Abstract
Multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE) with SmaI were used to subtype 55 isolates of Campylobacter jejuni from a diverse range of human and animal sources previously characterized by multilocus enzyme electrophoresis (MEE). MEE and MLST targeted 11 and 7 loci, respectively, and all loci were unique to each method. MEE, MLST, and PFGE identified 40, 37, and 48 discrete subtypes, respectively, with many of the subtypes occurring only once within the data set. Simpson's indices of diversity were calculated to be 0.979, 0.966, and 0.994 for MEE, MLST, and PFGE, respectively, demonstrating that MEE and MLST had similar discriminatory powers but that PFGE was more discriminatory. Allele diversity was higher in the MLST loci; individual single-locus diversities for the 11 MEE loci and the 7 MLST loci were 0.491 and 0.854, respectively. The clonal complexes recognized by MLST correlated with the strain associations previously recognized by MEE and contained some isolates indistinguishable by PFGE. Many clusters contained isolates from diverse geographical regions and from both humans and animals. These results demonstrate the usefulness of MLST for investigation of the global epidemiology of this important pathogen and illustrate its potential to identify indistinguishable strains or clones in geographically distinct regions.
Campylobacter jejuni is the most common bacterial cause of gastroenteritis in the United States; the number of cases of gastroenteritis attributed to C. jejuni has been estimated to exceed 4 million per year (29). Although C. jejuni is a common pathogen, outbreaks are relatively rare (35) and the majority of infections appear to be sporadic. However, large outbreaks of gastroenteritis have occurred and have been associated with a number of vehicles, particularly untreated milk (10) and untreated water (8). Many other foods including salads (39) and stir-fried food (9) have been shown to be the vehicles of infection in outbreaks following cross-contamination from raw or undercooked products. Poultry, cattle, pigs, domestic pets, and wild birds carry campylobacters in their intestinal tracts; and epidemiological evidence indicates that these host animals may be a reservoir for the strains infecting humans (2, 23). Rapid, discriminatory typing methods are required to identify sources of human infection and to determine the routes of infection from animal to human hosts.
Many phenotypic and genotypic methods for the subtyping of C. jejuni have been described. Phenotypic methods include biotyping (3), resistotyping (37), phage typing (24, 40), and serotyping on the basis of heat-stable antigens (14, 36) and heat-labile antigens (26). Biotyping and resistotyping methods have low discriminatory powers but may be useful if they are used in conjunction with serotyping (34). The usefulness of phage typing and serotyping is limited by the occurrence of nontypeable organisms and problems with cross-reactivity. In addition, serotyping and phage typing require the production and maintenance of panels of reagents, which are labor-intensive and costly, making these methods impractical for most clinical laboratories.
Molecular methods for the subtyping of Campylobacter species have been recently reviewed by Wassenaar and Newell (47) and include multilocus enzyme electrophoresis (MEE) (1, 31), flaA restriction fragment length polymorphism typing (32, 33), amplified fragment length polymorphism typing (4, 7), random amplified polymorphic DNA analysis (18, 27), ribotyping (12, 15), and pulsed-field gel electrophoresis (PFGE) (48). Many of these molecular methods are highly discriminatory; however, the lack of standardization of experimental protocols has made interlaboratory comparisons difficult. PFGE with the restriction enzyme SmaI has been demonstrated to be useful in the investigation of outbreaks of C. jejuni enteritis (13, 25). The standardization of experimental protocols for PFGE and normalization of the restriction fragment length polymorphism patterns produced have assisted with the development of a nationwide network known as PulseNet within the United States and have facilitated interlaboratory comparisons (44). However, such comparisons of PFGE data require strict adherence to standardized experimental protocols and labor-intensive normalization of the patterns obtained to produce comparable results.
The increasing availability of automated DNA sequencing technology has led to the development of molecular subtyping methods based on the sequencing of one or more gene loci. DNA sequencing has the advantage of minimal experimental variation in the results, which facilitates direct interlaboratory comparisons. The sequencing of a single locus, the short variable region of the flagellin A gene (flaA), may provide adequate discrimination for epidemiological investigations of outbreaks of C. jejuni enteritis (30). However, it may not be suitable for longitudinal epidemiological studies, since this gene has been demonstrated to undergo recombination events, raising concerns about the stability of the gene for use in subtyping methods (17). Multilocus sequence typing (MLST) is a technique that uses comparative DNA sequencing of conserved housekeeping genes to characterize haploid organisms (28). This method indexes the neutral genetic variation at several housekeeping gene loci and is therefore analogous to MEE (28). The sequencing of 400- to 500-bp segments of seven housekeeping genes of Nesseria meningitidis by MLST provided the same level of discrimination as typing 15 to 20 loci by MEE (28).
Dingle and colleagues (5) described an MLST scheme for the characterization of C. jejuni based on the sequencing of seven housekeeping genes. In that scheme, each of the alleles is assigned an allele number on the basis of those already present in the database, and the alleles form a seven-digit code that is then assigned a sequence type (ST). The characterization of 194 isolates by this MLST method demonstrated that C. jejuni is genetically diverse with a weakly clonal population structure (5); this finding was further substantiated in a second study of 814 isolates (6). The population structure of weakly clonal bacteria is thought to consist of clonal complexes or lineages in which the isolates are considered to be derived from a common ancestor (19). The STs of C. jejuni could be grouped into clonal lineages that were defined as groups of two or more isolates that shared identical alleles at four or more loci (5). Lineages were named after the ST identified as the putative founder of the lineage (central genotype) and were named complexes (e.g., the ST-45 complex) (5).
A recent MEE study reported that C. jejuni has a clonal framework, with some segments of the genome involved in genomic reassortment and random exchange of the segments contributing to the generation of C. jejuni diversity (31). A number of clonal groups were also identified within the C. jejuni population sampled (31). A comparison of the strain associations recognized by MLST with those previously documented by MEE has not been reported.
The aim of this study was to determine the congruence between the strain associations or clonal groupings recognized by MLST with those previously determined by MEE. A set of 55 C. jejuni isolates that had previously been characterized by MEE by using 11 loci were characterized by the MLST method. The groupings identified by MEE and MLST were also compared to the groupings identified by PFGE, the present “gold standard” for the subtyping of C. jejuni. The subtyping results of all three methods were compared, and the diversity of types recognized and the discriminatory power of each method were determined. The clusters of isolates identified by each method were compared to determine the congruence between the strain associations identified by each of the three methods.
MATERIALS AND METHODS
Bacterial isolates and culture conditions.
The 55 C. jejuni isolates used in this study are listed in Table 1. All cultures were obtained from the collection of the National Campylobacter and Helicobacter Reference Laboratory, Centers for Disease Control and Prevention. The isolates represent a diverse collection of strains that had previously been characterized by MEE (31). Forty-two isolates were from human cases of campylobacteriosis; and the other 13 isolates were from poultry, bovines, lambs, and goats. The geographic origins of the isolates included the United States, Canada, Scotland, Japan, Belgium, and England. C. jejuni isolates were stored at −70°C and were cultured on heart infusion agar containing 5% (vol/vol) defibrinated rabbit blood (Becton Dickinson Diagnostic Systems, Sparks, Md.). All cultures were incubated at 37°C for 48 h under microaerobic conditions obtained by the evacuation replacement method.
TABLE 1.
Results of subtyping of 55 C. jejuni isolates by MEE, MLST, and PFGE
| Clonal complex | Isolate no. | Sourcea | MEE data
|
MLST data
|
PFGE type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ET | Allele profileb | ST | Allele profile
|
||||||||||
| asp | gln | glt | gly | pgm | tkt | unc | |||||||
| ST-48 | D0128 | H (Japan) | 17 | 5.6c.4.4.1.8.6.10.4.2.8 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | 32 |
| D0132 | H (Japan) | 32 | 7.4.6.4.4.8.7.10.4.2.5 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | 29 | |
| D1038 | C (United States) | 33 | 7.4.6.4.4.8.7.10.4.2.8 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | 30 | |
| D2577 | H (Scotland) | 33 | 7.4.6.4.4.8.7.10.4.2.8 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | 31 | |
| D0424 | H (United States) | 7 | 5.4.6.4.4.8.6.10.3.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 17 | |
| D0603d | H (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 15 | |
| D0835d | C (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 15 | |
| D0712d | H (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 18 | |
| D0941d | GH (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 19 | |
| D0996d | C (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 19 | |
| D0977d | C (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 19 | |
| D1916d | H (United States) | 24 | 5.6.6.4.6.8.6.9.4.2.5 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 | 4 | |
| ST-45 | D0121 | H (Canada) | 14 | 5.5.6.5.4.8.6.2.3.2.5 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | 37 |
| D2589 | H (Scotland) | 15 | 5.5.6.5.4.8.6.2.5.2.5 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | 38 | |
| D2583 | H (United States) | 15 | 5.5.6.5.4.8.6.2.5.2.5 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | 39 | |
| D0125 | H (Canada) | 15 | 5.5.6.5.4.8.6.2.5.2.5 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | 42 | |
| D0123 | H (Canada) | 13 | 5.5.6.4.4.8.6.2.5.2.5 | 137 | 4 | 7 | 10 | 4 | 42 | 7 | 1 | 34 | |
| D0141 | G (Canada) | 16 | 5.5.8.5.4.8.6.2.5.2.5 | 25 | 4 | 7 | 10 | 1 | 1 | 7 | 1 | 35 | |
| D0131 | H (Canada) | 2 | 5.8e.6.4.4.8.6.2.5.2.5 | 418 | 4 | 7 | 2 | 4 | 1 | 7 | 1 | 42 | |
| D2588 | H (Belgium) | 31 | 7.8e.6.5.4.8.6.2.5.2.5 | 2 | 4 | 7 | 51 | 4 | 1 | 7 | 1 | 40 | |
| ST-460 | D0113 | H (Japan) | 27 | 5.6.6.5.3.8.7.8.4.2.8 | 460 | 24 | 30 | 2 | 2 | 89 | 59 | 6 | 12 |
| D1420 | C (United States) | 27 | 5.6.6.5.3.8.7.8.4.2.8 | 461 | 24 | 65 | 2 | 2 | 89 | 59 | 6 | 13 | |
| ST-443 | D2587 | H (United States) | 26 | 5.6.6.5.2.8.6.10.4.2.6 | 51 | 7 | 17 | 2 | 15 | 23 | 3 | 12 | 7 |
| D2578 | HB (United States) | 28 | 5.6.6.5.4.8.6.10.4.2.6 | 463 | 24 | 17 | 2 | 15 | 23 | 12 | 12 | 6 | |
| ST-42 | D2585 | H (United States) | 37 | 7.5.5.4.2.8.7.8.4.2.8 | 459 | 1 | 2 | 3 | 3 | 5 | 9 | 3 | 44 |
| D1887 | H (United States) | 38 | 7.5.5.4.4.8.7.8.4.2.8 | 469 | 1 | 2 | 3 | 4 | 5 | 9 | 6 | 43 | |
| ST-21 | D2627 | H (United States) | 11 | 5.5.6.4.2.8.4.10.3.2.5 | 21 | 2 | 1 | 1 | 3 | 2 | 1 | 5 | 28 |
| D0142 | L (United States) | 4 | 5.4.6.4.2.8.4.10.3.2.5 | 50 | 2 | 1 | 12 | 3 | 2 | 1 | 5 | 25 | |
| D0135 | H (Canada) | 4 | 5.4.6.4.2.8.4.10.3.2.5 | 50 | 2 | 1 | 12 | 3 | 2 | 1 | 5 | 25 | |
| D2582 | H (Belgium) | 5 | 5.4.6.4.2.8.4.10.4.2.5 | 50 | 2 | 1 | 12 | 3 | 2 | 1 | 5 | 21 | |
| D0140 | H (Japan) | 10 | 5.5.6.4.7e.8.7.10.4.2.8 | 451 | 2 | 1 | 2 | 3 | 2 | 3 | 5 | 14 | |
| D2586 | H (England) | 12 | 5.5.6.4.2.8.6.10.6.2.5 | 43 | 2 | 1 | 5 | 3 | 4 | 1 | 5 | 26 | |
| D0144 | H (Canada) | 3 | 5.4.6.6.2.8.4.10.3.2.5 | 455 | 2 | 1 | 12 | 3 | 2 | 12 | 5 | 27 | |
| D2584 | HB (United States) | 6 | 5.4.6.4.4.8.4.10.4.2.8 | 454 | 2 | 1 | 1 | 5 | 22 | 1 | 8 | 36 | |
| D2761 | H (United States) | 6 | 5.4.6.4.4.8.4.10.4.2.8 | 454 | 2 | 1 | 1 | 5 | 22 | 1 | 8 | 36 | |
| D0116 | H (Japan) | 4 | 5.4.6.4.2.8.4.10.3.2.5 | 456 | 59 | 1 | 12 | 3 | 92 | 1 | 5 | 23 | |
| ST-353 | D1917 | C (United States) | 22 | 5.6.6.4.4.8.6.9.4.2.3 | 353 | 7 | 17 | 5 | 2 | 10 | 3 | 6 | 5 |
| D0129 | H (Japan) | 23 | 5.6.6.4.4.8.6.9.4.2.5 | 404 | 7 | 17 | 5 | 2 | 2 | 3 | 6 | 2 | |
| D0597d | H (United States) | 23 | 5.6.6.4.4.8.6.9.4.2.5 | 452 | 7 | 17 | 12 | 2 | 10 | 3 | 6 | 3 | |
| D1713 | H (United States) | 25 | 5.6.6.4.6.8.6.9.4.2.8 | 452 | 7 | 17 | 12 | 2 | 10 | 3 | 6 | 3 | |
| D2600 | H (United States) | 9 | 5.4.6.4.4.8.6.10.4.3.6 | 452 | 7 | 17 | 12 | 2 | 10 | 3 | 6 | 16 | |
| D2737 | H (United States) | 19 | 5.6.6.4.4.8.6.10.4.2.5 | 462 | 7 | 17 | 5 | 2 | 11 | 3 | 6 | 1 | |
| D0983d | C (United States) | 34 | 7.4.6.4.6.8.6.10.6.2.8 | 457 | 7 | 4 | 5 | 2 | 13 | 3 | 26 | 22 | |
| ST-22 | D0109 | H (Japan) | 36 | 7.5.4.2.2.8.7.8.1.2.8 | 22 | 1 | 3 | 6 | 4 | 3 | 3 | 3 | 45 |
| D2580 | HB (United States) | 35 | 7.5.4.2.1.8.7.8.1.2.8 | 504 | 1 | 3 | 6 | 4 | 11 | 3 | 3 | 46 | |
| D0143 | H (Japan) | 18 | 5.6.6.4.2.8.4.10.4.2.5 | 458 | 8 | 1 | 12 | 4 | 3 | 3 | 3 | 24 | |
| ST-403 | D0133 | BFf | 1 | 4.3.8.6e.4.8.6.10.1.2.8 | 403 | 10 | 27 | 16 | 19 | 10 | 5 | 7 | 48 |
| ST-206 | D2817 | H (United States) | 8 | 5.4.6.4.4.8.6.10.4.2.8 | 122 | 6 | 4 | 5 | 2 | 2 | 1 | 5 | 33 |
| ST-49 | D2581 | HB (United States) | 29 | 5.6.6.5.4.8.7.8.2.2.8 | 467 | 3 | 1 | 5 | 84 | 11 | 11 | 6 | 8 |
| ST-52 | D0114 | H (Japan) | 40 | 9.6.6.4.4.8.6.8.4.2.5 | 305 | 9 | 53 | 2 | 10 | 11 | 3 | 3 | 20 |
| NAg | D0139 | H (Japan) | 20 | 5.6.6.4.4.8.6.10.4.2.6 | 464 | 24 | 2 | 2 | 2 | 10 | 3 | 1 | 9 |
| NA | D2683 | H (United States) | 21 | 5.6.6.4.4.8.6.11.4.2.8 | 503 | 9 | 17 | 2 | 2 | 2 | 1 | 5 | 10/PICK> |
| NA | D0117 | H (United States) | 30 | 6.7.7.4.4.8.3.6.2.2.5 | 468 | 10 | 2 | 50 | 62 | 91 | 73 | 45 | 41 |
| NA | D2579 | C (United States) | 39 | 9.6.5.4.3.8.5.10.7e.2.5 | 40 | 9 | 2 | 5 | 15 | 89 | 3 | 1 | 47 |
| NA | D0127 | U (Canada) | 17 | 5.6.4.4.1.8.6.10.4.2.8 | 301 | 9 | 17 | 5 | 10 | 2 | 1 | 3 | 11 |
H, human; L, lamb; HB, human blood; C, chicken; GH, game hen; BF, bovine feces; U, unknown; G, goat.
The MEE-targeted loci for which allele numbers are presented are MDH, ME, IDH, THD, CAT, ADK, ALD, PLP, PPP, FUM, and ACO, respectively.
Bold-face type indicates alleles different from the reference genotype.
Hippuricase-negative isolates.
Null alleles.
ATCC 33560.
NA, not applicable.
MEE.
MEE was performed as described previously (31). The 11 enzymes used were malate dehydrogenase (MDH), malic enzyme (ME), isocitrate dehydrogenase (IDH), threonine dehydrogenase (THD), catalase (CAT), adenylate kinase (ADK), alkaline phosphatase (ALD), phenylalanyl-leucine peptidase (PLP), phenylalanyl-proline peptidase (PPP), fumarase (FUM), and aconitase (ACO). The program ETDIV (www.foodsafe.msu.edu/whittam/programs) was used to assign MEE types (ETs) and to calculate allele diversity scores (h). Four loci (ME, THD, CAT, and PPP) had null alleles (no enzyme activity was detected); the null alleles were included in the analyses and scored as separate alleles.
MLST.
The isolates were characterized by the MLST scheme described by Dingle and colleagues (5). Briefly, DNA was extracted from bacterial cultures with a Puregene DNA isolation kit according to the instructions of the manufacturer (Gentra Systems, Minneapolis, Minn.). Approximately 10 ng of the DNA extracts was used as the template in the PCRs with the primers described previously (5) to generate amplicons from the seven MLST gene loci. Amplification products of the correct size and similar concentrations, based on visual inspection of the gels, were obtained from all 55 isolates and were then purified by using a QiaAmp PCR purification kit (Qiagen Inc., Valencia, Calif.) according to the instructions of the manufacturer. The nucleotide sequences of both DNA strands were determined by using internal nested primers and BigDye Reaction mix (Applied Biosystems, Foster City, Calif.) according to the instructions of the manufacturer. The reaction products were separated from unincorporated dye terminators by column purification (Centri-Sep; Princeton Separations, Adelphia, N.J.) and were separated and detected with an ABI Prism 377 automated DNA sequencer (Applied Biosystems). The chromatograms were exported into SeqMan II (DNAStar, Madison, Wis.), and the forward and reverse sequences were assembled. The sequences of each of the seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) were edited to the previously described allele lengths (between 402 and 507 bp). These were then assigned allele numbers based on those already described in the Campylobacter MLST database (http://campylobacter.mlst.net). An ST was assigned to each isolate on the basis of the combination of the seven alleles. New sequences for each allele and new STs (not previously recognized) were added to the database. Isolates were assigned to clonal complexes by using the Campylobacter MLST database (http://campylobacter.mlst.net), and the program ETDIV (www.foodsafe.msu.edu/whittam/programs) was used to calculate h values and allelic frequencies for all seven loci. The data were used to draw an unweighted pair group mean average (UPGMA) dendrogram by using the program START (http://outbreak.ceid.ox.ac.uk/software.htm) (22).
PFGE.
Isolates were characterized by the PulseNet PFGE protocol with the restriction enzyme SmaI and the electrophoretic running conditions described previously (38). Electrophoresis was carried out on a CHEF Mapper system (Bio-Rad Laboratories, Hercules, Calif.). Following electrophoresis, the fragments were visualized by ethidium bromide staining and UV transillumination, with gel images recorded with a Gel Doc 2000 image analysis system (Bio-Rad Laboratories). PFGE gel images were normalized, and the PFGE profiles were grouped together by using the Dice coefficient and UPGMA clustering with the BioNumerics program (version 3.0; Applied Maths, Austin, Tex.). PFGE profile types were assigned to isolates which clustered at greater than 95% similarity (0.41% optimization and 0.5% position tolerance) and were numbered arbitrarily.
RESULTS
Comparison of diversity identified by each subtyping method.
The data from each of the three subtyping methods are presented in Table 1. MEE identified 40 discrete types among the 55 C. jejuni isolates tested; 32 types occurred only once in the data set. The types that occurred more than once in the data set were ET-9 (seven isolates), ET-4 and ET-15 (three isolates each), and ETs 6, 17, 23, 27, and 33 (two isolates each). MLST identified 37 allelic profiles (STs) among the 55 isolates tested; 31 of the types occurred only once in the data set. Several of the profiles had not been previously described in the database at http://campylobacter.mlst.net. The types that occurred more than once in the data set were ST-429 (eight isolates), ST-45 and ST-48 (four isolates each), STs 50 and 452 (three isolates each), and ST-454 (two isolates). PFGE with SmaI demonstrated 48 macrorestriction profiles within the data set; 42 profiles occurred only once. The most common PFGE profiles were 19 (three isolates) and 3, 15, 25, 36, and 42 (two isolates each). The SmaI profiles contained 4 to 10 bands ranging in size from 30 to 1,000 kb; the average number of bands per profile was 7.5. Simpson's index of diversity was calculated for each of the three subtyping techniques by the method of Hunter and Gaston (20); and these were found to be 0.979, 0.966, and 0.994 for MEE, MLST, and PFGE, respectively.
Allelic diversity of MEE and MLST loci.
The numbers of MEE- and MLST alleles and h values are presented in Table 2. The number of MEE alleles per locus varied between 1 for ADK and 7 for PPP, with a mean number of 4.6. h values varied between 0.000 for ADK and 0.736 for ME, with an average h value for the 11 loci of 0.491. The total number of MLST alleles per locus varied between 9 for tkt and unc to 15 for pgm; the mean number was 10.6. The h values for the MLST loci varied between 0.792 and 0.919; the mean h value was 0.854.
TABLE 2.
Total numbers of MEE and MLST alleles and h values for the 55 C. jejuni isolates
| Assay | Locus | Total no. of alleles/locusa | h |
|---|---|---|---|
| MEE | MDH | 5 | 0.478 |
| ME | 6 | 0.736 | |
| IDH | 5 | 0.395 | |
| THD | 4 | 0.477 | |
| CAT | 6 | 0.623 | |
| ADK | 1 | 0.000 | |
| ALD | 5 | 0.610 | |
| PLP | 6 | 0.677 | |
| PPP | 7 | 0.672 | |
| FUM | 2 | 0.097 | |
| ACO | 4 | 0.633 | |
| Mean | 4.6 | 0.491 | |
| MLST | asp | 11 | 0.889 |
| gln | 10 | 0.863 | |
| glt | 10 | 0.855 | |
| gly | 10 | 0.814 | |
| pgm | 15 | 0.919 | |
| tkt | 9 | 0.792 | |
| unc | 9 | 0.829 | |
| Mean | 10.6 | 0.854 |
Includes null alleles for MEE data.
Comparison of clustering of C. jejuni strains by MLST, PFGE, and MEE.
Dendrograms illustrate the clustering of the 55 isolates on the basis of MLST and PFGE (Fig. 1 and 2); clustering of the isolates on the basis of the MEE data was previously reported by Meinersmann and colleagues (31). The isolates were highly diverse; hence, in each dendrogram many isolates were represented by single branches. Twenty-five isolates were found to be unique by all three methods, and seven isolates were indistinguishable by all three methods (see below). Isolates were arranged into clonal complexes on the basis of the MLST results, with 47 isolates being grouped into clonal complexes containing two or more isolates (Table 1).
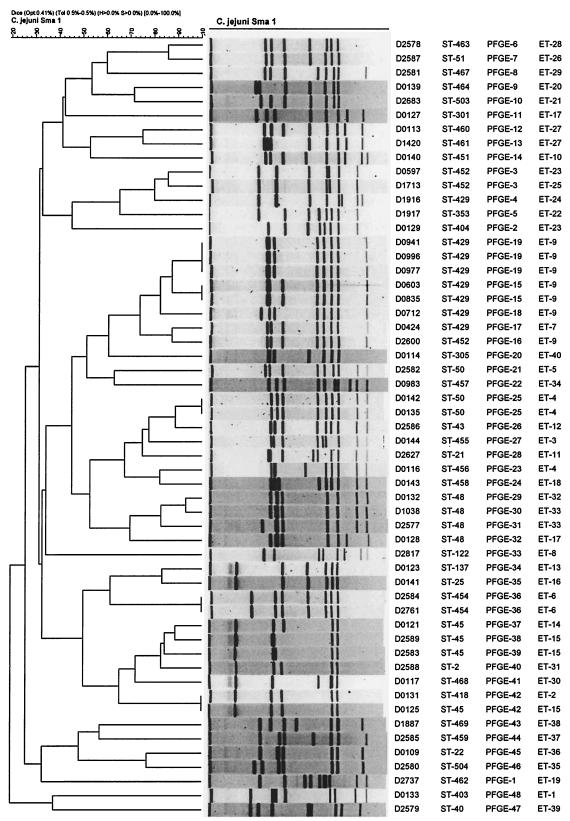
FIG. 1.
UPGMA clustering of MLST data for 55 isolates of C. jejuni.
FIG. 2.
Clustering of 55 C. jejuni isolates grouped by UPGMA clustering of PFGE profiles.
The ST-48 clonal complex was the largest group, with 12 isolates; however, the isolates in this group were not as diverse as the rest of the panel of strains, with 7 of the isolates being geographically and temporally related. These seven isolates were isolated from King County, Washington, in 1982 as part of a community survey (21); all were hippurate test-negative C. jejuni strains (45). At the time of the MEE study, they could not easily be differentiated from C. coli and were included in the MEE study for that reason. The ST-48 complex contained two STs (ST-48 and ST-429) and six ETs (ETs 7, 9, 17, 24, 32, and 33); three isolates (isolates D0941, D0996, and D0977) were found to be indistinguishable by all three methods (ET-9, ST-429, PFGE profile 19). Four isolates were found to be indistinguishable by MLST (ST-48) and had closely related PFGE profiles (similarity, >70%) (Fig. 2). Three of these isolates were very similar by MEE (one allele was different); however, the fourth isolate (isolate D0128) was quite distinct by MEE (four alleles were different) but was indistinguishable from another isolate (isolate D0127) by MEE. Isolate D0127 was very different from isolate D0128 by PFGE and MLST (at six of the seven alleles) and had an ST (ST-301) that was unique to this study and that was not previously represented in the Campylobacter MLST database at http://campylobacter.mlst.net. Eight isolates of the ST-48 complex were indistinguishable by MLST (ST-429), and seven of these isolates were very similar by MEE (one allele was different); the PFGE profiles of seven of the isolates were also closely related (similarity, >70%). The eighth isolate (isolate D1916) was very different by MEE (five alleles were different) and had a very different PFGE profile.
The ST-45 clonal complex contained eight isolates; four were indistinguishable by MLST (ST-45) and were very similar by MEE (one allele was different) and PFGE (similarity, >70%). The other four isolates were single-locus variants (SLVs) of the central genotype (ST-48) and were similar by MEE (one to three alleles were different, with two alleles being null) and had different PFGE profiles (similarity, <60%). The ST-460 clonal complex contained two isolates which were indistinguishable by MEE (ET-27) and which had STs that differed at one locus (gln), with one single-nucleotide polymorphism (SNP) within the 477 nucleotides analyzed. The isolates had closely related PFGE profiles (types 12 and 13) that had a similarity of >70%. The ST-443 clonal complex contained two isolates with very similar ET types (one allele was different) and STs (two alleles were different) and closely related PFGE profiles (similarity, >80%). The ST-42 clonal complex contained two isolates with closely related MEE types (one allele was different) and STs (two alleles were different) but with different PFGE profiles (similarity, <60%).
The ST-21 clonal complex contained 10 isolates, with 9 isolates being single-, double-, or triple-locus variants of the central genotype. Three isolates were indistinguishable by MLST (ST-50) but were very similar by MEE (one or two alleles were different); and two were indistinguishable by PFGE (PFGE type 25), but the other had a different PFGE profile (similarity, <60%). Isolate D0116 was indistinguishable from two of these ST-50 isolates by MEE, had a related PFGE profile (similarity, >70%), and differed at two MLST loci (one SNP in each of the asp and pgm loci). Two isolates (isolates D2584 and D2761) were indistinguishable by all three methods and were triple-locus variants of the central genotype. Three isolates (isolates D0140, D2586, and D0144) were all double-locus variants of the central genotype, differed at six MEE loci, and had different PFGE profiles.
The ST-353 clonal complex contained seven isolates; five were SLVs of the central genotype, and one was a triple-locus variant. Three isolates were indistinguishable by MLST (ST-452); two of the three isolates were indistinguishable by PFGE (type 3) and were similar by MEE. The third isolate (isolate D2600) was very different by both MEE and PFGE. This isolate was indistinguishable from isolates in the ST-48 clonal complex by MEE (ET-9) but was similar to isolates in the ST-48 clonal complex by PFGE; however, it shared only one of seven MLST alleles with ST-48. One isolate was a SLV (ST-404) and was very similar to isolate D1917 by MEE, differing at a single MEE allele, but it was very different from D1917 by PFGE. Another SLV (isolate D2737) differed at two MEE loci and was very different by PFGE. One isolate (isolate D0983) was a triple-locus variant, differed at six MEE-targeted loci, and was very different by PFGE.
The ST-22 clonal complex contained three isolates; isolate D2580 differed from the central genotype at one locus (pgm), was very similar by MEE (one allele different), and was similar by PFGE (similarity, >70%). Isolate D0143 differed at eight MEE loci and three MLST loci and had a very different PFGE profile.
Five isolates were unique to this study and could not be assigned to a clonal complex, and four isolates were assigned to clonal complexes that occurred only once in this data set (Table 1). Interestingly, isolate D0127 had an MEE profile (ET-17) that was indistinguishable from that of an isolate of the ST-48 complex (isolate D0128), but was distinct by both MLST (six of seven loci) and PFGE (Fig. 2).
DISCUSSION
Epidemiological subtyping methods are used to identify common types among suspected cases and possible sources of infection during the investigation of outbreaks of disease (short-term or epidemic epidemiology). In addition, they are used for investigation of the longer-term, global epidemiology of disease, which is important for recognizing emerging or reemerging strains or clones of pathogens that cause human disease. Both applications require accurate and discriminatory subtyping methods that can differentiate between strains descended from distinct clonal ancestors; however, individual methods may be more appropriate to address these different problems. Highly discriminatory methods such as PFGE (42) index variation in the C. jejuni genome that may be unstable due to genomic rearrangements, making such methods unsuitable for longitudinal epidemiological studies. MEE indexes changes that affect the electrophoretic mobility of the gene product in highly conserved housekeeping genes; these genes evolve slowly and are not under evolutionary pressure. MLST is analogous to MEE, but it uses DNA sequencing to directly detect the differences in genes at the nucleotide level rather than characterizing the enzymes themselves. The relative simplicity of the MLST method over MEE has led to an explosion of interest in this new technique. MLST, MEE, and PFGE all measure different characteristics that may change independently of each other; thus, the relationships between subtypes may not always be congruent when one typing method is compared to the others. In addition, these three methods index variations in the genome that have different levels of phylogenetic relevance. MEE and MLST measure variations that occur relatively slowly and without selective pressure and thus are more appropriate for phylogenetic studies. PFGE offers very good strain discrimination, but it is often too variable to provide definitive phylogenetic information.
MLST has a number of advantages over other subtyping methods. There is minimal experimental variation in results, which facilitates interlaboratory comparisons and provides the potential for the establishment of a standardized nomenclature for DNA sequence data. Sequencing provides precise information on the differences between strains, and because sequence data and not gel images are compared, the text data can be exchanged electronically via the World Wide Web and global databases (such as http://www.MLST.net) can be easily developed for comparison of isolates. The study had two aims: first, to subtype by MLST and PFGE a phenotypically diverse collection of C. jejuni isolates which had been previously characterized by MEE and, second, to compare the clustering and group associations determined by the MLST and PFGE methods to the groupings previously established by MEE. The MEE and MLST methods were based on established protocols and targeted completely different sets of housekeeping enzymes. Thus, while the two methods are generally analogous, the different evolutionary histories of the loci targeted by each method may result in incongruence between the clustering observed by each method.
All three methods differentiated the isolates into many subtypes, the majority of which occurred in the data set only once. MEE and MLST recognized 40 and 37 types among the 55 isolates, respectively, and had similar indices of discrimination. PFGE with the SmaI enzyme recognized 48 distinct subtypes and had a higher index of discrimination compared to those of the other two methods. For MEE and MLST, the same alleles occurred in multiple isolates; however, the alleles occurred in many different combinations, leading to the diversity of subtypes that were recognized. Suerbaum and colleagues (43) sequenced six other housekeeping gene loci plus a region of the uncA locus (F1α ATP synthase), which overlapped part of the segment targeted in the MLST scheme of Dingle and colleagues (5), from 32 C. jejuni isolates and reported similar findings, with relatively small numbers of alleles occurring in many combinations.
In this study, the single-locus and mean diversities as well as the number of alleles for the MLST loci were found to be higher than those for the MEE loci. This was to be expected, because changes in the nucleotide sequences of the housekeeping genes may not lead to changes in the net charge of the protein (a conservative substitution or silent genetic changes); therefore, there may be more alleles that cannot be detected by MEE. Additionally, proteins coded for by genes from distinctly different ancestral clones may fortuitously share the same electrophoretic properties and be considered indistinguishable by MEE (28). Even though the number of alleles detected by MEE was lower than the number detected by MLST, the numbers of ETs and STs within the data set were very similar by the MLST method, providing a level of discrimination similar to that provided by MEE for the strains in this data set. The greater number of alleles detected by MLST implies that it has the potential to detect more subtypes than the number that can be detected by MEE. MLST is less labor-intensive and faster than MEE and is amenable to automation; however, it still requires DNA to be extracted from the bacterial isolates prior to testing. PFGE was also more rapid than MEE; however, interlaboratory comparisons of the PFGE patterns require strict adherence to standardized protocols and labor-intensive normalization of the gel images that are produced. In addition, the significance of the differences in the presence or positions of restriction sites within the genome has not been established. Studies have also demonstrated that PFGE profiles may not provide a stable fingerprint, and chromosomal rearrangements may make this method unsuitable for long-term or global epidemiological studies of C. jejuni (16, 46). In the present study, some of the clonal complexes identified by MLST contained isolates that were indistinguishable by PFGE; however, overall there was considerable diversity in the PFGE types within each complex. This diversity may be due to the effects of C. jejuni genomic instability on PFGE profiles that limit their usefulness for long-term longitudinal studies of the epidemiology of this organism.
In the present study, several pairs of alleles that differed by a single base were identified. For example in the ST-21 clonal complex, ST-456 and ST-50 differed at the asp and pgm loci by one SNP in each allele (asp-2 and asp-59, pgm-2 and pgm-92). These differences could arise by point mutations or recombinational replacement between similar sequences that introduce only a single nucleotide change. Distinguishing between these events is not possible; however, a single point mutation will most likely result in a variant allele that is unique within the database (41). The accuracy of this assumption is dependent on the database containing most of the alleles that are present at a significant frequency in the population. More rare alleles will occur, but they will have a low frequency and will rarely be exchanged by recombination. The asp-59 and pgm-92 alleles had not previously been detected in the Campylobacter MLST database at http://campylobacter.mlst.net. Therefore, it is most likely that these two SNPs arose by spontaneous mutation in the allele sequences rather than through genetic exchange by recombination. Similarly, clonal complex ST-460 contained isolates with two STs (ST-460 and ST-461) that differed at the gln locus, with a single nucleotide difference between the gln-30 and gln-65 alleles. The gln-65 allele had also not previously been described in the MLST database, leading to the conclusion that spontaneous mutation rather than recombinational exchange was more likely to be the mechanism for this difference. In the ST-353 clonal complex, ST-404 and ST-452 differed at two loci, glt and pgm. The two glt and two pgm alleles were different from each other by 2 and 19 SNPs, respectively, and all four alleles are common in the database at http://campylobacter.mlst.net. In this case, we can conclude that the mechanism responsible for these differences is likely to be recombinational exchange rather than spontaneous mutation.
The MEE, MLST, and PFGE results were incongruent in three instances. Isolate D1916 (ST-429) was indistinguishable from the seven other ST-429 isolates by MLST but was very different by PFGE and MEE. Isolate D2600 (ST-452) was related to members of the ST-48 complex by MEE (ET-9) but was distinct by MLST. Isolate D0127 possessed an MEE type (ET-17) indistinguishable from that of isolate D0128; however, D0128 possessed a very different ST, which differed at six of the seven alleles, and also had a very different PFGE profile. The following are possible explanations for these results. (i) The MEE subtyping was performed several years ago; the isolates were recovered from frozen storage and were then concomitantly tested by MLST and PFGE. Therefore, it is possible that the differences observed between the MEE results and the MLST and PFGE results for these strains were due to a mix-up of strains, in which the strains tested by MEE were different from those tested by PFGE and MLST. (ii) The MEE results may be inaccurate due to difficulties in the interpretation of electrophoretic motilities by MEE analysis or due to an inability to differentiate proteins coded for by genes from distinctly different ancestral clones which fortuitously share the same electrophoretic properties (28). (iii) The PFGE differences may be due to genomic rearrangements that lead to changes in the PFGE profiles in closely related strains, a phenomenon that has been documented for C. jejuni (16, 46). We sequenced 500- to 600-bp fragments from six additional housekeeping genes and the flaA short variable region (30) from the isolates giving incongruent results and found that the clustering based on the results for these additional loci is completely congruent with the MLST groupings reported in this study (data not shown). Thus, it is unlikely that the incongruent results observed for these isolates are due to incorrect clustering by MLST.
The ST-21, -45, -48, and -353 clonal complexes all contained groups of indistinguishable isolates from diverse geographical regions, including five countries in North America, Europe, and Asia, and were isolated from both humans and animals. This fact demonstrates the usefulness of MLST for investigation of the global epidemiology of this important pathogen and illustrates its potential to identify indistinguishable strains or clones in geographically distinct regions.
The genetic heterogeneity demonstrated in the STs identified in this study may in part reflect the diversity of sources, disease etiologies, and geographic origins of the 55 isolates studied. Application of the MLST method to prospective studies of isolates from human patients with gastroenteritis may demonstrate less variability in the combinations of loci (STs) detected. In a recent study, 63% (318 of 501) of human isolates of C. jejuni belonged to one of six clonal complexes (6). This apparent lack of diversity among clinical isolates may limit the usefulness of this technique for short-term epidemiological investigations. In order to enhance the utility of the MLST method for short-term or epidemic investigations, we are applying the scheme to isolates of C. jejuni from recent sporadic cases of human gastroenteritis and to previously characterized isolates from outbreaks of gastroenteritis. The sequencing of more-variable genes in conjunction with MLST has proven useful in investigations of outbreaks of N. meningitidis infection and may increase the discriminatory power of the method (11). We are therefore also investigating more-variable genes in conjunction with MLST in an effort to increase the discriminatory power of MLST to make it useful for short-term epidemiological investigations of C. jejuni.
Acknowledgments
We thank Kate Dingle of The Peter Medawar Building for Pathogen Research and Department of Zoology, Oxford University, for technical advice and discussion of the MLST method. This publication made use of the Campylobacter Multilocus Sequence Typing website (http://campylobacter.mlst.net) developed by Man-Suen Chan and sited at the University of Oxford. Initial development of this site was funded by the Wellcome Trust; maintenance is funded by DEFRA.
The use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services.
REFERENCES
- 1.Aeschbacher, M., and J. C. Piffaretti. 1989. Population genetics of human and animal enteric Campylobacter strains. Infect. Immun. 57:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, M. J., D. N. Taylor, and R. A. Feldman. 1983. Epidemiology of Campylobacter jejuni infections. Epidemiol. Rev. 5:157-176. [DOI] [PubMed] [Google Scholar]
- 3.Bolton, F. J., D. R. A. Wareing, M. B. Skirrow, and D. N. Hutchinson. 1992. Identification and biotyping of Campylobacters, p. 151-162. In R. G. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied and environmental microbiology. Blackwell Scientific Publications, Oxford, United Kingdom.
- 4.Desai, M., J. M. Logan, J. A. Frost, and J. Stanley. 2001. Genome sequence-based fluorescent amplified fragment length polymorphism of Campylobacter jejuni, its relationship to serotyping, and its implications for epidemiological analysis. J. Clin. Microbiol. 39:3823-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. J. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingle, K. E., F. M. Colles, R. Ure, J. A. Wagenaar, B., Duim, F. J. Bolton, A. J. Fox, D. R. A. Wareing, and M. C. Maiden. 2002. Molecular characterization of Campylobacter jejuni clones: a basis for epidemiologic investigation. Emerg. Infect. Dis. 8:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duim, B., T. M. Wassenaar, A. Rigter, and J. Wagenaar. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 65:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke, L. A., A. S. Breathnach, D. R. Jenkins, B. A. Harkis, and A. W. Codd. 1996. A mixed outbreak of cryptosporidium and campylobacter infection associated with a private water supply. Epidemiol. Infect. 116:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, M. R., W. Lane, J. A. Frost, and G. Nylen. 1998. A campylobacter outbreak associated with stir-fried food. Epidemiol. Infect. 121:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahey, T., D. Morgan, C. Gunneburg, G. K. Adak, F. Majid, and E. Kaczmarski. 1995. An outbreak of Campylobacter jejuni enteritis associated with failed milk pasteurization. J. Infect. 31:137-143. [DOI] [PubMed] [Google Scholar]
- 11.Feavers, I. M., S. J. Gray, R. Urwin, J. E. Russell, J. A. Bygraves, E. B. Kaczmarski, and M. C. Maiden. 1999. Multilocus sequence typing and antigen gene sequencing in the investigation of a meningococcal disease outbreak. J. Clin. Microbiol. 37:3883-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald, C., R. J. Owen, and J. Stanley. 1996. Comprehensive ribotyping scheme for heat-stable serotypes of Campylobacter jejuni. J. Clin. Microbiol. 34:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald, C., L. O. Helsel, M. A. Nicholson, S. J. Olsen, D. L. Swerdlow, R. Flahart, J. Sexton, and P. I. Fields. 2001. Evaluation of methods for subtyping Campylobacter jejuni during an outbreak involving a food handler. J. Clin. Microbiol. 39:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost, J. A., A. N. Oza, R. T. Thwaites, and B. Rowe. 1998. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens. J. Clin. Microbiol. 36:335-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, J. R., C. Fitzgerald, and R. J. Owen. 1995. Comparison of PFGE, ribotyping and phage-typing in the epidemiological analysis of Campylobacter jejuni serotype HS2 infections. Epidemiol. Infect. 115:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanninen, M. L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 65:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington, C. S., F. M. Thomson-Carter, and P. E. Carter. 1997. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J. Clin. Microbiol. 35:2386-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez, J., A. Fayos, M. A. Ferrus, and R. J. Owen. 1995. Random amplified polymorphic DNA fingerprinting of Campylobacter jejuni and C. coli isolated from human faeces, seawater and poultry products. Res. Microbiol. 146:685-696. [DOI] [PubMed] [Google Scholar]
- 19.Holmes, E. C., R. Urwin, and M. C. Maiden. 1999. The influence of recombination on the population structure and evolution of the human pathogen Neisseria meningitidis. Mol. Biol. Evol. 16:741-749. [DOI] [PubMed] [Google Scholar]
- 20.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, K. E., and C. M. Nolan. 1985. Community-wide surveillance of Campylobacter jejuni infection. Evaluation of a laboratory-based method. Diagn. Microbiol. Infect. Dis. 3:389-396. [DOI] [PubMed] [Google Scholar]
- 22.Jolley, K. A., E. J. Feil, M. S. Chan, and M. C. Maiden. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230-1231. [DOI] [PubMed] [Google Scholar]
- 23.Jones, D. M., J. D. Abbott, M. J. Painter, and E. M. Sutcliffe. 1984. A comparison of biotypes and serotypes of Campylobacter sp. isolated from patients with enteritis and from animal and environmental sources. J. Infect. 9:51-58. [DOI] [PubMed] [Google Scholar]
- 24.Khakhria, R., and H. Lior. 1992. Extended phage-typing scheme for Campylobacter jejuni and Campylobacter coli. Epidemiol. Infect. 108:403-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner, A., C. Schneck, G. Feierl, P. Pless, A. Deutz, E. Brandl, and M. Wagner. 2000. Epidemiologic application of pulsed-field gel electrophoresis to an outbreak of Campylobacter jejuni in an Austrian youth centre. Epidemiol. Infect. 125:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lior, H., D. L. Woodward, J. A. Edgar, L. J. Laroche, and P. Gill. 1982. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J. Clin. Microbiol. 15:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madden, R. H., L. Moran, and P. Scates. 1996. Sub-typing of animal and human Campylobacter spp. using RAPD. Lett. Appl. Microbiol. 23:167-170. [DOI] [PubMed] [Google Scholar]
- 28.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinersmann, R. J., L. O. Helsel, P. I. Fields, and K. L. Hiett. 1997. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J. Clin. Microbiol. 35:2810-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meinersmann, R. J., C. M. Patton, G. M. Evin, I. K. Wachsmuth, and P. I. Fields. 2002. Diversity and relationships of Campylobacter species and subspecies. Int. J. Syst. E vol. Microbiol. 52:1789-1797. [DOI] [PubMed] [Google Scholar]
- 32.Nachamkin, I., K. Bohachick, and C. M. Patton. 1993. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 31:1531-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen, R. J., C. Fitzgerald, K. Sutherland, and P. Borman. 1994. Flagellin gene polymorphism analysis of Campylobacter jejuni infecting man and other hosts and comparison with biotyping and somatic antigen serotyping. Epidemiol. Infect. 113:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton, C. M., I. K. Wachsmuth, G. M. Evins, J. A. Kiehlbauch, B. D. Plikaytis, N. Troup, L. Tompkins, and H. Lior. 1991. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J. Clin. Microbiol. 29:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pebody, R. G., M. J. Ryan, and P. G. Wall. 1997. Outbreaks of campylobacter infection: rare events for a common pathogen. Commun. Dis. Rep. Rev. 7:R33-R37. [PubMed] [Google Scholar]
- 36.Penner, J. L., and J. N. Hennessy. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J. Clin. Microbiol. 12:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro, C. D., M. T. Thomas, D. Kembrey, J. T. Magee, and Z. North. 1996. Resistotyping of campylobacters: fulfilling a need. Epidemiol. Infect. 116:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roels, T. H., B. Wickus, H. H. Bostrom, J. J. Kazmierczak, M. A. Nicholson, T. Kurzynski, and J. P. Davis. 1998. A foodborne outbreak of Campylobacter jejuni (O:33) infection associated with tuna salad: a rare strain in an unusual vehicle. Epidemiol. Infect. 121:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salama, S. M., F. J. Bolton, and D. N. Hutchinson. 1990. Application of a new phagetyping scheme to campylobacters isolated during outbreaks. Epidemiol. Infect. 104:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spratt, B. G., W. P. Hanage, and E. J. Feil. 2001. The relative contributions of recombination and point mutation to the diversification of bacterial clones. Curr. Opin. Microbiol. 4:602-606. [DOI] [PubMed] [Google Scholar]
- 42.Steinbrueckner, B., F. Ruberg, and M. Kist. 2001. Bacterial genetic fingerprint: a reliable factor in the study of the epidemiology of human campylobacter enteritis? J. Clin. Microbiol. 39:4155-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suerbaum, S., M. Lohrengel, A. Sonnevend, F. Ruberg, and M. Kist. 2001. Allelic diversity and recombination in Campylobacter jejuni. J. Bacteriol. 183:2553-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and The CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Totten, P. A., C. M. Patton, F. C. Tenover, T. J. Barrett, W. E. Stamm, A. G. Steigerwalt, J. Y. Lin, K. K. Holmes, and D. J. Brenner. 1987. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J. Clin. Microbiol. 25:1747-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 64:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan, W., N. Chang, and D. E. Taylor. 1991. Pulsed-field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J. Infect. Dis. 163:1068-1072. [DOI] [PubMed] [Google Scholar]