prM and E proteins of TBEV strain Kumlinge A52. The sequence (2,163 nucleotides), submitted to GenBank with accession number AY268437, was 98% identical to that of the reference strain Neudoerfl (accession number U27495) (26). The E protein amino acid sequence was identical, and the nucleotide sequence differed at three residues from that previously reported for 1,142 nucleotides of strain Kumlinge A52 (40).
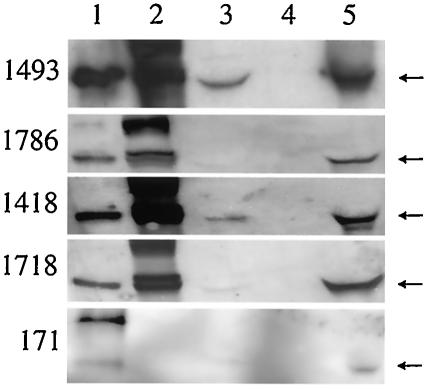
Both infected cell lines expressed equivalent amounts of a 55-kDa protein when cultured in Sf-900 medium including 10% fetal calf serum and antibiotics. The expressed protein was recognized by four out of five anti-TBEV E MAbs (29) from the cells in IFA and immunoblotting and by all five MAbs from the cell culture medium in immunoblotting (Fig. 2), showing that the E protein was secreted. In the IFA test and in immunoblotting, MAb 171 did not recognize the recombinant protein from the infected cells. Experiments of expression of the proteins in serum-free Sf-900 medium (including antibiotics) led to slower growth of the insect cells and lower levels of the secreted TBEV envelope proteins in the medium (data not shown). The recombinant prM and E proteins did not constitute more than a few percent of the total cell protein, as estimated from an SDS-PAGE gel stained with Coomassie blue.
FIG. 2.
Immunoblotting of baculovirus TBEV-prME-infected Sf9 cells and cell culture supernatants with five monoclonal antibodies. The samples were reduced with 2-mercaptoethanol and separated in SDS-10% PAGE. Lanes: 1, TBEV-infected Vero cells (a positive control); 2, baculovirus TBEV prME-infected Sf9 cells; 3, supernatant from baculovirus TBEV prME-infected Sf9 cells; 4, uninfected Sf9 cells (a negative control); 5, concentrated supernatant from baculovirus TBEV prME-infected Sf9 cells. An arrow indicates the position of the E protein.
Sucrose gradient centrifugation.
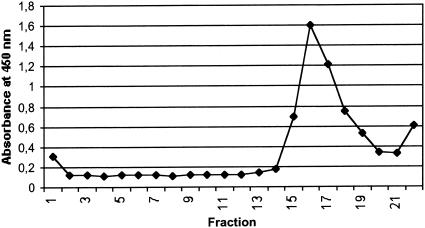
The cell culture medium of insect cells expressing TBEV prM and E proteins was centrifuged in a 10 to 60% (wt/vol) sucrose gradient, and fractions of 250 μl were analyzed by EIA. A peak in antigenicity was measured in a fraction with 36% sucrose, corresponding to a buoyant density of 1.15 g/cm3 (Fig. 3).
FIG. 3.
Antigenicity of different sucrose gradient fractions measured by μ-capture EIA with peroxidase-conjugated anti-TBEV MAb 1786 as a conjugate. After ultracentrifugation in a sucrose gradient, fractions of 250 μl were separated by upward displacement. Fraction number 1 is from the top, and number 22 is from the bottom. A peak in antigenicity was observed in fractions with 36% (wt/vol) sucrose.
μ-Capture IgM assay.
A μ-capture IgM assay was set up based on baculovirus-expressed TBEV prM/M and E antigens and peroxidase-conjugated anti-TBEV E MAb 1786 (29) for determination of IgM antibodies in human serum samples. A panel of 150 serum samples (50 IgM positive and 100 IgM negative) were studied, and the results were compared to those obtained with a commercially available IgM test (Progen), which is based on directly coated purified TBEV antigen and is routinely used in our diagnostic laboratory (Table 1). Forty negative control serum samples were first used to adjust the cutoff value of the assay, and the mean absorbance plus three standard deviations was 0.083 + 3 × 0.0486 ≈ 0.229. The cutoff was adjusted to a 25% higher value, to 0.29. Fifty serum samples previously found positive in the Progen TBEV IgM assay with native TBEV antigen were analyzed. Forty-eight of them were positive in this assay with recombinant TBEV antigen, and the absorbance values ranged from 0.500 to 2.448. The two negative samples were derived within an interval of 1 month from the same patient who was positive for rheumatoid factor and had a constant low level of hemagglutination inhibition antibodies to TBEV and no symptoms of tick-borne encephalitis. All of the 100 IgM serum samples that were negative in the reference test were also negative in our test.
TABLE 1.
Comparison of results of μ-capture IgM test based on the baculovirus-expressed antigen and Progen Immunozym FSME IgM and Focus Technologies dengue fever virus IgM capture ELISA
| Reference test resulta | TBEV ME IgM result
|
No. of samples | |
|---|---|---|---|
| IgM negative | IgM positive | ||
| TBEV IgM negative, HI negative | 88 | 0 | 88 |
| TBEV IgM negative, HI positive | 12 | 0 | 12 |
| TBEV IgM positive, HI positive | 2b | 48 | 50 |
| Dengue virus IgM positive, TBEV IgM negative | 6 | 1 | 7 |
HI, hemagglutination inhibition.
Includes two sera from one person positive for rheumatoid factor (see text).
We calculated the correlation between the absorbance values of our IgM test and the Progen FSME IgM from a panel of 103 serum samples analyzed originally with the same lot. The cutoff values varied between different lots of the reference test. The correlation coefficient was 0.93 (Fig. 4).
FIG. 4.
Comparison of μ-capture IgM EIA test and Progen IgM EIA adjusted absorbance values for 103 serum samples that were analyzed with the same Progen Immunozym FSME IgM test lot and thus are comparable to each other. The correlation coefficient between these two tests was 0.93. The cutoff value of our test was adjusted to 0.29, and for Progen's test in this lot, it was 0.55.
The sensitivity of our TBEV IgM test was 100% when calculated as the ratio of correct positive results (n = 48) to positive results in reference Progen tick-borne encephalitis IgM test (n = 48) and false-negative results (none). The two serum samples positive for rheumatoid factor and negative in our IgM test were not included in these calculations because they were apparently false-positives in the reference test. Thus, the sensitivity for this panel was 100%.
We also included acute-phase serum samples from patients infected with another flavivirus, dengue virus. Out of seven serum samples positive for dengue virus IgM antibodies in a commercial μ-capture IgM test (Focus Technologies), six were negative in our TBEV IgM test and one gave a low positive result. When those were included in the calculation of the specificity, when calculated as the ratio of the negative results from our test (n = 106) to the correct negative results (n = 106) and the false-positive results (n = 1), the specificity was 99%. If the cross-reacting flavivirus serum samples were excluded, the specificity was 100%.
IgG-IFA results.
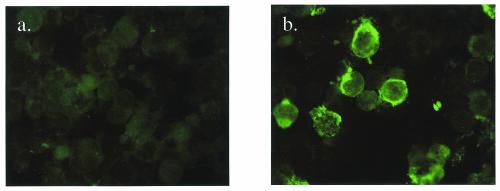
IgG antibodies to TBEV in serum samples were detected by IFA with baculovirus TBEV ME-infected acetone-fixed insect cells as the antigen (Fig. 5). Of 88 serum samples, 84 (95%) that were negative (titer < 10) in the hemagglutination inhibition test were also negative (titer < 10) in the IgG-IFA. All acute-phase tick-borne encephalitis serum samples (n = 48) were also positive in the IgG-IFA; however, only 7 of 12 samples likely to represent old immunity (hemagglutination inhibition titer ≥ 10, IgM negative) were positive in the IgG-IFA.
FIG. 5.
Immunofluorescence. High Five cells were infected with recombinant baculovirus expressing TBEV prM and E proteins and used in the IgG IFA test. (a) IgG-negative serum samples; (b) IgG-positive serum samples.
DISCUSSION
Most of the medically important flaviviruses are transmitted by arthropods and replicate readily in insect or tick cells. However, there are no previous reports of production of properly folded antigen in the baculovirus system in insect cells, although fully characterized virus-like particles from mammalian cells have been described (1, 14, 33). Previously, when expressed in insect cells, the TBEV E protein did not fold properly and was demonstrated to be recognized only by IgG antibodies in serum from vaccinated people but not by serum from tick-borne encephalitis patients in the acute or convalescent phase (27).
In the present study, we expressed recombinant TBEV prM/M and E proteins in insect cells. The recombinant E protein was recognized in concentrated cell culture supernatant by five different monoclonal antibodies directed against TBEV E protein and in diluted (1:5) cell culture supernatant by 48 TBEV IgM-positive human serum samples, suggesting that the proteins were antigenically active. One of the monoclonal antibodies, 171, did not recognize the recombinant protein from the infected cells by immunofluorescence test or by immunoblotting, although it did recognize the E protein from the concentrated cell culture supernatant. The reason for this may be in the sensitivity, since the antigen in the supernatant was concentrated for immunoblotting, or potentially in a qualitative difference in the epitope affected by the modification steps during the glycoprotein maturation and budding of virus-like particles from the cells.
The proteins were secreted from the cells into the culture medium. Because the transmembrane regions are included in the protein construct, they can only be secreted as membrane-bound, supposedly virus-like particles, including lipid membrane and envelope proteins but lacking the nucleocapsid. After sucrose gradient ultracentrifugation, a peak in antigenicity was measured in a fraction where the buoyant density was 1.15 g/cm3. This is close to what was found earlier for TBEV virus-like particles produced by expression of the same proteins in mammalian cells (33). The buoyant density of whole virions is 1.19 g/cm3 (33). It has been shown that, for Japanese encephalitis virus virus-like particles produced in mammalian cells, the buoyant density in sucrose gradient is 1.15 g/cm3 (18).
The supernatant from TBEV-prME expressing baculovirus-infected insect cells performed well as an antigen in the μ-capture IgM test. The supernatant was merely diluted, and the antigen did not need to be purified. In this material, with cross-reacting flavivirus serum samples included, the sensitivity was 100% and the specificity was 99%. In the case of the two serum samples with rheumatoid factor, our μ-capture test based on recombinant antigen gave negative results and the commercial reference test gave low positive results. It seems likely that the latter results are false-positives in the reference test because the serum samples were from the same person and collected within a 1-month interval and there was no rise in antibody level between these two samples and no clinical symptoms of tick-borne encephalitis were observed.
Vaccination is the most effective measure in the prevention of tick-borne encephalitis, as demonstrated by the high vaccination coverage of the Austrian population and subsequent significant reduction in the number of severe encephalitis cases (39; http://www.tbe-info.com). There are two commercially available formaldehyde-inactivated tick-borne encephalitis vaccines, FSME Immun Inject (Baxter Immuno) and Encepur (Chiron Behring). However, there are thousands of cases of tick-borne encephalitis annually in Europe. In Finland, about 30 to 40 are reported annually, and the number has been increasing in recent years (11; http://www.tbe-info.com). The highest number of cases has been reported from the Baltic states and eastern central Europe (e.g., in Latvia, Lithuania, and the Czech Republic, there are several hundred cases annually), and all three known subtypes are circulating in Latvia at least (25).
The European subtype of TBEV is the only subtype endemic in Finland (11), so in our material we did not have tick-borne encephalitis patients infected with the Far Eastern or Siberian subtypes. However, it is most likely that an antigen based on one of the TBEV subtypes recognizes the antibodies formed against any TBEV strain, as suggested by the facts that the formaldehyde-inactivated vaccine based on the European subtype protects from a disease caused by any of the three TBEV subtypes (4, 15, 16, 21, 34, 41) and that mice immunized with the western subtype and then challenged with different western and Far Eastern strains show no significant difference in cross-protection experiments (16). Also, variation in E protein at the amino acid level between different subtypes is only 5.6% (5).
Cross-reactions between different flaviviruses are typical in serological tests. We included seven dengue virus IgM-positive serum samples in this study. Six of these serum samples were negative in our μ-capture TBEV IgM test, indicating weak cross-reactivity with other flaviviral infections.
The antigen in infected cells could also be used for detection of TBEV IgG antibodies in an IFA format. This was not the actual focus of our study, and the results were compared only to the results of total antibody (hemagglutination inhibition) and IgM-EIA tests. All acute-phase serum samples were also detected by IgG-IFA, but the sensitivity for old-immunity serum samples (with TBEV hemagglutination inhibition antibodies but not IgM) was lower.
In conclusion, the IgM test based on recombinant noninfectious antigen produced in insect cells seems to be adequate for diagnostic use. In Europe, there are thousands of confirmed cases of tick-borne encephalitis annually, and there is a need for recombinant noninfectious antigens.
In view of the relatively low yields of TBEV diagnostic antigen in insect cells, in the future we aim to attempt to increase the expression level to produce more concentrated antigen for use in, e.g., an immunochromatographic test format (17). A possibility is the expression of antigens in stable cell lines, as reported for mammalian cell lines continuously producing Japanese encephalitis virus-like particles (18, 23) and dengue virus type 2 virus-like particles (22).
Acknowledgments
We thank Tytti Manni, Raija Leveelahti, Leena Kostamovaara, and Irja Luoto for expert technical assistance.
We thank TEKES (no. 40133/01) for funding.
REFERENCES
- 1.Allison, S. L., K. Stadler, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummer-Korvenkontio, M., P. Saikku, P. Korhonen, and N. Oker-Blom. 1973. Arboviruses in Finland. I. Isolation of tick-borne encephalitis (tick-borne encephalitis) virus from arthropods, vertebrates, and patients. Am. J. Trop. Med. Hyg. 22:382-389. [PubMed] [Google Scholar]
- 3.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 4.Dumpis, U., D. Croo, and J. Oksi. 1999. Tick-borne encephalitis. Clin. Infect. Dis. 28:882-890. [DOI] [PubMed] [Google Scholar]
- 5.Ecker, M., S. L. Allison, T. Meixne, and F. X. Heinz. 1999. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J. Gen. Virol. 80:179-185. [DOI] [PubMed] [Google Scholar]
- 6.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 7.Gritsun, T. S., K. Venugopal, P. M. de A. Zanotto, M. V. Mikhailov, A. A. Sall, E. C. Holmes, I. Polkinghorne, T. V. Frolova, V. V. Pogodina, V. A. Lashkevich, and E. A. Gould. 1997. Complete sequence of two tick-borne flaviviruses isolated from Siberia and the UK: analysis and significance of the 5′ and 3′-UTRs. Virus Res. 49:27-39. [DOI] [PubMed] [Google Scholar]
- 8.Gritsun, T. S., V. A. Lashkevich, and E. A. Gould. 2003. Tick-borne encephalitis. Antivir. Res. 57:129-146. [DOI] [PubMed] [Google Scholar]
- 9.Günther, G., M. Haglund, L. Lindquist, M. Forsgren, and B. Sköldenberg. 1997. Tick-borne encephalitis in Sweden in relation to aseptic meningo-encephalitis of other etiology: a prospective study of clinical course and outcome. J. Neurol. 244:230-238. [DOI] [PubMed] [Google Scholar]
- 10.Haglund, M., M. Forsgren, G. Lindh, and L. Lindquist. 1996. A 10-yer follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand. J. Infect. Dis. 28:217-224. [DOI] [PubMed] [Google Scholar]
- 11.Han, X., M. Aho, S. Vene, M. Peltomaa, A. Vaheri, and O. Vapalahti. 2001. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in Finland. J. Med. Virol. 64:21-28. [DOI] [PubMed] [Google Scholar]
- 12.Heinz, F. X. 1986. Epitope mapping of flavivirus glycoproteins. Adv. Virus Res. 31:103-168 [DOI] [PubMed] [Google Scholar]
- 13.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, R. J. M. Moorman, C. M. Rice, and H.-J. Thiel. 2000. Flaviviridae, p. 859-878. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 14.Heinz, F. X., and S. L. Allison. 2001. The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol. 4:450-455. [DOI] [PubMed] [Google Scholar]
- 15.Holzmann, H., M. Kundi, K. Stiasny, J. Clement, P. McKenna, C. Kunz, F. X. Heinz. 1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 48:102-107. [DOI] [PubMed] [Google Scholar]
- 16.Holzmann, H., M. S. Vorobyova, I. P. Ladyzhenskaya, E. Ferenczi, M. Kundi, C. Kunz, and F. X. Heinz. 1992. Molecular epidemiology of tick-borne encephalitis virus: cross-protection between European and Far Eastern subtypes. Vaccine 10:345-349. [DOI] [PubMed] [Google Scholar]
- 17.Hujakka, H., V. Koistinen, P. Eerikäinen, I. Kuronen, I. Mononen, M. Parviainen, Å. Lindkvist, A. Vaheri, A. Närvänen, and O. Vapalahti. 2001. New immunochromatographic rapid test for diagnosis of acute Puumala virus infection. J. Clin. Microbiol. 39:2146-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt, A. R., C. B. Cropp, and G.-J. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97:133-149. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser, R. 1999. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994-98. A prospective study of 656 patients. Brain 122:2067-2078. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser, R., and H. Holzmann. 2000. Laboratory findings in tick-borne encephalitis — correlation with clinical outcome. Infection 28:78-84. [DOI] [PubMed] [Google Scholar]
- 21.Klockmann, U., K. Krivanec, J. R. Stehenson, and J. Hilfenhaus. 1991. Protection against European isolates of tick-borne encephalitis virus after vaccination with a new tick-borne encephalitis vaccine. Vaccine 9:210-212. [DOI] [PubMed] [Google Scholar]
- 22.Konishi, E., and A. Fujii. 2002. Dengue type 2 subviral extracellular particles produced by a stably transfected cell line and their evaluation for a subunit vaccine. Vaccine 20:1058-1067. [DOI] [PubMed] [Google Scholar]
- 23.Konishi, E., A. Fujii, and P. W. Mason. 2001. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J. Virol. 75:2204-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz, I. C., S. L. Allison, F. X. Heinz, and A. Helenius. 2002. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 76:5480-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundkvist, Å., S. Vene, I. Golovljova, V. Mavtcchoutko, M. Forsgren, V. Kalnina, and A. Plyusnin. 2001. Characterization of tick-borne encephalitis virus from Latvia: evidence for cocirculation of three distinct subtypes. J. Med. Virol. 65:730-735. [DOI] [PubMed] [Google Scholar]
- 26.Mandl, C. W., F. X. Heinz, and C. Kunz. 1988. Sequence of the structural proteins of tick-borne encephalitis virus (Western subtype) and comparative analysis with other flaviviruses. Virology 166:197-205. [DOI] [PubMed] [Google Scholar]
- 27.Marx, F., T. S. Gritsun, B. Grubeck-Loebenstein, and E. A. Gould. 2001. Diagnostic immunoassays for tick-borne encephalitis virus based on recombinant baculovirus protein expression. J. Virol. Methods 91:75-84. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura, Y., R. D. Possee, H. A. Overton, and D. H. L. Bishop. 1987. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 68:1233-1250. [DOI] [PubMed] [Google Scholar]
- 29.Niedrig, M., U. Klockmann, W. Lang, J. Roeder, S. Burk, S. Modrow, and G. Pauli. 1994. Monoclonal antibodies directed against tick-borne encephalitis virus with neutralizing activity in vivo. Acta Virol. 38:141-149. [PubMed] [Google Scholar]
- 30.Niedrig, M., D. Vaisviliene, A. Teichman, U. Klockmann, and S. S. Biel. 2001. Comparison of six different commercial IgG-ELISA kits for detection of TBEV-antibodies. J. Clin. Virol. 20:179-182. [DOI] [PubMed] [Google Scholar]
- 31.Puchhammer-Stöckl, E., C. Kunz, C. W. Mandl, and F. X. Heinz. 1995. Identification of tick-borne encephalitis virus ribonucleic acid in tick suspensions and in clinical specimens by a reverse transcription-nested polymerase chain reaction assay. Clin. Diagn. Virol. 4:321-326. [DOI] [PubMed] [Google Scholar]
- 32.Russell, P. K., W. E. Brandt, and J. M. Dalrymple. 1980. Chemical and antigenic structure of flaviviruses, p. 503-529. In R. W. Schlesinger (ed.), The togaviruses. Academic Press, New York, N.Y.
- 33.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmaljohn, C., L. Vanderzanden, M. Bray, D. Custer, B. Meyer, D. Li, C. Rossi, D. Fuller, J. Fuller, and J. Haynes. 1997. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J. Virol. 71:9563-9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomazic, J., M. Poljac, P. Popovic, M. Maticic, B. Beovic, T. Avsic-Zupanc, S. Lotric, M. Jereb, F. Pikelij, and N. Gale. 1997. Tick-borne encephalitis: possibly a fatal disease in its acute stage. PCR amplification of tick-borne encephalitis RNA from postmortem brain tissue. Infection 25:41-43. [DOI] [PubMed] [Google Scholar]
- 37.Vapalahti, O., Å. Lundkvist, H. Kallio-Kokko, K. Paukku, I. Julkunen, H. Lankinen, and A. Vaheri. 1996. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J. Clin. Microbiol. 34:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vene, S., M. Haglund, O. Vapalahti, and Å. Lundkvist. 1998. A rapid fluorescent focus inhibition test for detection of neutralizing antibodies to tick-borne encephalitis virus. J. Virol. Methods 73:71-75. [DOI] [PubMed] [Google Scholar]
- 39.Vutuc, C., and M. Kunze. 1994. Tick-borne encephalitis in Austria: incidence 1990 and 1991. Eur. J. Epidemiol. 10:343-344. [DOI] [PubMed] [Google Scholar]
- 40.Whitby, J. E., A. D. Jennings, and A. D. Barret. 1993. Nucleotide sequence of the envelope protein gene of the tick-borne flavivirus, Kumlinge A52. Virus Genes 7:145-149. [DOI] [PubMed] [Google Scholar]
- 41.Zent, O., J. Beran, W. Jilg, T. Mach, and A. Banzhoff. 2003. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine 21:738-741. [DOI] [PubMed] [Google Scholar]