Abstract
In order to assess the efficacy of oral Helicobacter pylori heat shock protein 60 (HSP60) as a vaccine, protection against H. pylori infection in specific-pathogen-free (SPF) C57BL/6 and germfree (GF) IQI mice was examined. Prophylactic oral vaccination of these two strains of mice with either H. pylori HSP60 or Escherichia coli GroEL inhibited H. pylori colonization by 90 to 95% at 3 weeks postinfection (p.i.). However, these mice were only partially protected because bacterial loads increased in all animals at 10 weeks p.i. Anti-H. pylori HSP60 immunoglobulin G was detected in serum at 3 weeks p.i. in mice vaccinated with either H. pylori HSP60 or GroEL. Significant increases in the gastritis scores were observed only in SPF mice immunized with H. pylori HSP60. These results indicate that oral vaccination with H. pylori HSP60 has partial protective effects on subsequent H. pylori infection but also induces postimmunization gastritis. However, GF mice immunized with H. pylori HSP60 did not suffer from severe gastritis. Therefore, the presence of bacterial flora appears to contribute to the induction of postimmunization gastritis.
Helicobacter pylori infection is associated with the occurrence of chronic gastritis and is implicated in causing peptic ulcer diseases and gastric malignancies, such as mucosa-associated B-cell lymphoma and adenocarcinoma of the stomach (2, 11, 28, 39). This organism was recently categorized in carcinogen group I by the World Health Organization (17). Although serological studies have shown H. pylori infection in approximately half of the world's population, it is not clear how this pathogen persists in the stomach.
Eradication of chronic H. pylori infection with antibiotics obviously influences treatment for gastroduodenal diseases and reduces clinical symptoms. However, several problems are associated with antimicrobial therapy, including its side effects, as well as the generation of resistant strains of H. pylori (19, 25). Therefore, the development of a prophylactic vaccine would be an attractive strategy against H. pylori infection. Although the use of crude or purified H. pylori antigens has been explored in several animal models for the induction of protective immunity against Helicobacter species, these efforts have not resulted in the development of an optimal vaccine (20, 21, 26).
H. pylori heat shock protein 60 (HSP60) is known to be well conserved among different bacterial strains and is a strong immunogen (36, 38). Our previous studies showed that H. pylori HSP60, located on the bacterial surface, was involved in adhesion to gastric epithelial cells (35, 36). Moreover, Ferrero et al. have reported that vaccination with H. pylori HSP60 reduced colonization by H. felis in mice (9). Thus, these findings imply that H. pylori HSP60 has the potential to induce protective immunity against H. pylori infection.
In the present study, the efficacy of H. pylori HSP60 as an oral vaccine against H. pylori infection was assessed both in specific-pathogen-free (SPF) C57BL/6 and germfree (GF) IQI mice.
MATERIALS AND METHODS
Bacterial strains and preparation of antigens.
H. pylori clinical isolate 1402 (vacA+ cagA+), obtained from a patient with gastritis, was used. H. pylori clinical isolate TK1029 (vacA+ cagA+), obtained from a patient with gastric ulcer, was used for the amplification of the hsp60 gene and for the preparation of native H. pylori HSP60 by affinity purification methods described previously (34). E. coli pop2136 was used for the amplification of the groEL gene and for the transformation of expression plasmid pEX, which is capable of producing a β-galactosidase fusion protein (32). Construction of the E. coli expressing fusion proteins and the preparation of recombinant fusion protein was done by previously described methods (34, 37). cDNA fragments encoding E. coli GroEL and H. pylori HSP60 were amplified by PCR with the following primer sets: upstream for E. coli groEL, 5′-GAA TTC ATG GCA GCT AAA GAC GTA AA-3′; for H. pylori hsp60, 5′-GAA TTC ATG GCA AAA GAA ATC AAA TT-3′; and downstream for both genes, 5′-GAA TTC TTA CAT CAT GCC GCC CTA GC-3′. The GAATTC sites recognized by the EcoRI enzyme were added to both 5′ and 3′ sides for subcloning into the plasmid. All sequences of the amplified cDNAs inserted into the plasmid were confirmed by direct sequencing. These plasmids were then used to transform E. coli pop2136. The bacteria were cultivated at 30°C and shifted to 42°C for 2 h to induce the expression of the recombinant fusion proteins. Each transformed E. coli clone was disrupted by sonication (SONIFER 250; Bronson Ultrasonics Corp., Danbury, Conn.), and the insoluble fractions were collected. The pellet was incubated in 50 mM Tris-HCl (pH 8.0) containing 1 mM EDTA, 1 mM dithiothreitol, and 8 M urea for 1 h at 37°C. After the fusion proteins had been dialyzed against phosphate-buffered saline (PBS) and sterilized by filtration, they were applied as antigens for oral vaccination of mice against H. pylori infection. The fusion antigens containing E. coli GroEL and H. pylori HSP60 were designated rGalEcGroEL and rGalHpHSP60, respectively. The extract from E. coli transformed with pEX only was used as a control (rGal).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were carried out according to the methods previously described (34). Mouse monoclonal antibody (MAb) H9 (immunoglobulin G2a [IgG2a]), cross-reacting with E. coli GroEL and H. pylori HSP60 (34), was used as the fusion protein to detect antibody in Western blotting analyses.
Animal experiments.
SPF C57BL/6 and GF IQI mice (both female, 6 weeks old) were purchased from Nipon CLEA (Tokyo, Japan). SPF and GF mice were housed under SPF conditions and in a sterilized isolator, respectively. Each group of mice (SPF [n = 20] and GF [n = 14]) was orally inoculated five times on a weekly schedule with 100 μg of recombinant antigen (rGal, rGalEcGroEL, or rGalHpHSP60) and 5 μg of heat-inactivated cholera toxin (CT; Sigma, St. Louis, Mo.) as a mucosal adjuvant or CT alone in 500 μl of PBS. After the last inoculation, 500 μl of H. pylori TK1402 (5 × 109 CFU/ml) was inoculated orally three times on a daily schedule.
Assessment of gastric inflammation.
At 3 and 10 weeks after the final inoculation with H. pylori, mice were sacrificed, blood was collected for measurement of anti-H. pylori HSP60 IgG levels in serum, and stomachs were removed for determination of the number of H. pylori colonies and for the histological assessment of gastritis scores. The stomachs were washed in sterile PBS and cut longitudinally into two pieces: one half for bacterial cultures and the other half for histology. Longitudinal sections of gastric tissues from the esophageal-cardial junction to the duodenum were fixed in neutral 10% buffered formalin and embedded in paraffin. Sections of 4 μm thickness were stained with hematoxylin and eosin and viewed at magnifications of ×100 to ×400. Two histologists independently examined the gastric sections in a blind fashion. Gastritis was scored according to the method described by Dubois et al. (6) as follows: 0, intact mucosal lining and no infiltration of the lamina propria with mononuclear and polymorphonuclear cells; 1, mild increase of mononuclear or polymorphonuclear cell infiltration localized to the upper half of the mucosa; 2, mononuclear and polymorphonuclear cell infiltration extending from the surface into the lamina propria; and 3, either marked mononuclear and polymorphonuclear cell infiltration extending from the surface into the lamina propria or surface erosion.
Assessment of H. pylori in gastric tissue.
Gastric mucosa (width, 3 mm; thickness, 0.5 mm) was suspended in Hanks balanced salt solution (Nikken Seibutu, Tokyo, Japan), by vortexing the sample until the mucosa was disrupted, and then plated on M-BHI PYLORI agar (Nikken Seibutu), a selective agar medium. After cultivation for 4 days at 37°C under microaerophilic conditions, gold-colored colonies were counted.
Determination of antibody levels against H. pylori HSP60.
Enzyme-linked immunosorbent assay (ELISA) was performed as previously reported (34). Briefly, microplates were coated with affinity-purified H. pylori HSP60 and blocked with 1% skim milk-PBS. Mouse serum diluted 1:25 was added and visualized with goat anti-mouse IgG peroxidase conjugate (Cappel Research, Durham, N.C.) and OPD buffer (pH 5.0; 0.1 M citric acid, 0.07 M sodium phosphate dibasic 12-hydrate, and 0.015% [vol/vol] H2O2).
Statistical methods.
Statistical significance for the differences between groups was assessed by using the Student t test.
RESULTS
Effect of oral vaccination against H. pylori infection.
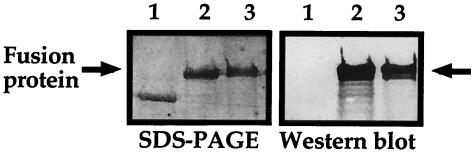
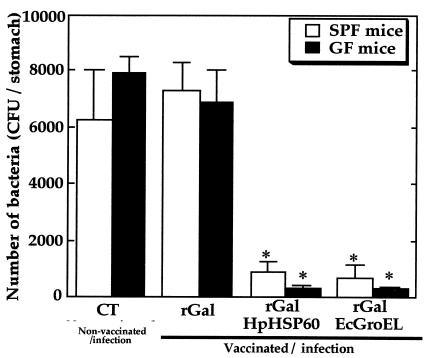
As shown in Fig. 1, H9 MAb reacted with a band of approximately 180 kDa in the fusion protein preparation. We first examined colonization of H. pylori in the stomach after vaccination. As shown in Fig. 2, oral vaccination with either rGalEcGroEL or rGalHpHSP60 significantly inhibited H. pylori colonization in the stomachs of both strains of mice by 3 weeks after infection. Compared to the control rGal-inoculated mice, vaccination resulted in reduction of infection by 90 to 95%. However, at 10 weeks postinfection (p.i.), the bacterial loads in both strains of mice were similar to the control CT group, regardless of which vaccine had been applied (data not shown).
FIG. 1.
Profiles of fusion protein on SDS-PAGE (left panel) and Western blot analysis with H9 MAb (right panel). A 1-μg portion of rGal (lane 1), rGalHpGroEL (lane 2), or rGalEcGroEL (lane 3) was loaded in each lane.
FIG. 2.
Effect of vaccination on bacterial colonization in SPF and GF mice at 3 weeks p.i. The number of bacteria per stomach was determined by a bacterial culturing system as described in the text. The data represent the means plus the standard deviations for 10 (SPF) or 7 (GF) mice. ✽, P < 0.05 (i.e., significantly different from the control CT group [Non-vaccinated/infection]).
H. pylori HSP60-specific antibody levels in sera.
As shown in Table 1, elevated levels of IgG in serum against H. pylori HSP60 were present at 3 weeks p.i. in both SPF and GF mice vaccinated with either rGalEcGroEL or rGalHpHSP60. There were no significant differences between any of these groups. At 10 weeks after infection, there were still no significant differences among the groups (average ELISA values against H. pylori HSP60, 0.241 [SPF] versus 0.304 [GF]). The levels of IgG in serum were similar in all groups of infected mice, as well as controls (data not shown).
TABLE 1.
IgG response to purified H. pylori HSP60 in sera of SPF and GF mice
| Group | Mean ELISA value (OD490)a ± SD in:
|
|
|---|---|---|
| SPF mice | GF mice | |
| Nonvaccinated with infection (CT only) | 0.145 ± 0.041 | 0.234 ± 0.091 |
| Vaccinated with infection | ||
| rGal | 0.286 ± 0.028 | 0.261 ± 0.022 |
| rGalHpHSP60 | 0.521 ± 0.081b | 0.552 ± 0.063b |
| rGalEcGroEL | 0.651 ± 0.122b | 0.566 ± 0.063b |
Data represent the mean values for each group at 3 weeks p.i. (SPF mice, n = 10; GF mice, n = 7). OD490, optical density at 490 nm.
P < 0.05 (versus the CT group animals).
Histological examination of gastric tissue.
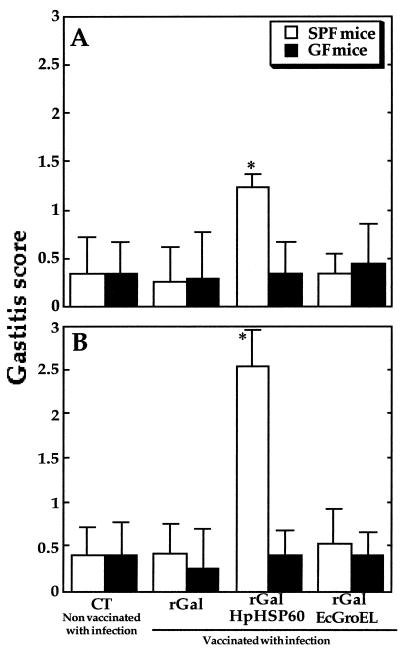
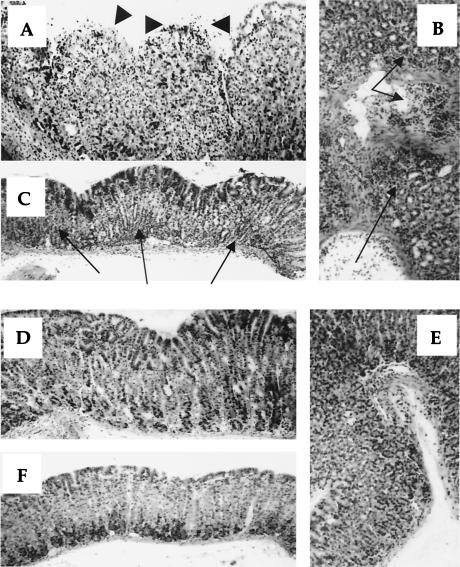
It is known that after infection, previous oral vaccination frequently results in postimmunization gastritis (10, 12, 30). Therefore, we assessed whether H. pylori infection after oral vaccination causes gastric inflammation in these mice. As shown in Fig. 3, mild and severe gastritis was observed only in SPF mice preimmunized with rGalHpHSP60, at 3 and 10 weeks p.i., compared to the other groups. Furthermore, no significant increase of the gastric inflammation score was observed in any GF mice for up to 10 weeks p.i. Figure 4 shows representative photomicrographs of gastric tissues of rGalHpHSP60-vaccinated SPF and GF mice at 10 weeks p.i. Severe gastritis disrupting the glandular structure, surface erosion, and marked inflammatory cell infiltration was frequently observed in the SPF mice vaccinated with rGalHpHSP60 (Fig. 4A to C). However, the same vaccination with rGalHpHSP60 did not cause gastric inflammation in GF mice (Fig. 4D to F). Oral vaccination with rGalEcGroEL and a after infection also did not cause any severe gastric inflammation in either group of mice up to 10 weeks p.i. No inflammatory cell infiltration was observed in the stomachs of immunized mice without H. pylori infection up to 12 weeks after the last vaccination (data not shown). This finding suggests that H. pylori HSP60 immunization does not have any harmful effects per se. H. pylori infection alone without any other treatment caused inflammatory cell infiltration in either strain, similar to mice immunized with CT, rGal, or rGalEcGroEL (data not shown). In addition, none of the mice showed any atrophic changes (data not shown).
FIG. 3.
Gastritis scores in vaccinated SPF and GF mice at 3 (A) and 10 (B) weeks p.i. Hematoxylin-eosin-stained gastric sections were scored for grade of inflammation (grades 0 to 3) as described in the text. The data represent the means plus the standard deviations for 10 (SPF) or 7 (GF) mice. ✽, P < 0.05 (i.e., significantly different from the control CT group [Nonvaccinated with infection]).
FIG. 4.
Photomicrographs of hematoxylin-eosin-stained gastric tissues of SPF (A, B, and C) and GF (D, E, and F) mice prevaccinated with H. pylori HSP60 at 10 weeks p.i. Cardia mucosa of SPF mice frequently showed severe gastritis with disruption of the gland structure, severe inflammatory cell infiltration, and erosion (A). GF mice did not show these symptoms (D). Severe inflammatory cell infiltration was observed in mucosa in SPF mice (B) but not in GF mice (E). Antral mucosa of SPF mice also showed severe inflammatory cell infiltration (C), but GF mice did not (F). Arrows indicate inflammatory cell infiltration, and arrowheads indicate erosion. Magnification, ×200.
DISCUSSION
Our results showed that oral vaccination with H. pylori HSP60 or GroEL reduced subsequent bacterial colonization 3 weeks after H. pylori infection. However, these protective effects were transient, with the bacterial load recovering by 10 weeks p.i. Several previous studies had shown that oral vaccination with H. pylori-derived antigens, including H. pylori whole-antigen or purified antigen, together with an appropriate adjuvant such as CT, have at least partial protective effects, reducing or eliminating colonization of H. pylori in animal models. These protective effects in vaccinated mice can be of extended duration (9, 10, 12, 20, 21, 30). Although the reason for the difference between the present results and these earlier findings is still not clear, the decreasing H. pylori HSP60-specific IgG levels in serum at 10 weeks p.i. might be associated with transient protection. It may also be speculated that the extent of bacterial load depends on the kind of vaccine.
The bacterial loads in both strains of mice recovered by 10 weeks p.i., despite prior vaccination. It is known that Th2 immune responses are dominant in H. pylori infection, and have a potentially protective role for bacterial infection (5, 22, 33). In this regard, several reports showed that Th2 immune responses lead to an increase in the number of H. pylori colonies in the stomach (4, 23, 30). These findings seem to be a possible explanation for increased bacterial load in mice vaccinated with E. coli GroEL. However, it is still not clear how the bacterial load increased in mice immunized with H. pylori HSP60 and GroEL.
In SPF mice vaccinated with H. pylori HSP60, severe gastric inflammation, including erosion, was observed 10 weeks p.i. However, these findings did not apply in SPF mice vaccinated with E. coli GroEL. Thus, the results indicated that the postimmunization gastritis caused by H. pylori HSP60 was an antigen-specific phenomenon. A number of investigators have also reported that gastric inflammation occurs in immunized mice after challenge (postimmunization gastritis) (10, 12, 30). However, the mechanism by which vaccination induces gastritis is still not understood. In the present study, GF mice lacking normal bacterial flora did not show gastric inflammation. Therefore, the presence of bacterial flora seems to be important for the induction of postimmunization gastritis in the H. pylori vaccination mouse model. However, the SPF and GF mice used here belonged to different strains, which may also have accounted for the differences.
Numerous studies indicate that alterations of bacterial flora relate to critical inflammatory illnesses through the stimulation of the immune system (1, 24, 31). Interestingly, vaccinated GF piglets suffered only slight gastritis without erosion after H. pylori infection (8). Eaton et al. recently reported that immunodeficient SCID mice permit higher levels of H. pylori colonization and fail to develop gastritis compared to wild-type mice, suggesting that the host gastric inflammatory response is mediated by lymphocytes stimulated through gut-associated lymph nodes and not via direct bacterial contact (7). In the present study, we also showed that GF mice exhibit only a few inflammatory changes without erosion and atrophy after H. pylori challenge. Thus, these findings suggest that the signals to immune cells through intestinal bacterial flora after H. pylori infection might be responsible for the postimmunization gastritis. On the other hand, it is known that several receptors such as CD14, Toll-like receptors (TLRs), and CARD4 contribute to innate immune responses to bacterial pathogens in mucosal surfaces that constitute the first line of defense against microbial pathogens (3, 16, 18, 29). A recent study showed that upregulated stimulation through TLR2 and TLR4, which recognize bacterial cell wall components, is associated with the induction of intestinal inflammation (13). TLR5 was recently identified as the mediator of bacterial flagellin recognition and subsequent induction of innate immune responses (14). The upregulated stimulation via CARD4 was also associated with the induction of inflammatory bowel diseases, including Crohn's disease (15, 27). Although the reasons for such postimmunization gastritis in SPF mice are still unknown, it may be speculated that upregulated signaling to innate immunity through receptors stimulated by the combined intestinal bacterial flora, bacterial HSP60 antigen, and H. pylori might be important.
In summary, our data indicate that prophylactic immunization with H. pylori HSP60 results not only in a partial reduction of bacterial colonization but also in postimmunization gastritis in SPF mice. Because GF mice did not show this severe gastric inflammation, we conclude that the presence of bacterial flora might contribute to the induction of postimmunization gastritis after vaccination with H. pylori HSP60. Many questions remain unanswered, including questions about the possible mechanisms of bacterial clearance and gastric inflammation caused by vaccination with H. pylori HSP60. However, the presence of bacterial flora may determine the usefulness and/or harmfulness of H. pylori HSP60 as a vaccine. The data in the present study might be valuable for the development of a vaccine for complete prevention of H. pylori without postimmunization gastritis.
Acknowledgments
We thank Catherine A. Newton (University of South Florida College of Medicine, Department of Medical Microbiology and Immunology) for careful reading and editing of the manuscript.
REFERENCES
- 1.Bengmark, S. 1998. Ecological control of the gastrointestinal tract: the role of probiotic flora. Gut 42:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser, M. J., and J. Parsonnet. 1994. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J. Clin. Investig. 94:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podoisky. 2000. Lipopolysaccharide activates during signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:443-451. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., D. Shu, and V. S. Chadwick. 1999. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand. J. Gastroenterol. 34:987-992. [DOI] [PubMed] [Google Scholar]
- 5.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 6.Dubois, A., C. K. Lee, N. Fiala, H. Kleanthous, P. T. Mehlman, and T. Monath. 1998. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect. Immun. 66:4340-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton, K. A., S. S. Ringler, and S. Krakowka. 1998. Vaccination of gnotobiotic piglets against Helicobacter pylori. J. Infect. Dis. 178:1399-1405. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero, R. L., J. M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, D. Y., G. M. Lew, P. D. Klein, D. G. Evans, D. J. Evans, Jr., Z. A. Saeed, and H. M. Malaty. 1992. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric and duodenal ulcers: a randomized controlled study. Ann. Intern. Med. 116:705-708. [DOI] [PubMed] [Google Scholar]
- 12.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausmann, M., S. Kiessling, S. Mestermann, G. Webb, T. Spottl, T. Andus, J. Scholmerich, H. Herfarth, K. Ray, W. Falk, and G. Rogler. 2002. Toll-like receptors 2 and 4 are upregulated during intestinal inflammation. Gastroenterology 122:1987-2000. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:599-603. [DOI] [PubMed] [Google Scholar]
- 15.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 16.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer, World Health Organization. 1994. Schistosomes, liver flukes, and Helicobacter pylori. Monogr. Eval. Carcinog. Risks Hum. 61:218-220. [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 19.Lahaie, R. G., and C. Gaudreau. 2000. Helicobacter pylori antibiotic resistance: trends over time. Can. J. Gastroenterol. 14:895-899. [DOI] [PubMed] [Google Scholar]
- 20.Lee, A., and M. Chen. 1994. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 62:3594-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, C. K., R. Weltzin, W. D. Thomas, Jr., H. Kleanthous, T. H. Ermak, G. Soman, J. E. Hill, S. K. Ackerman, and T. P. Monath. 1995. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J. Infect. Dis. 172:161-171. [DOI] [PubMed] [Google Scholar]
- 22.Londono-Arcila, P., D. Freeman, H. Kleanthous, A. M. O'Dowd, S. Lewis, A. K. Turner, E. L. Rees, T. J. Tibbitts, J. Greenwood, T. P. Monath, and M. J. Darsley. 2002. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect. Immun. 70:5096-5106. [DOI] [PMC free article] [PubMed]
- 23.Luzza, F., T. Parrello, L. Sebkova, L. Pensabene, M. Imeneo, M. Mancuso, A. M. La Vecchia, G. Monteleone, P. Strisciuglio, and F. Pallone. 2001. Expression of proinflammatory and Th1 but not Th2 cytokines is enhanced in gastric mucosa of Helicobacter pylori infected children. Dig. Liver Dis. 33:14-20. [DOI] [PubMed] [Google Scholar]
- 24.Marshall, J. C. 1999. Gastrointestinal flora and its alterations in critical illness. Curr. Opin. Clin. Metab. Care. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 25.Megraud, F. 1994. H. pylori resistance to antibiotics, p. 570-583. In R. H. Hunt and G. N. J. Tytgat (ed.), Helicobacter pylori: basic mechanism to clinical cure. Kluwer Academic Publishers, Dordecht, The Netherlands.
- 26.Montgomery, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 27.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 28.Peterson, W. L. 1991. Helicobacter pylori and peptic ulcer disease. N. Engl. J. Med. 324:1043-1048. [DOI] [PubMed] [Google Scholar]
- 29.Philpott, D. J., S. E. Girardin, and P. J. Sansonetti. 2001. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr. Opin. Immunol. 13:410-416. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan, S., A. M. Svennerholm, and J. Holmgren. 2002. Effects of oral vaccination and immunomodulation by cholera toxin on experimental Helicobacter pylori infection, reinfection, and gastritis. Infect. Immun. 70:4621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnani, S. 1999. Th1/Th2 cells. Inflamm. Bowel Dis. 5:285-294. [DOI] [PubMed] [Google Scholar]
- 32.Stanly, K. K., and J. P. Luzio. 1984. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 3:1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smythies, L. E., K. B. Waites, J. R. Lindesy, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi, H., T. Osaki, M. Kai, H. Taguchi, and S. Kamiya. 2000. Immune response against a cross-reactive epitope on the heat shock protein 60 homologue of Helicobacter pylori. Infect. Immun. 68:3448-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi, H., T. Osaki, N. Kurihara, H. Taguchi, T. Yamamoto, and S. Kamiya. 1997. Heat-shock protein 60 homologue of Helicobacter pylori is associated with adhesion of H. pylori to human gastric epithelial cells. J. Med. Microbiol. 46:825-831. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi, H., T. Osaki, H. Taguchi, T. Hanawa, T. Yamamoto, and S. Kamiya. 1996. Flow-cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J. Med. Microbiol. 45:270-277. [DOI] [PubMed] [Google Scholar]
- 37.Yasukawa, T., C. Kanei-Ishii, T. Maekawa, J. Fujimoto, T. Yamamoto, and S. Ishii. 1995. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J. Biol. Chem. 270:25138-25231. [DOI] [PubMed] [Google Scholar]
- 38.Young, D. B. 1990. Chaperonins and immune response. Semin. Cell Biol. 1:27-35. [PubMed] [Google Scholar]
- 39.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]