Abstract
In order to analyze the characteristics of the inflammatory response occurring in blood during pneumonia, we studied 38 patients with severe community-acquired pneumonia. Venous and arterial blood samples were collected at study entry and on days 1, 2, 3, 5, and 7 after inclusion. The concentrations of proinflammatory (tumor necrosis factor alpha [TNF-α], interleukin 1β [IL-1β], IL-6, and IL-8) and anti-inflammatory (IL-10) cytokines were determined in order to detect differences related to the origin of the sample, the causative organism, the clinical variables, and the final outcome of the episode. Legionella pneumonia infections showed higher concentrations of TNF-α, IL-6, IL-8, and IL-10. After 24 h, plasma IL-6, IL-8, and IL-10 concentrations in pneumococcal episodes increased, whereas in the same time interval, cytokine concentrations in Legionella episodes markedly decreased. The characteristics of the inflammatory response in bacteremic pneumococcal episodes were different from those in nonbacteremic episodes, as indicated by the higher plasma cytokine concentrations in the former group. Finally, our analysis of cytokine concentrations with regard to the outcome—in terms of the need for intensive care unit admittance and/or mechanical ventilation as well as mortality—suggests that there is a direct relationship between the intensity of the inflammatory response measured in blood and the severity of the episode.
Severe community-acquired pneumonia (SCAP) remains an important cause of mortality in spite of effective antibiotics (2, 3, 4). The lung inflammatory response during pneumonia is a complex process involving the coordinated expression of both pro- and anti-inflammatory cytokines. The adequate balance between both maintains the inflammatory response compartmentalized in the lungs and within the limits of homeostasis. If this balance is altered in favor of proinflammatory cytokines, the final response can involve the whole organ, leading to adult respiratory distress syndrome or even to the development in the entire organism of systemic inflammatory response syndrome. There is increasing evidence that an adequate cytokine balance plays a crucial role in determining the outcome of pneumonia. Even after successful eradication of causative organisms with appropriate antibiotic treatment, neither the transition from an inflamed lung to one with normal architecture nor the patient's survival is always ensured.
Better knowledge of the inflammatory host response may be of clinical importance in determining more efficacious therapy for SCAP. However, the relationship between the cytokine profile and the clinical outcome in pneumonia remains unclear, probably due to the complexity of the cytokine network and the different variables involved in clinical studies; the latter variables include (i) the timing of cytokine measurements in relation to the onset of infection, (ii) the effect of antibiotic therapy on cytokine production, and (iii) the compartmentalization of the inflammatory response within the lungs, which makes difficult the close monitoring of these phenomena.
The aim of our study was to assess the profile of the inflammatory response during the clinical course of SCAP by serial measurements. Several studies have evaluated inflammatory mediators in bronchoalveolar lavage (BAL) fluid (6, 13, 17) mainly because at least initially, the inflammatory response in pneumonia is confined to the lungs and this approach would therefore provide more reliable information than an analysis of inflammatory mediators in blood. Nevertheless, bronchoscopic BAL is a difficult procedure in nonintubated patients with respiratory failure and is associated with clinically relevant complications (fever, worsening of hypoxemia, and sepsis-like manifestations). Furthermore, some authors have found a positive correlation between the levels in serum of specific cytokines, such as interleukin 6 (IL-6), and their BAL fluid concentrations (6). For all of these reasons, we have analyzed this phenomenon by determining cytokine concentrations in blood samples, as has been done elsewhere (14).
We carried out a prospective study of SCAP in order to (i) determine the sequential changes in the expression of proinflammatory and anti-inflammatory cytokines in blood during pneumonia and (ii) analyze the relationship between these cytokine profiles and the etiology, severity, and outcome of the infection.
MATERIALS AND METHODS
Setting and study design.
This study was conducted at Bellvitge Hospital, a 1,000-bed university hospital in Barcelona, Spain, which serves an area of 1,100,000 inhabitants. A total of 38 consecutive patients with SCAP and with extensive radiographic consolidations (affecting at least two lobes) and respiratory failure (ratio of partial O2 pressure to the fraction of inspired O2, <300) were prospectively included. Pneumonia was diagnosed on the basis of a lung radiographic opacity and at least two of the following conditions: fever (>38.5°C), purulent expectoration, pleuritic chest pain, or leukocytosis (white blood cell count of >10,000/mm3). This prospective observational study was approved by our Institutional Review Board.
Microbiological studies.
For all patients, two sets of blood cultures were obtained. When available, sputum samples were processed for Gram staining and culturing. Investigation of pathogens in other specimens, such as normally sterile fluids, was also performed by conventional procedures. Isolation of Legionella pneumophila from sputum and other respiratory samples was attempted by using selective media (BCYE-α; Oxoid, Basingstoke, England). For all patients, the detection of L. pneumophila serogroup I antigen in urine was performed by an enzyme-linked immunosorbent assay (Legionella Urinary Antigen; Binax, Portland, Maine). Paired serum samples from the acute and convalescent phases (separated by 4 to 8 weeks) were also obtained for serological studies. Standard serological methods were used to detect antibodies to the following pathogens: Mycoplasma pneumoniae (indirect agglutination), Chlamydia psittaci (immunofluorescence), Chlamydia pneumoniae (microimmunofluorescence), Coxiella burnetii (immunofluorescence), L. pneumophila serogroups 1 to 6 (enzyme immunoassay), and influenza virus (enzyme immunoassay). Definite diagnosis for the infecitons caused by the aforementioned pathogens was based on the following parameters: fourfold increases in antibody titers for acute versus convalescence phases, with final titers for M. pneumoniae of ≥1:160, for C. psittaci of ≥1:256, for C. pneumoniae of ≥1:512, and for C. burnetti of ≥1:160, and for L. pneumophila serogroups 1 to 6, respiratory syncytial virus, influenza A virus, and parainfluenza 3 virus, indicative of seroconversion.
A definite microbiological diagnosis was obtained on the basis of blood cultures, pleural fluid cultures, serological tests, Legionella antigen in urine, and L. pneumophila in respiratory secretion cultures. A presumptive diagnosis was considered after the detection of a pure or predominant bacterial organism in the Gram stain of respiratory secretions and the subsequent cultures.
Collection of blood samples.
For all patients, serial venous blood samples were obtained at study entry and on days 1, 2, 3, 5, and 7 after inclusion. Because arterial blood comes directly from the lung, at least theoretically it could reflect the nature of local events more accurately than venous blood. Consequently, for a subgroup of patients, arterial and venous samples were simultaneously collected. The obtained blood was placed in tubes containing EDTA, immediately centrifuged, and stored at −80°C. Cytokine levels in the venous blood of eight healthy controls, adjusted for age and gender (five men and three women; age [mean ± standard deviation {SD}], 54.1 ± 3.3 years), were also determined.
Laboratory processing.
The concentrations of circulating proinflammatory (tumor necrosis factor alpha [TNF-α, IL-1β, IL-6, and IL-8) and anti-inflammatory (IL-10) cytokines were determined by using a commercial enzyme immunoassay technique (Genzyme, Cambridge, Mass.). Standard sensitivity assays were used, and the thresholds detectable in serum were 3 pg/ml for IL-6, 1 pg/ml for IL-8, 0.5 pg/ml for TNF-α, 3 pg/ml for IL-1β, and 4 pg/ml for IL-10.
Clinical and radiographic follow-up.
At inclusion, the following variables were recorded: age, smoking and alcohol habits, comorbidity, initial signs and symptoms. The severity of the pneumonia was evaluated by the Pneumonia Severity Index developed by the Pneumonia Outcome Research Team, which stratifies patients into risk classes showing a direct correlation between risk classes and death (8). Antibiotic treatment was prescribed according to our protocol for SCAP: 20 patients (52.6%) received an extended-spectrum cephalosporin by the intravenous route, whereas the remaining 18 patients (47.4%) were treated with the combination of an extended-spectrum cephalosporin and a macrolide (12 patients; 31.6%), both antibiotics plus rifampin (4 patients; 10.5%), and a macrolide plus rifampin (2 patients; 5.3%). The main clinical findings were monitored daily during the first 9 days of admission. A chest X-ray, routine venous blood test results, and arterial blood gas levels were obtained at entry and on days 1, 2, 3, 5, and 7.
Statistical analysis.
Statistical calculations were performed with the Statistical Package for the Social Sciences (SPSS) for Microsoft Windows. Results are expressed as the mean ± SD, median, interquartile range, and first and third quartiles. The significance levels were set to 0.05. The Mann-Whitney U test was used for comparisons of two groups. A comparison of serial cytokine measurements was made with the Kruskal-Wallis one-way analysis of variance nonparametric test. A comparison of arterial and venous cytokine concentrations was carried out with the Wilcoxon-Mann-Whitney test.
RESULTS
Characteristics of the included population.
The clinical and radiographic characteristics of the patients (27 men and 11 women; age [mean ± SD], 58.4 ± 17.7 years) at presentation are summarized in Table 1. A definitive etiological diagnosis was obtained for 21 cases (55.2%), and a presumptive diagnosis was obtained for 8 additional cases (21.0%). There was no etiological diagnosis for the remaining nine cases (23.6%). As indicated in Table 2, Streptococcus pneumoniae was the most frequent agent, isolated in 17 cases (44.7%) (11 definite and 6 presumptive), whereas L. pneumophila was the second most predominant organism, affecting 6 patients (15.7%).
TABLE 1.
Characteristics and outcomes of included cases
| Parameter | Valuea |
|---|---|
| No. of patients | 38 |
| Radiographic pattern | |
| Unilateral | 28 (74) |
| Bilateral | 10 (26) |
| Total duration of hospital stay (mean ± SD), days | 10.8 ± 5.7 |
| ICU admission | 13 (34.2) |
| Duration of ICU stay (mean ± SD), days | 2.1 ± 3.9 |
| Mechanical ventilation | 8 (21.05) |
| Total mortality | 11 (28.9) |
| Mortality at: | |
| 48 h | 2 (5.2) |
| 3-7 days | 4 (10.5) |
| >7 days | 5 (13.1) |
| Mortality according to Pneumonia Severity Index (deaths/cases) | |
| II | 2/12 (9.0) |
| III | 1/11 (16.6) |
| IV | 4/7 (57.1) |
| V | 4/8 (50) |
Reported as number (percentage), unless otherwise indicated.
TABLE 2.
Causative agents
| Diagnosis | No. (%) of cases | Microorganisms isolated | Diagnostic procedure | No. of cases |
|---|---|---|---|---|
| Definite | 21 (55.2) | Streptococcus pneumoniae | Blood culture | 10 |
| Transthoracic needle aspiration | 1 | |||
| Legionella pneumophila | Serology | 4 | ||
| Urine antigen | 2 | |||
| Haemophilus influenzae | Blood culture | 1 | ||
| Klebsiella pneumoniae | Blood culture | 1 | ||
| Streptoccocus pyogenes | Blood culture | 1 | ||
| Chlamydia psittaci | Serology | 1 | ||
| Presumptive | 8 (21) | Streptococcus pneumoniae | Sputum | 6 |
| Haemophilus influenzae | Sputum | 1 | ||
| Mixed oral flora | Sputum | 1 | ||
| None | 9 (23.6) |
After the initial evaluation, 25 patients (65.7%) were admitted into a conventional hospital ward, whereas the other 13 (34.2%) were transferred to an intensive care unit (ICU). The main outcome variables are summarized in Table 1.
Plasma cytokine concentrations.
As indicated in Table 3, all cytokines except for IL-1β could be detected in the plasma samples studied, although with a wide range of values; TNF-α and IL-6 showed higher values. When the variations in concentrations were analyzed over time, IL-6, IL-8, and IL-10 showed a statistically significant trend toward a rapid decrease after 24 to 48 h, whereas TNF-α remained basically unmodified throughout the study period. In the eight healthy controls, all blood cytokine levels were below the level of detection.
TABLE 3.
Plasma cytokine values in 38 patients with SCAP correlated to when the sample was obtained after admission to the hospital
| Cytokine | Median (interquartile rangea) pg/ml atb:
|
|||||
|---|---|---|---|---|---|---|
| Admission (n = 15) | Day
|
|||||
| 1 (n = 28) | 2 (n = 30) | 3 (n = 30) | 5 (n = 27) | 7 (n = 11) | ||
| TNF-α | 14.2 (11.9-28.06) | 12 (7.8-20.5) | 10.5 (7.4-20.2) | 14.4 (9.4-19.5) | 17 (8.5-32.2) | 20.4 (12.8-33.1) |
| IL-6 | 423 (162.1-1292) | 218 (100.3-218.3) | 83.8† (32-245) | 66.41† (33.8-171.6) | 29.51† (17.3-110.3) | 71† (29-155.6) |
| IL-8 | 4.8 (2.1-27.1) | 5.37 (1.9-17.9) | 1.74‡ (0.7-4.5) | 2.45‡ (1.03-4.9) | 3.1 (1.9-7.4) | 2.8 (2.5-14.4) |
| IL-10 | 11.85 (6.8-24.9) | 8.8 (4.3-23.9) | 6.4 (3.4-13) | 5.7* (1.8-8) | 5.5* (3.1-9.4) | 9.5‡ (3.5-15.8) |
First and third quartiles.
Statistically significant decreases over time in relation to basal levels of IL-6, IL-10, and IL-8 were determined with the Kruskal-Wallis nonparametric test and are indicated as follows: *, P < 0.01; †, P < 0.001; ‡, P < 0.05.
Cytokine concentrations in venous and arterial blood.
TNF-α and IL-6 achieved significantly higher levels in venous blood than in arterial blood; the opposite was found only for IL-8. Finally, IL-10 venous and arterial blood levels were similar.
Cytokine concentrations in relation to the etiological agent.
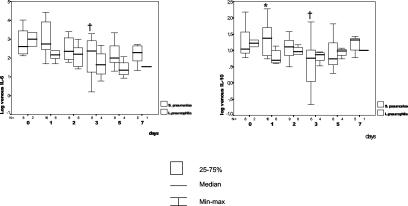
As expected, S. pneumoniae and L. pneumophila were the most common etiological agents, found in 17 and 6 episodes, respectively. Ten of the pneumoccocal episodes were bacteremic. A comparison between the two etiologies is shown in Fig. 1. Initial IL-6 and IL-10 levels were higher in Legionella episodes than in pneumococcal episodes, although these differences were not statistically significant. By day 1, however, IL-10 levels became higher in pneumoccocal pneumonia (mean, 42.7; median, 20.77; SD, 62.3) than in Legionella pneumonia (mean, 7.01; median, 5.12; SD, 3.9) (P = 0.02), and a marked but not statistically significant difference was found for IL-6 (respective means, medians, and SDs: 4,960.4 and 433.2, 605.1 and 154.1, and 8,923.8 and 723.3) (P = 0.07). On day 1, the third quartile for IL-10 (23.9 pg/ml) included 79.4% of patients with pneumoccocal pneumonia but none with Legionella pneumonia, whereas the third quartile for IL-6 (218.3 pg/ml) included 75% of patients with pneumoccocal pneumonia but 33% of patients with Legionella pneumonia. Finally, when changes in cytokine levels were compared with the initial values, by day 3 a significant reduction in IL-6 (P = 0.01) and IL-10 (P = 0.01) levels in plasma could be observed in the pneumococcal pneumonia group.
FIG. 1.
Pneumococcal and Legionella pneumonias. Comparative changes in the absolute daily plasma IL-6 and IL-10 concentrations and their variations over time in relation to the initial values are shown. An asterisk indicates a statistically significant difference in the comparisons of groups, as determined by the Mann-Whitney U test. A dagger indicates a statistically significant difference in relation to basal values, as determined by the Kruskal-Wallis one-way analysis of variance nonparametric test. For P values, see the text. The pairs of small numbers appearing just below the horizontal axis represent the numbers of observations in the subgroups of patients at different time intervals from admission. Min-max, minimum-maximum.
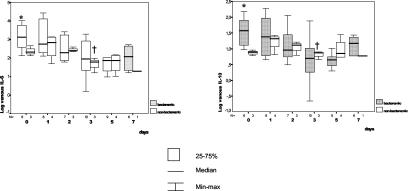
Cytokine concentrations in pneumococcal bacteremic and nonbacteremic episodes.
As shown in Fig. 2, bacteremic episodes of pneumococcal pneumonia showed higher TNF-α, IL-6, and IL-10 concentrations than nonbacteremic episodes. On admission, these differences were statistically significant for IL-6 (respective means, medians, and SDs for bacteremic and nonbacteremic episodes: 3,836.9 and 232.1, 1,337 and 172.2, and 4,210 and 153.6) (P = 0.02) and IL-10 (respective means, medians, and SDs for bacteremic and nonbacteremic episodes: 64.01 and 8.94, 47.4 and 8.36, and 56.6 and 2.9) (P = 0.02). The third quartile for IL-6 and IL-10 levels at admission (1,292 and 24.98 pg/ml, respectively) identified bacteremic pneumonia in 60 and 66% of the patients, whereas none of the nonbacteremic cases showed such concentrations. Finally, by day 2, there was a significant decrease in TNF-α concentrations (P = 0.05) in nonbacteremic cases, and the same was true by day 3 for IL-6 (P = 0.03) and IL-10 (P = 0.03).
FIG. 2.
Bacteremic and nonbacteremic pneumococcal pneumonias. Daily and time course comparisons of plasma IL-6 and IL-10 concentrations are shown. An asterisk indicates a statistically significant difference in the comparisons of groups, as determined by the Mann-Whitney U test. A dagger indicates a statistically significant difference in relation to basal values, as determined by the Kruskal-Wallis one-way analysis of variance nonparametric test. For P values, see the text. The pairs of small numbers appearing just below the horizontal axis represent the numbers of observations in the subgroups of patients at different time intervals from admission. Min-max, minimum-maximum.
Cytokine concentrations and pneumonia outcome.
The serum cytokine concentrations in patients admitted and not admitted to the ICU were similar initially, but significant differences became apparent from day 1 on. At this time, the patients needing ICU admittance showed higher values, and this finding was significant for IL-10 (respective means, medians, and SDs for ICU-admitted versus non-ICU-admitted patients: 109.7 and 17.1, 21.1 and 6.7, and 167.2 and 36.28) (P = 0.01). The tendency for lower values in non-ICU-admitted patients than in ICU-admitted patients was significant for IL-6 by day 2 (P = 0.001) and for IL-10 by day 3 (P = 0.003).
When ventilated and nonventilated patients were compared, the initial cytokine concentrations tended to be higher in the former group. On day 1, statistically significant higher levels were seen in ventilated patients than in nonventilated patients for TNF-α (respective means, medians, and SDs: 23.1 and 12.8, 15.8 and 10.9, and 15.7 and 8.7) (P = 0.05), IL-8 (respective means, medians, and SDs: 56.8 and 7.9, 17 and 4.41, and 75.7 and 10.5) (P = 0.04), and IL-10 (respective means, medians, and SDs: 178 and 17.5, 172.7 and 7.64, and 206.3 and 34.5) (P = 0.04). In contrast to ventilated patients, nonventilated patients showed significant reductions in plasma TNF-α levels by day 1 (P = 0.03), IL-6 levels by day 2 (P = 0.003), and IL-10 levels by day 3 (P = 0.01).
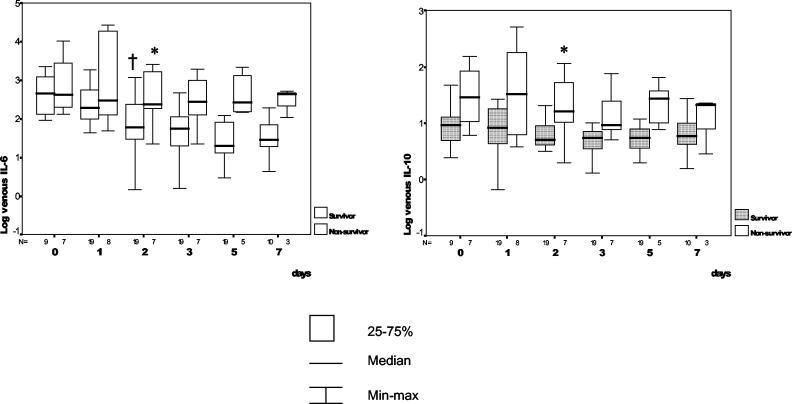
In general, serum cytokine concentrations were higher in nonsurvivors than in survivors (Fig. 3). The third quartile for IL-10 initial concentrations (38.2 pg/ml) identified 50% of nonsurvivors and only 11% of survivors. However, these initial differences did not reach statistical significance. In the following days and in contrast to nonsurvivors, survivors displayed significant reductions in IL-6 and IL-10 concentrations starting after day 2. Thus, at 48 h after admission, an IL-6 level of >87 pg/ml predicted mortality with a sensitivity of 85%, a specificity of 56%, a positive predictive value of 83%, and a negative predictive value of 94%. In addition, an IL-10 level of >14.7 pg/ml predicted mortality with a sensitivity of 71%, a specificity of 96%, a positive predictive value of 83%, and a negative predictive value of 91%.
FIG. 3.
Survivors and nonsurvivors. Daily changes as well as time course changes in plasma IL-6 and IL-10 concentrations are shown. An asterisk indicates a statistically significant difference in the comparisons of groups, as determined by the Kruskal-Wallis one-way analysis of variance nonparametric test. A dagger indicates a statistically significant difference in relation to basal values, as determined by the Kruskal-Wallis one-way analysis of variance nonparametric test. For P values, see the text. The pairs of small numbers appearing just below the horizontal axis represent the numbers of observations in the subgroups of patients at different time intervals from admission. Min-max, minimum-maximum.
DISCUSSION
The aim of this study was to analyze the time course profiles of inflammatory cytokines in the blood of patients with SCAP. The study was performed with a group of 38 patients admitted to our hospital during a 1-year period. This population can be considered representative of SCAP in terms of age, underlying diseases, etiology, initial severity, and outcome.
All studied cytokines except for IL-1β could be detected in venous blood samples. This finding disagrees with previous results comparing serum and lung cytokine levels in community-acquired pneumonia, which supported a lung compartmentalized response not detected in blood (6). In this study, only IL-6 showed higher levels in the plasma of patients with pneumonia than in controls; this finding probably was due to the lower degree of severity of the episodes in the study of Dehoux et al. (6) than in our study. It has been suggested that in severe infections, these cytokines can circulate at high levels, leading to either excessive production or saturation of target receptor sites or both (5). In SCAP episodes, the development of an intense and maintained inflammatory systemic response has been associated with high plasma TNF-α, IL-6, and IL-8 levels (17); however, as we found, IL-6 usually achieves higher levels (6, 17).
As arterial blood can reflect the nature of pulmonary events more accurately than venous blood (7), we compared the cytokine concentrations obtained in both arterial and venous blood samples. Although differences existed, their significance does not justify the collection of an arterial blood sample. Only IL-8 displayed significantly higher levels in arterial blood, probably because of more compartmentalized production. Therefore, we concluded that a venous blood sample is adequate for monitoring the inflammatory response generated in pneumonia.
In our study, serial measurements confirmed that plasma IL-6, IL-8, and IL-10 concentrations were time dependent, whereas those of TNF-α and IL-1β were not. Eventually, this time dependence of IL-6, IL-8, and IL-10 concentrations could be related to the clinical characteristics and outcome of the pneumonia. The fact that TNF-α and IL-1β concentrations did not change over time would appear paradoxical but, as already noted, because they play a crucial role in the early phase of the inflammatory cascade (11), their production is predominantly local and their mean life is probably much shorter than that of IL-6 and IL-8. Thus, there could be an early release of TNF-α and IL-1β from the lungs, detectable in venous blood but only during a premature and short period of time. The results obtained for our control group, in which blood cytokine levels were undetectable, support these interpretations.
Our experience suggests that blood cytokine concentrations are related to the etiological diagnosis of pneumonia. Legionella pneumonia infections displayed higher initial concentrations of TNF-α, IL-6, IL-8, and IL-10. After 24 h, serum IL-6, IL-8, and IL-10 concentrations increased in the pneumococcal infections but decreased markedly in the Legionella infections. In pneumoccocal pneumonia, the initial increase in cytokine levels has been related to bacterial lysis caused by β-lactams. It is known that the cell wall of S. pneumoniae is a potent inducer of inflammation, probably via the activation of complement and the induction of cytokines such as TNF-α and IL-1β (20, 22). With the onset of the process of antibiotic-induced bacterial death, cell wall components released by bacterial autolytic enzymes exacerbate inflammation early in the course of therapy (19). L. pneumophila differs from S. pneumoniae in its capability to interfere with the inflammatory response for its own benefit. Several observations have suggested an ability of L. pneumophila to stimulate a more moderate cytokine response as an adaptive mechanism to facilitate its intracellular survival (15). In the same way, some authors have hypothesized that L. pneumophila induces the expression of IL-10 in human lungs and that endogenous IL-10 facilitates the pathogenesis of Legionella pneumonia in the lungs (16).
In pneumococcal pneumonia, the characteristics of the inflammatory response appeared to differ between bacteremic and nonbacteremic episodes; the concentrations in serum were much higher in the former subgroup. This result agrees with previous reports suggesting a positive correlation between IL-6 and IL-10 levels and SCAP (9). The inflammatory response was also more prolonged in bacteremic episodes, and this finding may be related to the imbalance between pro- and anti-inflammatory responses (11).
As other authors have reported (12), our results suggest a direct relationship between the intensity of the inflammatory response and the severity of the episode. Some authors recently described the appearance of a sort of lung tissue damage related to mechanical ventilation that could enhance the release of inflammatory mediators (1, 8, 18). In our study, no correlation was found between the severity of the Simplified Acute Physiology Score and cytokine levels; however, a statistically significant correlation was observed between C-reactive protein and IL-6 levels only at day 3 (r = 0.9, P = 0.03). Finally, the potential interference of corticosteroid treatment with our data must have been rather small, considering that only 5 of 38 patients (13%) received this treatment and for a period shorter than 24 h.
In our experience, the initial serum cytokine concentrations tended to be higher in nonsurvivors than in patients with a favorable outcome, and this finding agrees with previous reports (16, 21). In the same way, the inflammatory response was significantly greater and more prolonged in nonsurvivors. In practice, blood IL-6 and IL-10 concentrations at day 2 predict mortality with a reasonable accuracy. Finally, our experience indicates that a fatal outcome was announced by a recrudescence of the inflammatory response.
So far, knowledge of the mechanisms involved in host-bacterium interactions is limited. For SCAP, there is an evident need to fill the gap in the understanding of pneumonia inflammatory mechanisms. At least hypothetically, the inappropriate balance of the mechanisms involved in inflammation may be responsible for a fatal outcome in some cases. A clearer understanding of the key events controlling these interactions could help to detect cases with a poorer prognosis earlier, to clarify the potential role of certain antibiotics in excessively exacerbated inflammation, and to develop appropriate tools for more effective control of the inflammatory response.
Acknowledgments
We thank Ana Fernández-Agüera for technical assistance in the collection of samples as well Jordi Bonete for laboratory processing. We also thank the staff and residents of our departments, as well as the patients and their relatives, for their valuable collaboration in this study.
This work was supported by grants awarded by the Sociedad Española de Neumología y Cirugía Torácica (SEPAR-FEPAR), 1997, and the Fondo de Investigaciones Sanitarias de la Seguridad Social (FIS) (grant no. 99/0838). S. F.-S.was the recipient of a grant from the Ciutat Sanitària i Universitària de Bellvitge for the year 1998.
REFERENCES
- 1.American Journal of Respiratory and Critical Care Medicine. 1999. International Consensus Conferences in Intensive Care Medicine: ventilator-associated lung injury in ARDS. Am. J. Respir. Crit. Care Med. 160:2118-2124. [DOI] [PubMed] [Google Scholar]
- 2.Austrian, R., and J. Gold. 1964. Pneumococcal bacteriemia with special reference to bacteriemic pneumococcal pneumonia. Ann. Intern. Med. 6:759-770. [DOI] [PubMed] [Google Scholar]
- 3.Bethmann, A. N., F. Brasch, R. Nüsing, K. Vogt, H. D. Volk, K. M. Müller, A. Wendel, and S. Uhlig. 1998. Hyperventilation induces release of cytokines from perfused mouse lung. Am. J. Respir. Crit. Care Med. 157:263-272. [DOI] [PubMed] [Google Scholar]
- 4.Busund, R., R. O. Lindsetmo, and I. T. Rasmussen. 1990. Tumor necrosis factor and interleukin-1 appearance in experimental Gram-negative septic shock. J. Lab. Clin. Med. 116:100-105. [DOI] [PubMed] [Google Scholar]
- 5.Creasey, A. A., P. Stevens, and J. Kenney. 1991. Endotoxin and cytokine profile in plasma baboons challenged with lethal and sublethal Escherichia coli. Circ. Shock 33:84-91. [PubMed] [Google Scholar]
- 6.Dehoux, M. S., A. Boutten, J. Ostinelli, N. Seta, M. Dombret, B. Crestani, M. Deschenes, J. Trouillet, and M. Aubier. 1994. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 150:710-716. [DOI] [PubMed] [Google Scholar]
- 7.Douzinas, E., P. D. Tsidemiadou, M. T. Piratidis, I. Andrianakis, A. Bobota-Chloraki, K. Katsouyanni, D. Sfyras, K. Malagari, and C. Roussos. 1997. The regional production of cytokines and lactate in sepsis-related multiple organ failure. Am. J. Respir. Crit. Care Med. 155:53-59. [DOI] [PubMed] [Google Scholar]
- 8.Fine, M. J., T. E. Auble, D. M. Yealy, B. H. Hanusa, L. A. Weissfeld, D. E. Singer, C. M. Coley, T. J. Marrie, and W. N. Kapoor. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243-250. [DOI] [PubMed] [Google Scholar]
- 9.Glynn, P., R. Coakley, I. Kilgallen, N. Murphy, and S O′Neill. 1999. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax 54:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutsky, A. S., and L. N. Tremblay. 1998. Multiple organ failure. Is mechanical ventilation a contributing factor? Am. J. Respir. Crit. Care Med. 157:1721-1725. [DOI] [PubMed] [Google Scholar]
- 11.Martin, T. R. 1997. Overview of cytokine networks in lung injury, p. 19-28. In M. R. Pratter and S. Nelson (ed.), The role of cytokines in systemic and pulmonary medicine. American Thoracic Society Continuing Education Monograph Series (Part II). American Thoracic Society, New York, N.Y.
- 12.Meduri, G. U., E. A. Tolley, G. P. Chrousos, and F Stentz. 2002. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 165:983-991. [DOI] [PubMed] [Google Scholar]
- 13.Monton, C., A. Torres, M. El-Ebiary, X. Filella, A. Xaubet, and J. P. de la Bellacasa. 1999. Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit. Care Med. 27:1745-1753. [DOI] [PubMed] [Google Scholar]
- 14.Örtqvist, A., J. Hedlund, B. Wretlind, A. Carlström, and M. Kalin. 1995. Diagnostic and prognostic value of interleukin-6 and C-reactive protein in community-acquired pneumonia. Scand. J. Infect. Dis. 27:457-462. [DOI] [PubMed] [Google Scholar]
- 15.Park, D. R., and S. J. Skerrett. 2000. Cytokines in Legionella pneumophila infections, p. 155-188. In S. Nelson and T. R. Martin (ed.), Cytokines in pulmonary disease. Lung Biology in Health and Disease, vol. 141. Marcel Dekker, New York, N.Y.
- 16.Park, D. R., and S. J. Skerrett. 1996. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ. J. Immunol. 157:2528-2538. [PubMed] [Google Scholar]
- 17.Shütte, M., J. Lohmeyer, S. Rosseau, S. Ziegler, C. Siebert, H. Kielisch, H. Pralle, F. Grimminger, H. Morr, and W. Seeger. 1996. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary edema. Eur. Respir. J. 9:1858-1867. [DOI] [PubMed] [Google Scholar]
- 18.Takata, M., J. Abe, H. Tanaka, Y. Kitano, S. Doi, T. Kohsaka, and K. Miyasaka. 1997. Intraalveolar expression of tumor necrosis factor-α gene during conventional and high-frequency ventilation. Am. J. Respir. Crit. Care Med. 156:272-279. [DOI] [PubMed] [Google Scholar]
- 19.Tuomanen, E. 1993. Breaching the blood-brain barrier. Sci. Am. 268:890-894. [DOI] [PubMed] [Google Scholar]
- 20.Tuomanen, E., R. Rich, and O. Zak. 1987. Induction of pulmonary inflammation by components of the pneumoccocal cell surface. Am. Rev. Respir. Dis. 135:869-874. [DOI] [PubMed] [Google Scholar]
- 21.van Dissel, J. T., P. van Langevelde, R. G. J. Westendorp, K. Kwappenberg, and M. Frölich. 1998. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351:950-953. [DOI] [PubMed] [Google Scholar]
- 22.Winkelstein, J. A., and A. Tomasz. 1978. Activation of the alternative complement pathway by pneumoccocal cell wall teichoic acid. J. Immunol. 120:174-178. [PubMed] [Google Scholar]