Abstract
The secreted aspartyl proteinases (Saps) of Candida albicans have been implicated as virulence factors associated with adherence and tissue invasion. The potential use of proteinases as markers of invasive candidiasis led us to develop a competitive binding inhibition enzyme-linked immunosorbent assay (ELISA) to detect Sap in clinical specimens. Daily serum and urine specimens were collected from rabbits that had been immunosuppressed with cyclophosphamide and cortisone acetate and infected intravenously with 107 C. albicans blastoconidia. Disseminated infection was confirmed by organ culture and histopathology. Although ELISA inhibition was observed when serum specimens from these rabbits were used, more significant inhibition, which correlated with disease progression, occurred when urine specimens were used. Urine collected as early as 1 day after infection resulted in significant ELISA inhibition (mean inhibition ± standard error [SE] compared with preinfection control urine, 15.7% ± 2.7% [P < 0.01]), and inhibition increased on days 2 through 5 (29.4% ± 4.8% to 44.5% ± 3.5% [P < 0.001]). Urine specimens from immunosuppressed rabbits infected intravenously with Candida tropicalis, Candida parapsilosis, Candida krusei, Cryptococcus neoformans, Aspergillus fumigatus, or Staphylococcus aureus were negative in the assay despite culture-proven dissemination. Nonimmunosuppressed rabbits receiving oral tetracycline and gentamicin treatment were given 2 × 108 C. albicans blastoconidia orally or intraurethrally to establish colonization of the gastrointestinal tract or bladder, respectively, without systemic dissemination; urine specimens from these rabbits also gave negative ELISA results. Dissemination to the kidney and spleen occurred in one rabbit challenged by intragastric inoculation, and urine from this rabbit demonstrated significant inhibition in the ELISA (mean inhibition ± SE by day 3 after infection, 32.9% ± 2.7% [P < 0.001]). The overall test sensitivity was 83%, the specificity was 92%, the positive predictive value was 84%, the negative predictive value was 91%, and the efficiency was 89% (166 urine samples from 33 rabbits tested). The specificity, positive predictive value, and efficiency could be increased to 97, 95, and 92%, respectively, if at least two positive test results were required for a true positive designation. The ELISA was sensitive and specific for the detection of Sap in urine specimens from rabbits with disseminated C. albicans infection, discriminated between colonization and invasive disease, reflected disease progression and severity, and has the potential to be a noninvasive means to diagnose disseminated candidiasis.
Despite the introduction of improved antifungal drugs for treatment and prophylaxis, invasive candidiasis remains a significant clinical problem. In a recent population-based active laboratory surveillance study, Candida species were responsible for 72.8 cases of invasive mycoses per million population per year, followed by species of Cryptococcus (65.5 cases per million population per year), Coccidioides (15.3 cases per million population per year), Aspergillus (12.4 cases per million population per year), and Histoplasma (7.1 cases per million population per year) (56). Although other Candida species were significant contributors to this problem, Candida albicans was the single most prevalent species associated with bloodstream infections in the hospital setting (28, 78). C. albicans was responsible for 59% of primary candidemia occurring between 1989 and 1999 among patients in 1,116 intensive care units participating in the National Nosocomial Infections Surveillance system (78) and for 55% of all bloodstream infections in a recent population-based candidemia surveillance study (28).
C. albicans can invade deep organs in immunocompromised patients, resulting in significant morbidity and mortality (18, 43). Risk factors for disseminated disease include indwelling catheters, administration of broad-spectrum antibacterial antibiotics, immunosuppressive drug regimens associated with bone marrow or organ transplantation, and cancer chemotherapy (17, 46). Diagnosis is difficult because clinical signs and symptoms of invasive disease are not specific and currently available serological tests often lack the desired sensitivity or specificity for a rapid and reliable diagnosis (45). Whereas histopathological examination of infected tissue is highly specific, the invasive procedures required to obtain deep organ biopsies are not recommended for immunocompromised patients, who are often thrombocytopenic (46). In the absence of a rapid and specific diagnosis, appropriate therapy is often delayed, contributing to increased morbidity and mortality.
Despite continued efforts to develop rapid and specific diagnostic tests to detect invasive candidiasis, most tests developed to date lack sensitivity or specificity. Detection of antibodies to Candida antigens can be unreliable, as healthy individuals have been shown to possess natural levels of anti-Candida antibodies. Further, antibody production in immunocompromised patients can fluctuate, depending upon the state of immune suppression, making interpretation of test results difficult (45). Whereas strides have been made in PCR-based methods to detect DNA from C. albicans in blood (13, 16, 71), these tests have not yet been standardized for general use. Detection of various C. albicans antigens or metabolites such as 1,3-β-d-glucan (52, 53), arabinitol (82, 83), enolase (81), and cell wall mannoprotein (11, 20, 70) have shown promise, but each test has limitations and most are not available outside of the research laboratory.
Another approach to the diagnosis of systemic candidiasis involves detection of the secreted aspartyl proteinases (Saps) of C. albicans in serum specimens (50, 64, 66). C. albicans, along with Candida tropicalis and Candida parapsilosis, secretes inducible aspartyl proteinases that are considered to be virulence factors which aid in the invasion of host tissue (39, 63, 68, 72). In addition, it has been demonstrated that clinical isolates express Sap at concentrations that correlate with the severity of human (8) and murine (36) infection. Sap production has also been associated with an increased capacity of C. albicans to adhere to and degrade epithelial cells (2, 12, 49, 54, 84) and to invade the stratum corneum, mucosal surfaces, and deep organs in murine models of candidiasis (7, 14, 15, 55). Sap expression has been demonstrated in vivo in C. albicans-infected mice or rats (10, 15, 33, 40), and disruption of Sap genes results in reduced virulence (9, 25, 33, 69). The usefulness of a test using an inducible enzyme as a marker of disease lies in its potential to differentiate colonization from invasive disease. It has been demonstrated that differential expression of Sap genes occurs in patients and in individuals who were carriers of C. albicans (51). Because extracellular production of Sap is inducible (40, 47) and since Sap has been shown to be produced during active tissue invasion (40, 54, 55), its extracellular concentration should correlate with invasive disease rather than simple colonization.
A limited number of tests to detect Sap or anti-Sap antibodies have been described for the immunodiagnosis of disseminated candidiasis (41, 50, 66, 74). These tests examined serum as a source of Sap antigen or anti-Sap antibodies, and the test sensitivity varied widely depending upon the antibodies or antigens used and the test format employed. No studies, however, have examined urine as a source of Sap antigen. The use of urine specimens, unlike that of serum specimens, would allow frequent, noninvasive collection of large sample volumes which could be readily concentrated to potentially increase test sensitivity. In addition, other researchers may have used unpurified Sap antigens as immunogens, leading to the production of antibodies that may cross-react with contaminating cell wall mannoprotein or extraneous proteins, reducing test specificity (3, 4, 39, 65, 74). We therefore used anti-Sap antibodies, raised against highly purified Sap and shown previously not to react with contaminating mannoproteins (47), to develop and evaluate an enzyme-linked immunosorbent assay to detect Sap in urine and serum specimens from rabbits experimentally infected with C. albicans.
MATERIALS AND METHODS
Microorganisms.
Lyophilized stock cultures of C. albicans strain CBS 2730 (a gift from R. Rüchel, University of Göttingen, Göttingen, Germany) (a variant of the type strain also known as DSM 6659) was used to produce purified Sap for the competitive binding inhibition enzyme-linked immunosorbent assay (ELISA) and to immunize mice for the production of anti-Sap antibodies. The following isolates, used for the infection of rabbits in an animal model of disseminated disease or for urinary tract or gastrointestinal tract colonization studies, were obtained from the Fungus Reference Unit, Mycotic Diseases Branch, Centers for Disease Control and Prevention (CDC): C. albicans strains 36B, Lecocq, and Q16 (B serotypes) and 3181A, 2730, and B311 (A serotypes); C. tropicalis 83-48062, C. parapsilosis B390, Candida krusei 83-056058, Cryptococcus neoformans 9759, and Aspergillus fumigatus 2570. A clinical isolate of Staphylococcus aureus, strain 255, was kindly supplied by Gary Hancock of the Division of Bacterial and Mycotic Diseases, CDC, as a bacterial control isolate. S. aureus was grown on Trypticase soy agar slants containing 5% sheep's blood (TSAB) (BBL, Becton Dickinson, Cockeysville, Md.) at 37°C for 24 h. Fungal isolates were grown on Sabouraud dextrose agar (SDA) slants (Emmon's modification; BBL) at 25°C for 48 h (yeasts) or at 35°C for 4 days (A. fumigatus).
Rabbit model of disseminated disease.
All animal models and methods were approved by the CDC Institutional Animal Care and Use Committee. Female New Zealand White rabbits, 3 to 4 kg in weight, were immunosuppressed by subcutaneous injection of 25 mg of cyclophosphamide (Cytoxan; Bristol Myers Squibb, Princeton, N.J.) per kg of body weight 2 days before infection and of 15 mg of cortisone acetate suspension (Eli Lilly and Co., Indianapolis, Ind.) per kg 2 days and 1 day before infection and again on the day of infection, as previously described (26). This immunosuppressive drug regimen resulted in a significant decline in total leukocytes as determined by hemacytometer counting. Rabbits were infected intravenously with 1 × 107 blastoconidia (Candida spp.) or conidia (A. fumigatus) or with 2 × 107 C. neoformans or 1 × 108 S. aureus cells harvested by washing surface growth from agar slants with 5 ml of sterile 0.01 M phosphate-buffered saline (PBS) (8.1 mM Na2HPO4, 1.9 mM KH2PO4, 0.85% NaCl [pH 7.2]), followed by centrifugation and washing with PBS at 2,700 × g for 10 min. Microorganisms were suspended to their final inoculating volume in 0.85% NaCl after enumeration in a hemacytometer. CFU per milliliter were calculated after serial dilutions of the inocula were plated onto SDA and incubated at 25°C (fungi) or plated onto TSAB and incubated at 37°C (S. aureus).
Rabbit models of urinary tract and gastrointestinal tract colonization.
Female New Zealand White rabbits, 3 to 4 kg in weight, were used in both urinary tract and gastrointestinal tract colonization models. Rabbits were treated with broad-spectrum antibiotics to facilitate mucosal colonization with fungi. Tetracycline (0.1 mg/ml) (The Butler Co., Columbus, Ohio) and gentamicin (1 mg/ml) (Sigma Chemical Co., St. Louis, Mo.) were administered ad libitum in the drinking water and were replaced with fresh antibiotics every 24 h. Antibiotic treatment began 1 day prior to administration of C. albicans blastoconidia and continued until euthanasia and necropsy on day 3 to 5 postadministration. A significant reduction in bacterial CFU from fecal cultures was noted following antibiotic treatment.
Intraurethral colonization was established by introducing 2 × 108 C. albicans blastoconidia through a tom cat catheter attached to a 1-ml tuberculin syringe. Rabbits were first sedated with 0.8 ml of a 7:2:1 (vol/vol) mixture of ketamine (10 mg/ml solution; Fort Dodge Animal Health, Fort Dodge, Iowa), xylazine (20 mg/ml solution; Ben Venue Laboratories, Bedford, Ohio), and acepromazine maleate (10 mg/ml; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Mo.) injected intramuscularly.
Gastrointestinal tract colonization was achieved by first tranquilizing rabbits by intramuscular injection with 0.5 ml of the ketamine-xylazine-acepromazine maleate solution described above and then feeding them via a 3-ml needleless syringe with 2 ml of a 50:50 mixture of light corn syrup (Karo; Best Foods, Englewood Cliffs, N.J.) and C. albicans blastoconidia suspended in PBS (final amount received per rabbit, 2 × 108 C. albicans blastoconidia).
Specimen collection and processing.
Rabbits were housed in metabolic cages that allowed collection of urine in one location and of fecal material in another. Blood and urine were collected daily before infection and during disease progression for use in the inhibition ELISA. In addition to serum and urine specimens collected for ELISA testing, daily blood, urine, and fecal specimens were also collected to determine CFU per milliliter of body fluid (blood or urine) or per gram of feces. Blood, urine, and feces were also collected from uninfected rabbits as negative controls. Blood for culture was collected daily from a central ear artery into Isolator lysis-centrifugation tubes (Wampole, Inc., Cranbury, N.J.) (1). The Isolator tubes were then centrifuged at 3,000 × g for 30 min, supernatants were removed, and pellets were plated onto SDA (fungi) or TSAB (S. aureus) and incubated at 25°C (fungi) or 37°C (S. aureus) for up to 1 week. Plates were examined daily for growth, and CFU were determined.
Urine was collected daily from each rabbit's metabolic cage, 0.1 ml was spread onto SDA plates (fungi) or mannitol salt agar (BBL) (S. aureus) for CFU determination, and the remainder was centrifuged at 5,000 × g for 10 min. Supernatants were then stored frozen at −40°C. At the time of euthanasia, additional clean-catch urine was collected from supine, anesthetized rabbits by gently pressing down on the area above the bladder to release residual urine. Urine specimens were dialyzed (3,500-molecular-weight-cutoff Spectra/Por dialysis membrane; Spectrum Medical Industries, Inc., Houston, Tex.) overnight against two changes of PBS and centrifuged at 5,000 × g for 10 min before supernatants were tested in the ELISA. For some experiments, urine specimens were concentrated 10-fold with 3,500-molecular-weight-cutoff concentrators (Centricon; Amicon, Beverly, Mass.) according to the manufacturer's instructions. A total of 166 urine specimens from 33 rabbits were collected and analyzed.
Fecal pellets (10 per rabbit) were collected daily, weighed, and then placed in a 50-ml conical centrifuge tube (Falcon no. 2098; Becton Dickinson). Twenty-five milliliters of PBS was added, the samples were allowed to sit for 10 min, and the tubes were then vortex mixed. Serial dilutions of fecal suspensions were spread onto SDA plates (fungi) or mannitol salt agar plates (S. aureus) for CFU determination after incubation at 25°C (fungi) or 37°C (S. aureus).
Kidneys, livers, and spleens were aseptically removed and homogenized in sterile PBS on day 3, 4, or 5 after infection. Kidneys were each homogenized separately in glass Waring blender bottles, and livers and spleens were each homogenized in a mechanical tissue homogenizer (Stomacher; Seward Medical, London, United Kingdom). Organ homogenates were serially diluted in PBS, and the CFU per gram of tissue was determined after plating on SDA (fungi) or TSAB (S. aureus) and incubating at 25°C (fungi) or 37°C (S. aureus). Invasion of deep tissues was also confirmed by histopathological staining of sections from paraffin-embedded tissue by using standard Gomori silver staining procedures.
Production of purified Sap for use in ELISA and Sap activity assays.
C. albicans strain 2730 (final concentration, 107 blastoconidia per ml) was grown in 300 ml of yeast carbon base-bovine serum albumin broth in 1-liter Erlenmeyer flasks rotating at 140 rpm (47, 48). Sap activity assays were performed on cultures grown at 25°C to maximize Sap production, as previously described (47, 88). Five-milliliter aliquots were removed daily, and Sap activity was determined spectrophotometrically at 280 nm following the degradation of substrate bovine serum albumin as previously described (47, 88). Cultures grown at 25°C were harvested when Sap activity reached a maximum (8 to 10 days of growth), and Sap was purified by serial anion-exchange and gel filtration column chromatographies as previously described (47).
Pepstatin A column chromatography.
Pepstatin A-agarose (Sigma) column chromatography was performed on urine specimens from uninfected rabbits (negative controls) and on preinfection and day 4 urine specimens from rabbits with disseminated candidiasis. Sham columns, prepared with agarose beads (Sigma) but without ligated pepstatin A, was used as a negative control. Chromatography was performed as previously described by Kregar et al. (32) and Morrison et al. (47). Briefly, 17 ml of urine was adjusted to pH 3.6 with 10% acetic acid and dialyzed (3,500-molecular-weight-cutoff Spectra/Por dialysis membrane; Spectrum Medical Industries, Inc.) for 3 h against 100 volumes of 0.1 M acetic acid-sodium acetate buffer (pH 3.6) containing 1 M NaCl. Urine was removed from the dialysis membrane and centrifuged at 2,700 × g and 4°C for 10 min to remove sediment, and the supernatant was applied to the column. Five milliliters of packed pepstatin A-agarose gel (Sigma), in a 0.6- by 9-cm glass column, was equilibrated with column buffer (0.1 M acetic acid-sodium acetate buffer [pH 3.6] containing 1 M NaCl). The sample was applied and washed into the column at a rate of 15 ml per h, using 3.5 volumes of column buffer. Samples were desorbed with elution buffer (0.1 M Tris-HCl [pH 8.5] containing 1 M NaCl), and fractions were collected into tubes containing 100 μl of neutralization buffer (0.1 M acetic acid-sodium acetate buffer [pH 3.6]). Peak fractions were pooled and dialyzed overnight against neutral 0.1 M Tris-HCl buffer by using a 3,500-molecular-weight-cutoff dialysis membrane (Spectra/Por; Spectrum Medical Industries, Inc.). Samples were concentrated 10-fold with Centricon-3 ultrafiltration concentrators (Amicon) before testing in the inhibition ELISA. Column fractions were also examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by Western blotting with the PhastSystem (Pharmacia, Piscataway, N.J.) as previously described (47).
Production of anti-Sap polyclonal antibody for use in ELISA and Western blotting.
Polyclonal mouse antibodies to purified Sap were raised in mice, and purified immunoglobulin G (IgG) antibodies were obtained as previously described (47). IgG antibodies were labeled with horseradish peroxidase (Sigma Chemical Co.) by the method of Wilson and Nakane (85) as modified by Gray (23), as previously described (26).
Competitive binding inhibition ELISA.
Microtitration plates (Immulon II; Dynatech, Inc., Chantilly, Va.) were precoated with 100 μl of a 0.2-μg/ml solution of purified Sap in 0.06 M sodium carbonate buffer (pH 9.6), sealed with plastic film, and incubated at 37°C for 3 h and then overnight at 4°C. Immediately before use, the plates were washed three times with PBS containing 0.05% Tween 20 (Sigma) (PBS-T). Two hundred microliters of serum or urine was combined with 200 μl of horseradish peroxidase-labeled anti-Sap antibody (diluted 1:250 in PBS-T) in 13- by 100-mm glass test tubes, and, after gentle agitation, the tubes were incubated in the dark for 30 min at 25°C. The mixture was then added to the washed plates (100 μl per well in triplicate) and incubated in the dark for 30 min at 25°C. Plates were washed three times with PBS-T to remove unbound antibody, and 100 μl of a 50:50 (vol/vol) mixture of 3′,3′-tetramethylbenzidine substrate and H2O2 solution (Kirkegaard and Perry, Gaithersburg, Md.) was added per well. The plates were read immediately in a kinetic microtitration plate reader (UVMax; Molecular Devices, Sunnyvale, Calif.) for 2 min at 650 nm. Results were reported as the percent inhibition relative to control wells containing preinfection serum (when assessing serum samples) or preinfection urine (when assessing urine samples) from the same rabbit. The percent ELISA inhibition by serum specimens from infected rabbits was significantly less than that by urine specimens from the same rabbits; therefore, the remainder of the inhibition ELISA testing was conducted with urine specimens. Samples giving inhibition values greater than 2 standard deviations above the mean for samples obtained from uninfected rabbits were considered positive. The cutoff value for positivity in the ELISA with urine as the test specimen was therefore assigned an inhibition value of 13.5% (i.e., the mean percent inhibition ± standard deviation for preinfection urine was 3.9 ± 4.8, and 3.9 + 4.8 + 4.8 = 13.5). It has been reported that C. albicans isolates can maintain 50% of their proteolytic activity at pH 7.25 (80) and that C. albicans Sap can digest immunoglobulins (27, 63, 65, 67); however, denaturation of the enzyme does not appear to interfere with antigenicity (8, 50). Therefore, purified Sap was boiled for 5 min to inactivate enzymatic activity before serial dilution (0 to 100 ng/ml) in PBS and use in the construction of a standard curve. Absorbance at 650 nm was inversely proportional to the amount of Sap present, and inhibition results could be converted into nanograms of Sap per milliliter by using the standard curve, which was linear over the concentration range used. A 50% reduction in absorbance was obtained with 25 ng of purified Sap per ml. A conjugate control (0.2 ml of PBS-T substituted for rabbit urine) was run in triplicate on each plate and represented 0 ng of Sap per ml.
Statistical analyses.
Means ± standard error (SEs) for the number of experiments or animals designated are reported. Values were considered to be significant by Student's t test, chi-square analysis, or Spearman's correlation coefficient (r) when the P value was <0.05.
RESULTS
Comparison of Sap concentrations in serum and in urine.
All intravenously infected rabbits demonstrated dissemination from the bloodstream to the deep organs. Preliminary testing suggested that although some ELISA inhibition occurred with serum specimens, the results were more significant and paralleled disease progression more closely when urine specimens were tested. Compared with preinfection serum specimens, serum specimens from rabbits infected intravenously with C. albicans gave the following mean percent inhibition results ± SE (n) for the indicated day postinfection: day 1, 8.2 ± 8.2 (2); day 2, 0 ± 0 (2); and day 3, 19.9 ± 2.5 (3). In contrast, urine specimens from the same rabbits gave the following inhibition results compared to preinfection urine: day 1, 12.2 ± 1.3 (2); day 2, 32.5 ± 3.7 (2); and day 3, 50.8 ± 1.9 (3). Given these results and the variable results obtained by others using serum (50, 64, 66), the remainder of the inhibition ELISA testing was conducted with urine specimens.
Inhibition ELISA results with urine correlate with disease progression.
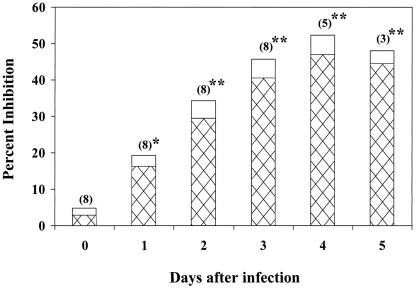
Figure 1 shows the detection of Sap antigenuria by the inhibition ELISA during the course of disease progression in rabbits infected systemically with C. albicans strain 36B. The ELISA detected an increase in the amount of Sap in urine specimens from infected rabbits which correlated with time after infection (days 1 to 5; Spearman's correlation coefficient [r] = 0.95). The percent inhibition by postinfection urine was significantly greater than that by preinfection urine as early as 1 day postinfection (P < 0.01), and significant ELISA inhibition continued with urine collected through day 5 (P < 0.001) (Fig. 1).
FIG. 1.
Time course for inhibition ELISA with C. albicans strain 36B-infected rabbits. Rabbits were infected on day 0, and urine was collected daily and tested in the inhibition ELISA. The number of rabbits tested for each day is shown in parentheses. *, P < 0.01 compared to day 0 inhibition; **, P < 0.001 compared to day 0 inhibition. Cross-hatched bars, mean percent ELISA inhibition values; open bars, SEs from the mean percent ELISA inhibition values.
ELISA positivity compared with culture results for organs and other specimens from C. albicans-infected rabbits during disease progression.
Table 1 shows the time course of disease progression in rabbits systemically infected with C. albicans strain 36B by organ burden compared with ELISA results. No rabbits were culture positive before infection on day 0, and ELISA results were also negative. By day 1 after infection, 38% of rabbits were positive by the ELISA, whereas 63, 75, and 38% were positive by blood, urine, and fecal culture, respectively (Table 1). ELISA results were positive for all rabbits from day 2 through day 5, whereas blood culture positivity declined with time. In contrast, urine and fecal cultures continued to increase in positivity over time. Therefore, blood culture positivity did not reflect disease progression over time, whereas ELISA results and urine and fecal culture positivity did.
TABLE 1.
Comparison of ELISA positivity and culture results for organs and other specimens from rabbits infected intravenously with C. albicans strain 36B during disease progressiona
| Day and parameter | ELISA result | Culture result
|
|||||
|---|---|---|---|---|---|---|---|
| Blood | Urine | Feces | Kidneys | Spleen | Liver | ||
| Day 0 | |||||||
| No. positive/total (% positive)b | 0/8 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 2.8 ± 2.1 (8) | 0 ± 0 (8) | 0 ± 0 (8) | 0 ± 0 (8) | |||
| Range | 0-12.1 | 0-0 | 0-0 | 0-0 | |||
| Day 1 | |||||||
| No. positive/total (% positive) | 3/8 (38) | 5/8 (63) | 6/8 (75) | 3/8 (38) | |||
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 15.7 ± 2.7 (8) | 0.6 ± 0.2 (8) | 51 ± 23 (8) | 73 ± 38 (8) | |||
| Range | 8.2-27.3 | 0-1 | 0-200 | 0-260 | |||
| Day 2 | |||||||
| No. positive/total (% positive) | 8/8 (100) | 5/8 (63) | 8/8 (100) | 6/7 (86) | |||
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 29.4 ± 4.8 (8) | 0.6 ± 0.2 (8) | 145 ± 83 (8) | 173 ± 34 (7) | |||
| Range | 16.7-52.6 | 0-1 | 10-720 | 0-280 | |||
| Day 3 | |||||||
| No. positive/total (% positive) | 8/8 (100) | 4/7 (57) | 8/8 (100) | 7/8 (88) | 4/4 (100) | 2/2 (100) | 2/2 (100) |
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 41.9 ± 4.1 (8) | 1.1 ± 0.6 (7) | 1,020 ± 700 (8) | 1,849 ± 732 (8) | 14.6 ± 6.4 (4) | 85 ± 85 (2) | 11.9 ± 11.1 (2) |
| Range | 27.9-63.7 | 0-4 | 11-5,800 | 0-6,450 | 4.5-32 | 0.1-170 | 0.8-23 |
| Day 4 | |||||||
| No. positive/total (% positive) | 5/5 (100) | 2/5 (40) | 5/5 (100) | 6/6 (100) | 6/6 (100) | 3/3 (100) | 3/3 (100) |
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 46.9 ± 5.4 (5) | 0.6 ± 0.4 (5) | 1,950 ± 1,462 (5) | 2,235 ± 1,328 (6) | 475 ± 215 (6) | 0.7 ± 0.7 (3) | 2.4 ± 1.9 (3) |
| Range | 30.8-64.7 | 0-2 | 8-9,700 | 62-8,640 | 82-1,500 | 0.03-2.1 | 0.009-6.2 |
| Day 5 | |||||||
| No. positive/total (% positive) | 3/3 (100) | 0/2 (0) | 3/3 (100) | 1/1 (100) | 6/6 (100) | 3/3 (100) | 3/3 (100) |
| Inhibition level or organism burdenc | |||||||
| Mean ± SE | 44.5 ± 3.5 (3) | 0 ± 0 (2) | 1,340 ± 658 (3) | 86 ± 0 (1) | 456 ± 259 (6) | 0.02 ± 0.01 (3) | 0.2 ± 0.1 (3) |
| Range | 37.5-54.3 | 0-0 | 24-2,000 | 86d | 17-1,700 | 0.006-0.04 | 0.004-0.44 |
Rabbits were infected intravenously on day 0 with 107 C. albicans strain 36B blastoconidia and necropsied on day 3, 4, or 5 after infection.
Number (percentage) of rabbits with a positive urine ELISA antigen detection result or with a culture-positive organ or other clinical specimen.
ELISA results are percent inhibition relative to preinfection urine. Culture results are numbers of CFU per milliliter (blood and urine) or per gram (wet weight) (organs and feces). Data are for the number of rabbits (given in parentheses as n). Organ data were multiplied by 10−6.
Feces were not available for two of the three remaining rabbits.
Homogenization and culture of deep organs (kidneys, liver, and spleen) demonstrated that 100% of rabbits were culture positive in all three organs by day 3 after infection and remained culture positive until day 5. The number of recoverable CFU per gram in the kidney increased with time, reflecting increased disease progression (Table 1). In contrast, the number of recoverable CFU per gram in the spleen and liver declined. Tissue invasion was confirmed by histopathology (Fig. 2).
FIG. 2.
Histopathological section of kidney tissue from a C. albicans-infected rabbit, showing tissue invasion with C. albicans (darkly staining material). Gormori silver stain was used. Approximate magnification, ×540.
Comparison of inhibition ELISA results with infectious burden by using different infecting C. albicans strains.
Table 2 demonstrates that significant ELISA inhibition (P < 0.01 compared to preinfection urine) was also observed with urine from rabbits infected intravenously with any of five strains of C. albicans, including both serotype A and serotype B strains. These data indicate that significant ELISA inhibition was not limited to urine obtained from rabbits infected with one particular C. albicans strain. Significant differences were noted, however, in the degree of inhibition observed for a given infecting strain (Table 2). For example, significantly more inhibition was noted when urine specimens from rabbits infected with C. albicans strain Lecocq, 36B, or 2730 were tested than when those from rabbits infected with strain Q16 (P = 0.02) or B311 (P < 0.01) were tested. Differences in inhibition with urine specimens from rabbits infected with different C. albicans strains paralleled the degree of pathogenicity for a given strain as demonstrated by organ burden data obtained on the day of necropsy (day 4 or 5 postinfection). Strains resulting in the highest ELISA inhibition values (Lecocq, 36B, 2730, and 3181A) were also those producing the greatest organ burden, and those resulting in the lowest ELISA inhibition values (Q16 and B311) produced the lowest organ burden (Table 2). Rabbits were infected in multiple experiments, and the correlation between organ data and ELISA results remained the same. The strongest correlation between ELISA inhibition results and organ burden was noted for the mean log units per gram of kidney data (r = 0.89 for the kidney, compared to r = 0.65 for the spleen and r = 0.77 for the liver).
TABLE 2.
Comparison of inhibition ELISA results and infectious burden with different C. albicans strains
| C. albicans strain | % Inhibition ± SE (n)a | Burdenb in:
|
|||||
|---|---|---|---|---|---|---|---|
| Kidney | Spleen | Liver | Blood | Urine | Feces | ||
| Lecocq | 56.8 ± 2.5 (5)c | 8.67 | 5.23 | 4.08 | 18 | 4,600 | 1,200 |
| 36B | 54.3 ± 5.1 (3)c | 8.97 | 3.76 | 3.60 | 0 | 24 | 86 |
| 2730 | 51.5 ± 2.0 (3)c | 8.83 | 3.71 | 4.87 | 0 | 530 | 11,680 |
| 3181A | 46.4 ± 6.3 (3)d | 7.05 | 3.46 | 4.26 | 0 | 620 | 850 |
| Q16 | 34.1 ± 5.9 (8)d | 4.61 | 0 | 1.69 | 0 | 0 | 0 |
| B311 | 26.0 ± 4.3 (3)d | 5.84 | 3.15 | 2.52 | 0 | 230 | 74 |
Mean percent ELISA inhibition ± SE for the number of experiments in parentheses; urine was collected on the day of necropsy (day 4 or 5 after infection). Mean percent ELISA inhibition ± SE by day 0 urine, 5.4 ± 1.0 (n = 33).
Mean log CFU per gram (kidney, spleen, and liver), mean CFU per gram (feces), or mean CFU per milliliter (blood and urine).
P < 0.001 compared to ELISA inhibition by preinfection urine.
P < 0.01 compared to ELISA inhibition by preinfection urine.
Table 2 also shows the recoverable CFU from blood, urine, and fecal specimens from C. albicans-infected rabbits on the day of necropsy. Unlike the tissue burden results, the number of CFU recovered from the blood, urine, or feces did not correlate with the relative rank order of ELISA inhibition results (i.e., r ranged from 0.33 to 0.54 for feces, blood, and urine). Indeed, blood cultures were negative in rabbits infected with five of the six C. albicans strains tested despite the use of lysis centrifugation blood culture tubes (1), and urine and fecal cultures were negative for one strain (Q16) despite a significant organ burden.
Specificity of the ELISA for detecting only disseminated candidiasis caused by C. albicans.
To determine the specificity of the inhibition ELISA for detecting invasive C. albicans infection, urine specimens were obtained from rabbits colonized intragastrically or intraurethrally with C. albicans strain 36B or from rabbits infected intravenously with non-C. albicans Candida species or with other agents (both fungal and bacterial). Urine inhibition ELISA values and culture results for these rabbits are shown in Table 3. As expected from the results presented above, urine specimens from immunosuppressed rabbits infected intravenously with C. albicans strain 36B demonstrated significant ELISA inhibition (P < 0.001 compared with preinfection urine), and these rabbits had significant invasion of the kidneys, spleen, and liver by C. albicans. Urine specimens from rabbits infected intravenously with C. albicans strain 36B but not immunosuppressed also demonstrated significant but somewhat lower ELISA inhibition results (41.9% ± 2.9%) compared to urine from systemically infected, immunosuppressed rabbits (50.2% ± 5.1%). Tissue involvement in the kidney was also somewhat reduced in nonimmunosuppressed rabbits (Table 3), whereas spleen and liver involvement was similar to that observed for immunosuppressed rabbits.
TABLE 3.
Inhibition ELISA results for rabbits infected with C. albicans compared with rabbits colonized with C. albicans or infected with non-C. albicans Candida species or other infectious agents
| Organism | Route of administrationa | % Inhibition ± SE (n)b | Burdenc in:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Kidney | Spleen | Liver | Blood | Urine | Feces | |||
| C. albicans strain 36B | Intravenous | 50.2 ± 5.1 (6)d | 8.73 | 4.54 | 5.67 | 2 | 470 | 355 |
| Intravenouse | 41.9 ± 2.9 (3)f | 6.71 | 4.92 | 5.89 | 1 | 72 | 0 | |
| Intragastricg | 32.9 ± 2.7 (3)h | 1.40 | 1.15 | 0 | 0 | 620 | NAi | |
| Intragastricj | 4.8 ± 1.7 (3) | 0 | 0 | 0 | 0 | 11 | 42,141 | |
| Intraurethral | 0 ± 0 (4) | 0 | 0 | 0 | 0 | 230 | 305 | |
| C. parapsilosis | Intravenous | 6.5 ± 1.6 (6) | 2.40 | 0 | 0 | 0 | 0 | 0 |
| C. tropicalis | Intravenous | 5.8 ± 2.2 (4) | 6.48 | 3.86 | 4.49 | 0 | 1,500 | 570 |
| C. krusei | Intravenous | 5.1 ± 1.8 (6) | 0 | 2.23 | 0 | 0 | 0 | 0 |
| A. fumigatus | Intravenous | 9.1 ± 3.1 (3) | 3.28 | 3.15 | 3.22 | 1 | 1 | 0 |
| C. neoformans | Intravenous | 7.5 ± 3.6 (6) | 2.26 | 2.79 | 2.11 | 0 | 0 | 10 |
| S. aureus | Intravenous | 6.4 ± 2.0 (3) | 9.47 | 0 | 2.19 | 15 | 10,000 | 190,000 |
All intravenously infected rabbits were immunosuppressed unless otherwise noted.
Mean percent ELISA inhibition ± SE for the number of experiments in parentheses compared with preinfection urine; specimens were collected on the day of necropsy (day 4) unless otherwise noted. Mean percent ELISA inhibition ± SE on day 0, 2.6 ± 0.4 (n = 48).
Mean log CFU per gram (kidney, spleen, and liver), mean CFU per gram (feces), mean CFU per milliliter (blood and urine).
P < 0.001 compared to inhibition by preinfection urine.
Rabbits were not immunosuppressed before infection.
P < 0.01 compared to ELISA inhibition by preinfection urine.
Rabbits were colonized by intragastric administration, and organisms disseminated to peripheral organs.
ELISA results for urine collected on day 3 after infection; P < 0.01 compared to ELISA inhibition by preinfection urine.
NA, none available.
Rabbits were colonized by intragastric administration, and organisms did not disseminate to peripheral organs.
C. albicans was found to disseminate to the peripheral organs in one rabbit after colonization by the intragastric route. Significant ELISA inhibition (32.9% ± 2.7%; P < 0.01) was demonstrated when urine from this rabbit was tested, but inhibition was somewhat lower than that observed for intravenously infected rabbits (50.2% ± 5.1%) (Table 3). Although dissemination to the kidney and spleen was observed, no liver involvement occurred. These data indicate that significant ELISA inhibition could still occur when urine from a rabbit with a less severe but disseminated C. albicans infection was assayed. In contrast, significant ELISA inhibition did not occur when urine specimens from intragastrically or intraurethreally colonized rabbits showing no dissemination of infection to peripheral organs were tested (Table 3). In addition, no significant ELISA inhibition occurred when urine specimens from rabbits infected with other fungi (including other Candida species) or bacteria were tested, despite proven dissemination of the microorganisms to one or more peripheral organs (Table 3). These data indicate that the inhibition ELISA is specific for invasive candidiasis caused by C. albicans and can differentiate between colonization and true infection by C. albicans.
Blood cultures were either negative or demonstrated only 1 or 2 CFU per ml for rabbits infected with any fungus by any route (Table 3). In contrast, urine and fecal cultures were positive for all rabbits infected or colonized by C. albicans (with the exception of fecal cultures from nonimmunosuppressed rabbits) and for all rabbits infected with C. tropicalis. These data indicate that blood cultures are not a consistently sensitive method to detect systemic infection by the fungi tested and that urine and fecal cultures cannot differentiate systemic infection from colonization. However, intragastric colonization produced a quantitatively higher number of recoverable CFU per gram of fecal material. In contrast, urine and fecal cultures for rabbits infected with C. parapsilosis or C. krusei were negative, and urine and fecal cultures, respectively, were negative for C. neoformans- and A. fumigatus-infected rabbits despite disseminated disease (Table 3).
Effect of urine concentration by ultrafiltration on ELISA results.
In an attempt to increase the sensitivity of the ELISA, particularly early after infection, urine specimens from rabbits infected intravenously with C. albicans strain 36B were concentrated 10-fold by ultrafiltration before testing. Concentration of preinfection urine did not increase the ELISA inhibition results (Table 4). In contrast, significant increases in ELISA inhibition were observed with urine collected on day 3 or 4 after infection, including urine collected by the clean-catch method (Table 4).
TABLE 4.
Effect of urine concentration by ultrafiltration on inhibition ELISA results over time with urine from rabbits infected with C. albicans strain 36B
| Day | Mean % inhibition ± SE (n)a
|
% Increase by 10× concentration | |
|---|---|---|---|
| 1 × urine | 10 × urine | ||
| 0 | 0 ± 0 (3) | 0.1 ± 2.5 (3) | |
| 1 | 12.0 ± 2.3 (3) | 13.9 ± 3.2 (3) | 15.8 |
| 2 | 16.7 ± 2.6 (3) | 17.8 ± 2.7 (6) | 6.6 |
| 3 | 43.1 ± 2.2 (4) | 67.3 ± 6.5 (4)b | 56.1b |
| 4 | 43.8 ± 2.3 (3) | 71.2 ± 1.0 (3)c | 62.6c |
| 4 (CC)d | 49.8 ± 2.0 (3) | 87.3 ± 0.6 (3)c,e | 75.3c |
Mean percent ELISA inhibition ± SE for the number of experiments in parentheses; the cutoff value for a positive ELISA result with unconcentrated urine was 13.5% (see Materials and Methods).
Significant increase in ELISA inhibition compared with 1× concentration results (P < 0.02).
Significant increase in ELISA inhibition compared with 1× concentration results (P < 0.001).
CC, clean-catch urine.
No significant difference in ELISA inhibition was noted for unconcentrated clean-catch urine compared with unconcentrated non-clean-catch urine; there was a significant difference between ELISA inhibition by 10× clean catch urine and that by 10× non-clean-catch urine (P < 0.001).
The increased ELISA inhibition observed after urine concentration was specific for urine from C. albicans-infected rabbits, as no significant increase in ELISA inhibition occurred after concentrating urine specimens from C. albicans-colonized rabbits or from rabbits infected with other fungi (Table 5). These data indicate that the component being concentrated is specific for systemic C. albicans infection. Although some increase in ELISA inhibition results occurred after concentrating urine specimens from rabbits infected with C. albicans strain Q16 or B311, the two least virulent C. albicans strains, no statistically significant increase in ELISA inhibition was observed (Table 5).
TABLE 5.
Effect of urine concentration by ultrafiltration on inhibition ELISA results with urine from colonized or intravenously infected rabbitsa
| Organism | % Inhibition ± SE (n)
|
Pb | |
|---|---|---|---|
| 1× urine | 10× urine | ||
| C. albicans | |||
| Lccocq | 62.4 ± 3.4 (3) | 91.5 ± 0.8 (3) | <0.001 |
| 36B | 43.8 ± 2.3 (3) | 71.2 ± 1.0 (3) | <0.001 |
| 3181A | 46.4 ± 3.6 (3) | 65.5 ± 0.7 (3) | <0.01 |
| Q16 | 38.2 ± 3.6 (3) | 40.1 ± 1.6 (3) | NSc |
| B311 | 26.0 ± 2.5 (3) | 30.9 ± 0.9 (3) | NS |
| Colonizedd | 0.6 ± 0.6 (6) | 0 ± 0 (6) | 0e |
| C. tropicalis | 6.2 ± 2.9 (6) | 0 ± 0 (3) | 0 |
| C. neoformans | 6.8 ± 3.3 (6) | 10.9 ± 0.8 (3) | NS |
| A. fumigatus | 8.8 ± 1.9 (6) | 4.5 ± 2.3 (6) | 0 |
Urine was collected from rabbits on the day of necropsy (day 4 or 5).
P value for a significant increase in ELISA inhibition results by 10× concentration of urine. Mean percent ELISA inhibition ± SE by column effluents, 3.2 ± 0.9 (n = 21).
NS, no significant increase in ELISA inhibition by 10× concentration of urine.
Rabbits were colonized by intraurethral installation of C. albicans strain 36B.
No increase in ELISA inhibition by 10× concentration of urine.
Use of pepstatin A-agarose column chromatography to capture Sap from urine specimens.
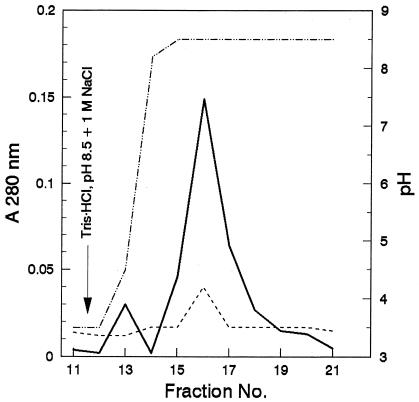
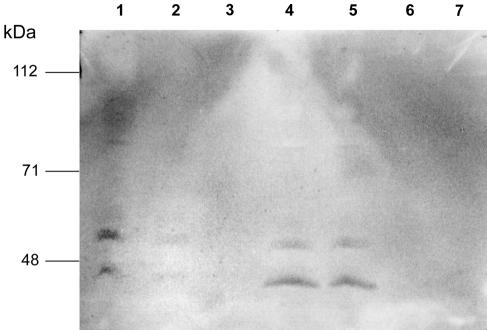
Pepstatin A is an inhibitor that is specific for aspartyl proteinases and acts by binding to the active site of the enzyme (2, 7, 33, 54). Therefore, urine from an ELISA-positive, C. albicans-infected rabbit was passaged over a pepstatin A-agarose column in an attempt to remove ELISA-inhibitory activity caused by Sap in the urine. Controls included urine from a normal rabbit and urine from the same C. albicans-infected rabbit described above passaged over a sham column of agarose beads without ligated pepstatin A. Figure 3 shows the elution profile for urine from the C. albicans-infected rabbit passaged over the pepstatin A-agarose column and urine from the same rabbit passaged over the sham column. Although an elution peak between fractions 15 and 17 was obtained for urine from the infected rabbit passaged over the pepstatin A-agarose column, pooled peak fractions did not result in increased inhibition when tested in the ELISA despite 10-fold concentration of the pooled peak fractions before testing. However, SDS-PAGE followed by Western blot analysis demonstrated two distinct protein bands of approximately 43 and 52 kDa in the lanes containing the pepstatin A eluate; lanes containing the sham column eluate did not reveal any detectable bands (Fig. 4). In addition, the effluent from pepstatin A-passaged urine from the C. albicans-infected rabbit was depleted of these two bands, whereas the effluent from sham-passaged urine demonstrated bands similar to those observed for the pepstatin A eluate (Fig. 4). The pepstatin A-agarose column removed ELISA-inhibitory activity from the urine of the infected rabbit, whereas the sham column did not (i.e., the effluent from the sham column inhibited the ELISA by 78.1% ± 2.4% [n = 3], the effluent from the pepstatin A column inhibited the ELISA by 31.6% ± 2.2% [n = 3] [P < 0.001 compared to the sham column], and effluent pepstatin A column fractions of urine from normal rabbits inhibited the ELISA by 13.8% ± 4.0% [n = 3] [P < 0.02 compared to either of the other two]). Therefore, passage of urine from the C. albicans-infected rabbit over the pepstatin A column resulted in the removal of approximately 60% of the ELISA-inhibitory activity. These data indicate that a significant amount of the ELISA-inhibitory agent binds to pepstatin A, a specific ligand for aspartyl proteinases. Normal rabbit urine passaged over the pepstatin A column had a slightly inhibitory activity, but this effect was minor, in contrast to the 60% difference in inhibition occurring between sham-passaged and pepstatin A-passaged urine specimens from infected rabbits.
FIG. 3.
Pepstatin A-agarose column chromatographic profiles for urine from a C. albicans-infected rabbit. Urine was applied to a pepstatin A-agarose column or a sham column (agarose support only, no pepstatin A), and effluent and eluate fractions were collected as described in Materials and Methods. Solid line, pepstatin A-agarose elution profile; dashed line, sham column elution profile; dotted and dashed line, pH elution gradient.
FIG. 4.
Western blot analysis of effluent and eluate fractions of urine from a C. albicans-infected rabbit passaged over pepstatin A-agarose or sham (agarose beads only, no pepstatin A) columns. Blots were tested with horseradish peroxidase-labeled polyclonal anti-Sap antibodies as described in Materials and Methods. Lane 1, sham column effluent (two bands noted); lane 2, pepstatin A column effluent (depleted, barely visible bands noted); lane 3, empty; lanes 4 and 5, duplicates of pepstatin A-agarose column eluate (two bands noted); lane 6, empty; and lane 7, sham column eluate (no bands noted). Molecular mass markers, BRL prestained high-molecular-mass standards (Bethesda Research Laboratories, Gaithersburg, Md.).
The ELISA was not inhibited by the extraneous addition of purified (61) cell wall mannan, because addition of up to 5 μg per ml in normal rabbit urine gave no significant ELISA inhibition (0 to 0.3% ± 0.1% inhibition by 0.005 to 0.5 μg/ml and 13.4% ± 0.7% inhibition by 5 μg/ml); these data indicate that the ELISA-inhibitory activity was not due to contaminating mannoprotein present in the urine and confirm the specificity of the anti-Sap antibodies (47, 48).
ELISA inhibition results and number of rabbits testing positive for antigen by a given day.
Table 6 shows the overall ELISA inhibition results by day postinfection and the number of C. albicans-infected rabbits testing positive compared to negative control rabbits. No rabbits were positive before infection on day 0 in either group. Fifty percent of C. albicans-infected rabbits were positive 1 day after infection (P < 0.01), and 6.0% of negative control rabbits gave false-positive results (P > 0.05). The number of ELISA-positive rabbits increased to 100% by day 4 for C. albicans-infected rabbits (P < 0.001), whereas negative control rabbits gave false-positive results in 6% of cases on day 4 (P > 0.05). False-positive results were skewed on day 5 by the fact that only three negative control rabbits were tested on this day and one of these three gave a false-positive result on this day only. Of those rabbits giving sporadic false-positive results, one was colonized intraurethrally with C. albicans, two were infected with S. aureus, two were infected with C. tropicalis, and one each was infected with C. neoformans and A. fumigatus. In all cases, negative control rabbits reverted to negative ELISA results on later days unless the single false-positive result was obtained on the last day that specimens were collected for a given rabbit. If two or more positive results were required for a rabbit to be considered positive by the ELISA (i.e., at least one positive result was obtained before the positive result for a given day), the test sensitivity declined somewhat for days 1, 2, and 4 but the specificity increased for all days (Table 6).
TABLE 6.
Overall ELISA inhibition results and number of rabbits testing positive for antigen by a given day
| Day | No. of rabbits ELISA positive/total no. of rabbits tested (% positive)a
|
Mean percent inhibition ± SEb (n)
|
||||
|---|---|---|---|---|---|---|
|
C. albicans-infected rabbits
|
Negative control rabbits
|
C. albicans-infected rabbits | Negative control rabbits | |||
| One positive resultc | Two or more positive resultsd | One positive result | Two or more positive results | |||
| 0 | 0/14 (0) | 0/14 (0) | 0/19 (0) | 0/19 (0) | 5.3 ± 1.3 (14) | 2.4 ± 0.8 (19) |
| 1 | 7/14 (50)e | 0/14 (0) | 1/18 (6) | 0/18 (0) | 16.3 ± 2.5 (14)e,f | 6.0 ± 2.0 (18) |
| 2 | 12/14 (86)e | 7/14 (50)e | 2/18 (11) | 0/18 (0) | 25.1 ± 3.4 (14)e,f | 6.7 ± 1.9 (18) |
| 3 | 13/14 (93)e | 13/14 (93)e | 4/19 (21) | 2/19 (11) | 34.4 ± 3.9 (14)e,f | 6.4 ± 2.0 (19) |
| 4 | 11/11 (100)e | 10/11 (91)e | 1/17 (6) | 0/17 (0) | 40.6 ± 5.2 (11)e,f | 5.2 ± 1.3 (17) |
| 5 | 5/5 (100)e | 5/5 (100)e | 1/3 (33) | 0/3 (0) | 37.3 ± 4.4 (5)e,g | 11.6 ± 7.7 (3) |
C. albicans-infected rabbits included those receiving C. albicans by the intravenous or intragastric route with evidence of dissemination to deep organs by positive culture; negative control rabbits included all uninfected rabbits, rabbits colonized with C. albicans intragastrically or intraurethrally, and rabbits intravenously infected with bacteria or fungi other than C. albicans.
Mean percent ELISA inhibition ± SE compared to preinfection urine for the number of rabbits in parentheses (positive cutoff, 13.5%).
Number of rabbits giving a positive result on a given day regardless of results obtained on other days.
Number of rabbits giving a positive result on a given day and on at least one day before the given day.
P < 0.01 or P < 0.001 compared to day 0 results.
P < 0.01 or P < 0.001 compared to urine from negative control rabbits obtained on the day indicated.
P < 0.05 compared to urine from negative control rabbits obtained on the day indicated.
Inhibition ELISA results paralleled the trend observed for the number of rabbits testing positive by a given day postinfection (i.e., the inhibition increased from 5.3% on day 0 to 40.6% on day 4) (Table 6). No positive mean ELISA inhibition was noted on any day for urine specimens from negative control rabbits, although urine from a single negative control rabbit on day 5 postinfection gave an abnormally high ELISA value, which skewed the mean ELISA inhibition value to 11.6% ± 7.7% (Table 6). Therefore, the sensitivity for detecting a positive rabbit by ELISA increased during disease progression, and the specificity remained high.
Overall sensitivity, specificity, positive and negative predictive values, and efficiency for the inhibition ELISA by day postinfection.
Table 7 shows the overall sensitivity, specificity, positive and negative predictive values, and efficiency of the inhibition ELISA when all specimens from all days were examined. The sensitivities and specificities were similar to those obtained on a per-rabbit basis, and the positive and negative predictive values generally increased with disease progression (Table 7). The overall sensitivity, specificity, and positive and negative predictive values for all days were 83, 92, 84, and 91%, respectively. The test efficiency ranged from 75 to 100%, with an overall efficiency of 89%. If positive test results were required on at least 2 days for assignment as a true positive case, test sensitivity declined somewhat on days 1 and 2 but test specificity increased for all days (Table 7); the positive predictive values increased on all days except day 1, and the negative predictive values and efficiency increased or remained the same for all days except day 2 (Table 7).
TABLE 7.
Overall sensitivity, specificity, positive and negative predictive values, and efficiency of the inhibition ELISA by day of infection
| Day | % Sensitivity/specificity for:
|
% PPV/NPVa for:
|
% Efficiency for:
|
|||
|---|---|---|---|---|---|---|
| One positive resultb | Two or more positive resultsc | One positive result | Two or more positive results | One positive result | Two or more positive results | |
| 0 | 0/100 | 0/100 | 100 | |||
| 1 | 50/94 | 0/100 | 88/71 | 0/100 | 75 | 100 |
| 2 | 86/89 | 50/100 | 86/89 | 100/72 | 88 | 78 |
| 3 | 93/79 | 93/89 | 77/94 | 87/94 | 85 | 91 |
| 4 | 100/94 | 100/100 | 92/100 | 100/100 | 96 | 100 |
| 5 | 100/67d | 100/100 | 83/100 | 100/100 | 88 | 100 |
| Overalle | 83/92 | 82/97 | 84/91 | 95/90 | 89 | 92 |
PPV, positive predictive value; NPV, negative predictive value.
Calculations based on a single positive result on a given day regardless of results obtained on other days; day 0 urine was collected before infection.
Calculations based on a positive result on a given day and on at least one additional positive result on a day before the given day.
These results are artificially low because only three negative control rabbits were tested on day 5 and one of these gave a false positive result on day 5 only.
Data based on a total of 166 urine specimens from 33 rabbits collected on days 0 through 5 from C. albicans-infected rabbits and all negative control rabbits.
DISCUSSION
The significant morbidity and mortality associated with invasive candidiasis in immunocompromised patients and in the intensive care unit setting have resulted in considerable efforts to develop rapid, reliable diagnostic tests to facilitate clinical treatment decisions. Theoretically, because Sap is an inducible enzyme and is associated with tissue adherence and invasion, its production should correlate with invasive disease rather than simple colonization. Rüchel et al. (66) used polyclonal anti-Sap antibodies in a sandwich ELISA format to detect circulating Sap antigen in serum specimens from patients with candidiasis. Sap was detected in only 50% of suspected plus confirmed cases (66), and it was later hypothesized that sensitivity may have been reduced by the formation of complexes between Sap and alpha-2-macroglobulin in the circulation (64). Later, Na and Song (50) compared two different antigen detection ELISAs for Sap in serum, a sandwich ELISA and an inhibition ELISA. The sensitivities of both ELISA formats were the same (94%), but the specificity of the inhibition ELISA was slightly greater (96 versus 92%).
In our own studies, detection of Sap antigen in serum specimens from rabbits infected systemically with C. albicans was not as sensitive as detection of Sap in urine specimens even though an inhibition ELISA format was used. The differences between our results and those of Na and Song (50) may be related to the fact that they used a monoclonal antibody and we used a polyclonal antibody; they also used a peroxidase-conjugated anti-mouse IgG to detect their anti-Sap monoclonal antibody, whereas we directly conjugated our anti-Sap antibodies with peroxidase. Traditionally, monoclonal antibodies have been less sensitive than polyclonal antibodies for detection of antigen; however, it may be that the CAP1 monoclonal antibody used by Na and Song detected the circulating form of Sap better than the polyclonal antibody used in our studies. In addition, the indirect detection of Sap, using an anti-Sap monoclonal antibody which was then detected with a peroxidase-conjugated anti-mouse IgG, may have increased the test sensitivity. Nonetheless, our detection of Sap in serum did not directly correlate with disease progression, whereas detection of Sap in urine did. Also, other researchers have had variably successful results with serum as the test fluid (50, 64, 66), and the potential to collect larger volumes of urine than of serum from patients and to concentrate the antigen from urine to increase test sensitivity made the use of urine instead of serum attractive. Therefore, all remaining studies were conducted with urine as the test fluid in an inhibition ELISA format.
In our studies, the results of the inhibition ELISA with urine directly correlated with disease progression, and this was best reflected by the increase in tissue burden for the kidneys of infected rabbits over time. In contrast, the tissue burden for the spleen and liver was somewhat reduced over time, suggesting that the rabbit model described in this report better reflects acute disseminated candidiasis (found in both oncologic and nononcologic patients [59]) rather than the chronic, hepatosplenic infection most often observed in patients treated for acute leukemia and in bone marrow transplant recipients who have recovered from neutropenia (31, 59, 62, 77). Most animal models of acute disseminated infection, particularly when animals are infected via the intravenous route, demonstrate an increased organ burden for the kidney with disease progression and a reduction in tissue burden for other organs (5, 21, 37, 38, 86). This result occurs because the osmotic pressure within the kidney tubules reduces the effectiveness of phagocytic cells to kill and remove C. albicans cells, and fungus balls can grow and obstruct kidney function (86). It would be of interest to reduce the size of the infecting inoculum to determine if this may produce a more chronic, hepatosplenic form of infection or to use a different route of infection. Nonetheless, in our present study, C. albicans disseminated to peripheral organs from the gastrointestinal tract of one colonized rabbit and, despite a tissue burden for the kidney that was 6 times lower than that in rabbits infected by the intravenous route, significant ELISA inhibition still occurred. Dissemination from the gastrointestinal tract is thought to be a common form of systemic infection in immunosuppressed patients whose mucosal barriers are compromised as a result of chemotherapy (7, 12, 15, 17, 59). Urine specimens from rabbits that were not immunosuppressed before infection demonstrated a lower kidney burden without any reduction in the burden in the spleen or liver, and significant ELISA inhibition also occurred. These data suggest that even a less severe infection, regardless of the site from which it disseminated, could still be detected by the inhibition ELISA.
The positive correlation between tissue burden, particularly for the kidney, and the inhibition ELISA results is in contrast to the lack of a correlation between CFU per milliliter of urine and ELISA inhibition results. Therefore, the simple presence of candiduria did not result in significant ELISA inhibition, suggesting that tissue invasion, rather than simple colonization, was necessary for positive ELISA results. This hypothesis was further validated by the data generated for intragastrically and intraurethrally colonized rabbits, where no significant ELISA inhibition occurred in the absence of tissue invasion. Therefore, tissue invasion appears to be necessary for significant ELISA inhibition. It is not known whether the ELISA described here could also detect localized tissue invasion in addition to disseminated infection, and further study of this possibility is warranted. In vivo Sap production, which is associated with tissue invasion, has been documented by other researchers using indirect immunofluorescence and anti-Sap antibodies applied to tissue sections from infected mice (40). In addition, Ray and Payne (55) described the formation of pits or craters surrounding C. albicans blastoconidia adhering to newborn mouse skin which could be eliminated by the exogenous addition of the specific aspartyl proteinase inhibitor pepstatin A. Because 60% of the ELISA-inhibitory activity could be removed by passage of urine from a C. albicans-infected rabbit over a pepstatin A column, it is apparent that an aspartyl proteinase was responsible for the majority of the ELISA-inhibitory activity.
Significant ELISA inhibition by urine specimens from rabbits infected with a variety of C. albicans strains was observed, and the inhibition results directly correlated with strain pathogenicity as determined by tissue burden, particularly for the kidney. There was not a direct correlation between the amount of in vitro Sap enzyme activity and ELISA inhibition observed among the different C. albicans strains, and this may be a reflection of the difference between enzyme activity and enzyme antigenicity. Some researchers have treated test specimens with trichloroacetic acid before use in Western blot or ELISA detection formats (8), finding that although enzymatic activity could be completely abolished before testing, antigenicity remained intact. Also, the effect of processing in the host may alter the configuration of the enzyme secreted in the urine such that different strains demonstrate somewhat altered antigenicity and therefore reactivity with the detector antibodies. Nonetheless, in our study, the correlation between organ burden and ELISA inhibition was maintained regardless of the infecting C. albicans strain used.
Processing of Sap antigen in vivo may explain the appearance of two immunoreactive protein bands in Western blots of pepstatin A-passaged urine from infected rabbits, whereas only a single protein band was detected after SDS-PAGE analysis of pepstatin A-passaged Sap derived from culture supernatants (47). The two bands detected in urine specimens from infected rabbits may represent either different isoenzymes of Sap (25, 33, 48, 69) or breakdown products of a single Sap (8). At least 10 Sap isoenzymes have been described, and each may play a distinct role in pathogenicity (25, 33, 51, 69).
Although both C. parapsilosis and C. tropicalis have been documented to produce Saps (4, 63, 65, 68, 72) and we also detected in vitro Sap enzyme activity for these Candida species as well as for C. krusei (data not shown), no significant ELISA inhibition by urine specimens from rabbits infected with any of these Candida species was observed. All three species disseminated to at least one organ after intravenous injection, and C. tropicalis infection resulted in significant organ burden in the kidney, spleen, and liver. These data indicate that non-C. albicans Candida species produce Saps that may differ antigenically from those of C. albicans. Conflicting data exist regarding the potential cross-antigenicity of Saps from the different Candida species (4, 39, 50, 65, 68), and it has been hypothesized that Candida Saps possessed common as well as species-specific antigenic sites. Nonetheless, if Sap was produced in vivo by the non-C. albicans Candida species used in the present study, our polyclonal antibodies raised against purified C. albicans Sap did not appear to cross-react with them. Although C. glabrata has been reported to be an emerging cause of Candida bloodstream infections (28; R. A. Hajjeh, A. Sofair, M. E. Brandt, L. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. Mirza, M. Phelan, J. Morgan, W. L. Yang, M. A. Ciblak, L. E. Benjamin, L. Thomson, S. Huie, L. Yeo, P. Pass, and D. W. Warnock, submitted for publication), it produces little or no extracellular Sap (89) and was not studied here.
The purity of the Sap antigen used to immunize animals may also account for the conflicting data in the literature regarding the cross-reactivity of anti-Sap antibodies among Candida species. It may be that contaminating cell wall mannoproteins in preparations used for immunizations are responsible for the observed cross-reactivity of anti-Sap antibodies, particularly between C. albicans and C. tropicalis, because of the shared antigenicity of their cell wall mannans (4, 42, 61). We previously demonstrated that our purified Sap preparations used to immunize animals for the production of polyclonal antibodies to Sap did not contain contaminating cell wall mannan and that our polyclonal anti-Sap antibodies did not react against purified cell wall mannans (47, 48). In the present study, we demonstrated that exogenously added mannan, at concentrations much higher than that obtained physiologically (60), did not interfere with the inhibition ELISA results. Therefore, the antibodies used in our study appear to be specific for the detection of Sap from C. albicans only and no other Candida species tested. This is not to say that detection of infections by other Candida species is unimportant; indeed, inclusion of antibodies specific for the detection of Saps produced by non-C. albicans Candida species may improve the overall utility of a test to detect systemic candidiasis.
Our polyclonal anti-Sap antibodies also did not detect infections caused by the other opportunistic fungi tested (A. fumigatus and C. neoformans) or by S. aureus despite dissemination of these organisms to two or more organs and despite a higher kidney burden in rabbits infected with S. aureus than in rabbits infected with C. albicans. C. neoformans does not produce Sap (89), although initial studies by Staib et al. (73) to detect proteinases in C. neoformans led to the discovery of Sap in C. albicans. In contrast, A. fumigatus and S. aureus have been reported to produce various proteolytic enzymes (30, 34, 44, 57, 58), but despite these reports, no significant ELISA inhibition by urine specimens from rabbits infected with these organisms was observed. Na and Song (50) reported that 6 of 12 patients with aspergillosis reacted positively in their Sap antibody detection ELISA and that 2 of 12 and 1 of 12 reacted positively in the antigen capture and inhibition ELISAs, respectively. These false-positive results were suggested to occur because of cross-reactivities between the aspartyl proteinases of Aspergillus species and C. albicans even though a monoclonal antibody was used in their studies (50). We did not observe such cross-reactivities despite our use of a polyclonal anti-Sap antibody. However, Na and Song (50) used single specimens collected retrospectively from each patient, whereas we tested serial specimens collected prospectively from each rabbit. In one A. fumigatus-infected rabbit, a single specimen did gave a false-positive result in our ELISA, and if this had been the only specimen tested from this rabbit, we would also have reported this rabbit as a positive case.
In our study, regardless of whether multiple deep tissue sites or only one tissue site was involved, blood cultures were often negative despite the use of lysis centrifugation tubes to optimize recovery (1). Therefore, blood cultures did not parallel disease progression or organ burden in this animal model, which suggests that nonculture methods to detect systemic candidiasis would be helpful. In contrast, urine and fecal cultures continued to increase in positivity over time to 100% for urine and fecal cultures of rabbits infected with C. albicans strain 36B. Therefore, blood culture positivity did not reflect disease progression over time, whereas inhibition ELISA results and urine and fecal cultures did. However, unlike the tissue burden results, the number of CFU recovered from the blood, urine, or feces did not correlate with the relative rank order of ELISA inhibition results among the various C. albicans strains tested. In addition, urine and fecal cultures could not discriminate between invasive infection and simple colonization, as intragastrically and intraurethrally colonized rabbits demonstrated consistently high numbers of recoverable CFU from fecal cultures and from urine cultures, respectively. Therefore, only tissue burden results (for the kidney in particular) and inhibition ELISA results were reliable measures of disease progression.
Whereas 10-fold concentration of urine increased the mean ELISA inhibition values obtained on and after day 3 of infection, concentration did not result in an earlier detection of infection, and in only one case did concentration change a negative result to a positive one. These data reflect the fact that urine was already positive for 40% of rabbits as early as 1 day after infection. Concentration of urine may be a more helpful adjunct in a less acute candidiasis model in which disease is more chronic and disease progression is of a longer duration. Although concentration of urine was not an effective method to increase test sensitivity early in infection, it may be useful as a means to rule out false-positive results, should they occur, because only concentration of urine from true cases resulted in an increased ELISA inhibition. Unlike concentration of rabbit urine, which may be more naturally concentrated than human urine, concentration of human urine may result in increased test sensitivity early in the infection process. Also, centrifugation columns, similar to those used in the present study, could be used not only to concentrate samples but to more easily dialyze urine by washing samples during centrifugation. The use of human urine in the inhibition ELISA, whether concentrated or not, may also need to include an internal control such as the measurement of urine creatinine or other factors to normalize results for kidney function. Such adjustments have been used for the accurate measurement of arabinitol in studies by others (6, 22, 35, 83, 87).
The use of urine as a test fluid instead of serum apparently overcame any problems related to the formation of complexes between Sap and alpha-2-macroglobulin, previously reported to occur in the circulation (64), and no antigen-antibody complexes needed to be removed before testing. It is possible that the pH of urine contributed to the dissociation of any complexes that may have formed in the circulation (3, 64) or that complexes were removed by the dialysis methods used in the present study. Nonetheless, neither we, using urine as the test fluid, nor Na and Song (50), using serum as the test fluid, found removal of these complexes to be necessary. It may be that we circumvented any problems with antigen-antibody complex formation because we used specimens derived from rabbits, which are not routinely colonized by C. albicans and do not have naturally occurring antibodies to components of C. albicans. Alternatively, such complexes may have been broken down prior to excretion as part of normal urine processing. Further studies using human urine will be required to fully assess these possibilities.
The overall test sensitivity and specificity were 83 and 92%, respectively, when only one positive sample was required for positivity and were 82 and 97%, respectively, when two or more positive samples were required for positivity. The test positive predictive value and efficiency could also be increased, from 84 to 95% and from 89 to 92%, when two or more positive samples were required for positivity. Requiring two or more positive test samples before a case is considered positive often improves the sensitivity or specificity of antigen or metabolite detection tests for candidiasis as well as for aspergillosis. This stems at least in part from the transient nature of some antigens (reducing sensitivity) or possible cross-reactive epitopes (reducing specificity). For example, multiple sampling for enolase, a metabolic enzyme of C. albicans, in an antigen detection format was found to improve test sensitivity to 75%, compared to 54% when only a single sample was tested (81). Daily sampling was also required to optimally detect the C. albicans metabolite arabinitol in patient serum (22, 35, 82, 83). In addition, the highest test sensitivity for detecting cell wall-derived mannoproteins of C. albicans occurred when cancer patients were screened at least weekly (19, 59). In contrast, multiple sampling improved the specificity of the PLATELIA, a test to detect circulating cell wall galactomannan of Aspergillus species, by decreasing false-positive results from 50 to 23% (26). Others have found multiple sampling to increase the specificity of the PLATELIA (76, 79), and it has been hypothesized that potential cross-reactivities of galactomannan with carbohydrate molecules from various sources may be responsible for transient false-positive results (24, 29, 75, 76). In our study, multiple sampling had little effect on test sensitivity but did increase test specificity.
In conclusion, the Sap inhibition ELISA described here was sensitive and specific for the detection of Sap in urine specimens from rabbits with disseminated C. albicans infection, discriminated between colonization and invasive disease, reflected disease progression and severity, could detect invasive disease as early as 1 day after infection in 50% of cases, and has the potential to be a noninvasive means to diagnose disseminated candidiasis.
Acknowledgments
We gratefully acknowledge James Gathany for his assistance with the graphics and Carlton Arnold and his staff for assistance with the animal model work.
REFERENCES
- 1.Berenguer, J., M. Buck, F. Witebsky, F. Stock, P. A. Pizzo, and T. J. Walsh. 1993. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Diagn. Microbiol. Infect. Dis. 17:103-109. [DOI] [PubMed] [Google Scholar]
- 2.Borg, M., and R. Rüchel. 1988. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect. Immun. 56:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg, M., D. Watters, B. Reich, and R. Rüchel. 1988. Production and characterization of monoclonal antibodies against secretory proteinase of Candida albicans CBS2730. Zentbl. Bakteriol. Mikrobiol. Hyg. A 268:62-73. [DOI] [PubMed] [Google Scholar]
- 4.Borg-von Zepelin, M., and V. Gruness. 1993. Characterization of two monoclonal antibodies against secretory proteinase of Candida tropicalis DSM 4238. J. Med. Vet. Mycol. 31:1-15. [PubMed] [Google Scholar]
- 5.Brieland, J., D. Essig, C. Jackson, D. Frank, D. Loebenberg, F. Menzel, B. Arnold, B. Di Domenico, and R. Hare. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensson, B., T. Wiebe, C. Pehrson, and L. Larsson. 1997. Diagnosis of invasive candidiasis in neutropenic children with cancer by determination of d-arabinitol/l-arabinitol ratios in urine. J. Clin. Microbiol. 35:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colina, A. R., F. Aumont, N. Deslauriers, P. Belhumeur, and L. de Repentigny. 1996. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect. Immun. 64:4514-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bernardis, F., L. Agatensi, I. K. Ross, G. W. Emerson, R. Lorenzini, P. A. Sullivan, and A. Cassone. 1990. Evidence for a role of secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J. Infect. Dis. 161:1276-1283. [DOI] [PubMed] [Google Scholar]
- 9.de Bernardis, F., S. Arancia, L. Morelli, B. Hube, D. Sanglard, W. Schafer, and A. Cassone. 1999. Evidence that members of the secretory aspartyl proteinase gene family, in particular, SAP2, are virulence factors for Candida vaginitis. J. Infect. Dis. 179:201-208. [DOI] [PubMed] [Google Scholar]
- 10.de Bernardis, F., A. Cassone, J. Sturtevant, and R. Calderone. 1995. Expression of Candida albicans SAP1 and SAP2 in experimental vaginitis. Infect. Immun. 63:1887-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Repentigny, L., R. J. Kuykendall, F. W. Chandler, J. R. Broderson, and E. Reiss. 1984. Comparison of serum mannan, arabinitol, and mannose in experimental disseminated candidiasis. J. Clin. Microbiol. 19:804-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Repentigny, L., F. Aumont, K. Bernard, and P. Belhumeur. 2000. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 68:3172-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einsele, H., H. Hebart, G. Roller, J. Loeffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J.-A. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallon, K., K. Bausch, J. Noonan, E. Huguenel, and P. Ramburin. 1997. Role of aspartic proteinases in disseminated Candida albicans infection in mice. Infect. Immun. 65:551-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felk, A., M. Kretschmar, A. Albrecht, M. Schaller, S. Beinhauer, T. Nichterlein, D. Sanglard, H. C. Korting, W. Schafer, and B. Hube. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flauhaut, M., D. Sanglard, M. Monod, J. Bille, and M. Rossier. 1998. Rapid detection of Candida albicans in clinical samples by DNA amplification of common regions from C. albicans-secreted aspartyl proteinase genes. J. Clin. Microbiol. 36:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming, R. V., and T. J. Walsh. 2002. Risk factors for Candida infection in the intensive care unit, p. 23-43. In R. A. Barnes and D. W. Warnock (ed.), Fungal infection in the intensive care unit. Kluwer Academic Publishers, Norwell, Mass.
- 18.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita, S., F. Matsubara, and T. Matsuda. 1986. Enzyme-linked immunosorbent assay measurement of fluctuations in antibody titer and antigenemia in cancer patients with and without candidiasis. J. Clin. Microbiol. 23:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita, S.-I., and T. Hashimoto. 1992. Detection of serum Candida antigens by enzyme-linked immunosorbent assay and a latex agglutination test with anti-Candida albicans and anti-Candida krusei antibodies. J. Clin. Microbiol. 30:3131-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulurija, A., R. B. Ashman, and J. M. Papadimitriou. 1996. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney mechanisms of host resistance. Microbiology 142:3487-3496. [DOI] [PubMed] [Google Scholar]
- 22.Gold, J. W., M. B. Wong, T. E. Bernard, T. E. Kiehn, and D. Armstrong. 1983. Serum arabinitol concentration and arabinitol/creatinine ratio in invasive candidiasis. J. Infect. Dis. 147:504-513. [DOI] [PubMed] [Google Scholar]
- 23.Gray, G. 1978. Antibodies to carbohydrates: preparation of antigens by coupling carbohydrates to proteins by reductive amination with cyanoborohydride. Methods Enzymol. 50:155-160. [DOI] [PubMed] [Google Scholar]
- 24.Hashiguichi, K., Y. Niki, and R. Soejima. 1994. Cyclophosphamide induces false-positive results in detection of Aspergillus antigen in urine. Chest 105:975-976. [DOI] [PubMed] [Google Scholar]
- 25.Hube, B., Sanglard, D., F. C. Odds, D. Hess, M. Monod, W. Schafer, A. J. P. Brown, and N. A. R. Gow. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurst, S. F., D. W. McLaughlin, G. H. Reyes, E. Reiss, and C. J. Morrison. 1999. Comparative evaluation of commercial latex agglutination and sandwich enzyme immunoassays with a competitive binding inhibition enzyme immunoassay to detect antigenemia and antigenuria for the rapid diagnosis of invasive aspergillosis. Clin. Diagn. Lab. Immunol. 7:477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminishi, H., H. Miyaguchi, T. Tamaki, N. Suenaga, M. Hisamatsu, I. Mihashi, H. Matsumoto, H. Maeda, and Y. Hagihara. 1995. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 63:984-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 29.Kappe, R., and A. Schulzeberge. 1993. New cause for false-positive results with the Pastorex Aspergillus latex agglutination test. J. Clin. Microbiol. 31:2489-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson, A., and S. Arvidson. 2002. Variation in extracellular protease production among clinical isolates of Staphylococcus aureus due to different levels of expression of the protease repressor Sar A. Infect. Immun. 70:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauffman, C. A., S. F. Bradley, S. C. Ross, and D. R. Weber. 1991. Hepatosplenic candidiasis: successful treatment with fluconazole. Am. J. Med. 91:137-141. [DOI] [PubMed] [Google Scholar]
- 32.Kregar, I., I. Urh, H. Umezawa, and V. Turk. 1977. Purification of cathepsin D by affinity chromatography on pepstatin Sepharose column. Croat. Chem. Acta 49:587-592. [Google Scholar]
- 33.Kretschmar, M., B. Hube, T. Bertsch, D. Sanglard, R. Merker, M. Schroder, H. Hof, and T. Nichterlein. 1999. Germ tubes and proteinase activity contribute to virulence of Candida albicans in murine peritonitis. Infect. Immun. 67:6637-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, J. D., and P. E. Kolattukudy. 1995. Molecular cloning of the cDNA and gene for an elastinolytic aspartic proteinase from Aspergillus fumigatus and evidence of its secretion by the fungus during invasion of the host lung. Infect. Immun. 63:3796-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lentonen, L., V.-J. Anttila, T. Ruutu, J. Salonen, J. Nikoskelainen, F. Ecrola, and P. Ruutu. 1996. Diagnosis of disseminated candidiasis by measurement of urine d-arabinitol/l-arabinitol ratio. J. Clin. Microbiol. 34:2175-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louie, A., D. M. Dixon, E. A. el-Maghrabi, J. W. Burnett, A. L. Baltch, and R. P. Smith. 1994. Relationship between Candida albicans epidermolytic proteinase activity and virulence in mice. J. Med. Vet. Mycol. 32:59-64. [PubMed] [Google Scholar]
- 37.Louie, A., W. Liu, D. A. Miller, A. C. Sucke, Q-F. Liu, G. L. Drusano, M. Mayers, and M. H. Miller. 1999. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob. Agents Chemother. 43:2831-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louie, A., P. Kaw, P. Banerjee, W. Liu, G. Chen, and M. H. Miller. 2001. Impact of the order of initiation of fluconazole and amphotericin B in sequential or combination therapy on killing of Candida albicans in vitro and in a rabbit model of endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 45:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macdonald, F. 1984. Secretion of inducible proteinase by pathogenic Candida species. Sabouraudia 22:79-82. [PubMed] [Google Scholar]
- 40.Macdonald, F., and F. C. Odds. 1980. Inducible proteinase of Candida albicans in diagnostic serology and pathogenesis of systemic candidiasis. J. Med. Microbiol. 13:431-438. [DOI] [PubMed] [Google Scholar]
- 41.Macdonald, F., and F. C. Odds. 1980. Purified Candida albicans proteinase in the serological diagnosis of systemic candidosis. JAMA 243:2409-2411. [PubMed] [Google Scholar]
- 42.McNeil, M. M., A. R. Gerber, D. W. MacLaughlin, R. A. Vega, K. Winn, L. Kaufman, H. L. Keyserling, and W. R. Jarvis. 1992. Mannan antigenemia during invasive candidiasis caused by Candida tropicalis. Pediatr. Infect. Dis. J. 11:493-496. [PubMed] [Google Scholar]
- 43.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2002. Trends in mortality due to invasive mycotic diseases in the United States 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 44.Miedzobrodzki, J., P. Kaszycki, A. Bialecka, and A. Kasprowicz. 2002. Proteolytic activity of Staphylococcus aureus strains isolated from the colonized skin of patients with acute-phase atopic dermatitis. Eur. J. Clin. Microbiol. Infect. Dis. 21:269-276. [DOI] [PubMed] [Google Scholar]
- 45.Morrison, C. J. 2002. Laboratory diagnosis of opportunistic infections in the intensive care unit, p. 55-104. In R. A. Barnes and D. W. Warnock (ed.), Fungal infection in the intensive care unit. Kluwer Academic Publishers, Norwell, Mass.
- 46.Morrison, C. J., and M. D. Lindsley. 2001. Serological approaches to the diagnosis of invasive fungal infections, p. 667-716. In R. Cihlar and R. Calderone (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, New York, N.Y.
- 47.Morrison, C. J., S. F. Hurst, S. L. Bragg, R. J. Kuykendall, H. Diaz, D. W. McLaughlin, and E. Reiss. 1993. Purification and characterization of the extracellular aspartyl proteinase of Candida albicans: removal of extraneous proteins and cell wall mannoprotein and evidence for lack of glycosylation. J. Gen. Microbiol. 139:1177-1186. [DOI] [PubMed] [Google Scholar]
- 48.Morrison, C. J., S. F. Hurst, S. L. Bragg, R. J. Kuykendall, H. Diaz, J. Pohl, and E. Reiss. 1993. Heterogeneity of the purified extracellular aspartyl proteinase from Candida albicans: characterization with monoclonal antibodies and N-terminal amino acid sequence analysis. Infect. Immun. 61:2030-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morschhauser, J., R. Virkola, T. K. Korhonen, and J. Hacker. 1997. Degradation of human subendothelial extracellular matrix by proteinase-secreting Candida albicans. FEMS Microbiol. Lett. 153:349-355. [DOI] [PubMed] [Google Scholar]
- 50.Na, B.-K., and C.-Y. Song. 1999. Use of monoclonal antibody in diagnosis of candidiasis caused by Candida albicans: detection of circulating aspartyl proteinase antigen. Clin. Diagn. Lab. Immunol. 6:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naglik, J., G. Newport, T. C. White, L. L. Fernandes-Naglik, J. S. Greenspan, D. Greenspan, S. P. Sweet, S. J. Challacombe, and N. Agabian. 1999. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect. Immun. 67:2482-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obayashi, T., M. Yoshida, H. Tamara, J. Aketagawa, S. Tanaka, and T. Kawai. 1992. Determination of plasma (1-3)-β-d-glucan: a new diagnostic aid to deep mycoses. J. Med. Vet. Mycol. 30:275-280. [DOI] [PubMed] [Google Scholar]
- 53.Obayashi, T., M. Yoshida, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshimi, S. Kohno, A. Horiuchi, and A. Ito. 1995. Plasma (1-3)-β-d-glucan measurement in diagnosis of invasive deep mycoses and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 54.Ray, T., C. D. Payne, and B. J. Morrow. 1991. Candida albicans acid proteinase: characterization and role in candidiasis. Adv. Exp. Med. Biol. 306:173-183. [DOI] [PubMed] [Google Scholar]
- 55.Ray, T. L., and C. D. Payne. 1988. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect. Immun. 56:1942-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rees, J. R., R. W. Pinner, R. A. Hajjeh, M. E. Brandt, and A. L. Reingold. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin. Infect. Dis. 27:1138-1147. [PubMed] [Google Scholar]
- 57.Reichard, U., M. Monod, F. Odds, and R. Rüchel. 1997. Virulence of an aspergillopepsin-deficient mutant of Aspergillus fumigatus and evidence for another aspartic proteinase linked to the fungal cell wall. J. Med. Vet. Mycol. 35:189-196. [DOI] [PubMed] [Google Scholar]
- 58.Reichard, U., G. T. Cole, R. Rüchel, and M. Monod. 2000. Molecular cloning and targeted deletion of PEP2, which encodes a novel aspartic proteinase from Aspergillus fumigatus. Int. J. Med. Microbiol. 290:85-96. [DOI] [PubMed] [Google Scholar]
- 59.Reiss, E., and C. J. Morrison. 1993. Nonculture methods for diagnosis of disseminated candidiasis. Clin. Microbiol. Rev. 6:311-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiss, E., R. J. Kuykendall, and L. Kaufman. 1986. Antigenemia in rabbits infected with Candida albicans serotype B: detection by enzyme immunoassay and preliminary characterization of the antigen. J. Med. Vet. Mycol. 24:259-269. [PubMed] [Google Scholar]
- 61.Reiss, E., L. de Repentigny, R. J. Kuykendall, A. W. Carter, R. Galindo, P. Auger, S. L. Bragg, and L. Kaufman. 1986. Monoclonal antibodies against Candida tropicalis mannan: antigen detection by enzyme immunoassay and immunofluorescence. J. Clin. Microbiol. 24:796-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rex, J. H., T. J. Walsh, and E. J. Anaissie. 1998. Fungal infections in iatrogenically compromised hosts. Adv. Intern. Med. 43:321-371. [PubMed] [Google Scholar]
- 63.Rüchel, R. 1984. A variety of Candida proteinases and their possible targets of proteolytic attack in the host. Zentbl. Bakteriol. Mikrobiol. Hyg. A 257:266-274. [PubMed] [Google Scholar]
- 64.Rüchel, R., and B. Boning. 1985. Detection of Candida proteinase by enzyme immunoassay and interaction of the enzyme with alpha-2-macroglobulin. J. Immunol. Methods 61:107-116. [DOI] [PubMed] [Google Scholar]
- 65.Rüchel, R., B. Boning, and M. Borg. 1986. Characterization of a secretory proteinase of Candida parapsilosis and evidence for the absence of the enzyme during infection in vitro. Infect. Immun. 53:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rüchel, R., B. Boning-Stutzer, and A. Mari. 1988. A synoptical approach to the diagnosis of candidosis, relying on serological antigen and antibody tests, on culture, and on evaluation of clinical data. Mycoses 31:87-106. [PubMed] [Google Scholar]
- 67.Rüchel, R., R. Tegeler, and M. Trost. 1982. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia 20:233-244. [DOI] [PubMed] [Google Scholar]
- 68.Rüchel, R., K. Uhlemann, and B. Boning. 1983. Secretion of acid proteinase by different species of the genus Candida. Zentbl. Bakteriol. Mikrobiol. Hyg. I Abt. Orig. A 255:537-548. [PubMed] [Google Scholar]
- 69.Sanglard, D., B. Hube, M. Monod, F. Odds, and N. A. R. Gow. 1997. A triple deletion of secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sendid, B., M. Tabouret, J. L. Poirot, D. Mathieu, J. Fruit, and D. Poulain. 1999. New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J. Clin. Microbiol. 37:1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin, J. H., F. S. Nolte, and C. J. Morrison. 1997. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J. Clin. Microbiol. 35:1454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sono, E., T. Masuda, K. Takesako, I. Kato, K. Uchida, S. Y. Murayama, and H. Yamaguchi. 1992. Comparison of secretory acid proteinases from Candida tropicalis, C. parapsilosis, and C. albicans. Microbiol. Immunol. 36:1099-1104. [DOI] [PubMed] [Google Scholar]
- 73.Staib, F. 1965. Serum proteins as nitrogen source for yeastlike fungi. Sabouraudia 4:187-193. [DOI] [PubMed] [Google Scholar]
- 74.Staib, F., S. Mishra, and T. L. Abel. 1977. Serodiagnostic value of extracellular antigens of an actively proteolysing culture of Candida albicans. Zentbl. Bakteriol. Mikrobiol. Hyg. I Abt. Orig. 238:284-287. [PubMed] [Google Scholar]
- 75.Sulahain, A., M. Tabouret, P. Ribaud, J. Sarfati, E. Gluckman, J. P. Latge, and F. Derouin. 1996. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 15:139-145. [DOI] [PubMed] [Google Scholar]
- 76.Swanink, C. M. A., J. F. G. M. Meis, A. J. M. M. Rijs, J. P. Donnelly, and P. E. Verweij. 1997. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 35:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thaler, M., B. Pastakia, T. H. Shawker, T. O'Leary, and P. A. Pizzo. 1988. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann. Intern. Med. 108:88-100. [DOI] [PubMed] [Google Scholar]
- 78.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 79.Verweij, P. E., D. Stynen, A. J. Rijs, B. E. de Pauw, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1995. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J. Clin. Microbiol. 33:1912-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner, T., M. Borg von Zepelin, and R. Rüchel. 1995. pH-dependent denaturation of extracellular aspartic proteinases from Candida species. J. Med. Vet. Mycol. 33:275-278. [PubMed] [Google Scholar]
- 81.Walsh, T. J., J. W. Hathorn, J. D. Sobel, W. G. Merz, V. Sanchez, S. M. Maret, H. R. Buckley, M. A. Pfaller, R. Schaufele, C. Sliva, E. Navarro, J. Lecciones, P. Chandrasekar, J. W. Lee, and P. A. Pizzo. 1991. Detection of circulating Candida enolase by immunoassay in patients with cancer and invasive candidiasis. N. Engl. J. Med. 324:1026-1031. [DOI] [PubMed] [Google Scholar]
- 82.Walsh, T. J., J. W. Lee, T. Sien, R. Schaufele, J. Bacher, A. C. Switchenko, T. C. Goodman, and P. A. Pizzo. 1994. Serum d-arabinitol measured by automated quantitative enzymatic assay for detection and therapeutic monitoring of experimental disseminated candidiasis: correlation with tissue concentrations of Candida albicans. J. Med. Vet. Mycol. 32:205-215. [DOI] [PubMed] [Google Scholar]
- 83.Walsh, T. J., W. G. Merz, J. W. Lee, R. Schaufele, T. Sein, P. O. Whitcomb, M. Ruddel, W. Burns, J. R. Wingard, A. C. Switchenko, T. Goodman, and P. A. Pizzo. 1995. Diagnosis and therapeutic monitoring of invasive candidiasis by rapid enzymatic detection of serum d-arabinitol. Am. J. Med. 99:164-172. [DOI] [PubMed] [Google Scholar]
- 84.Watts, H. J., F. S. Cheah, B. Hube, D. Sanglard, and N. A. Gow. 1998. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes. FEMS Microbiol. Lett. 159:129-135. [DOI] [PubMed] [Google Scholar]
- 85.Wilson, M. B., and P. K. Nakane. 1978. Recent developments in the periodate method of conjugating horseradish peroxidase (HRPO) to antibodies, p. 215. In W. Knapp, K. Holubar, and G. Wicks (ed.), Immunofluorescence and related staining techniques. Elsevier/North-Holland Biomedical Publishing Co., Amsterdam, The Netherlands.
- 86.Winbald, B. 1975. Experimental renal candidiasis in mice and guinea pigs. Acta Pathol. Microbiol. Scand. Sect. A 83:406-414. [DOI] [PubMed] [Google Scholar]
- 87.Wong, B., K. L. Brauer, J. R. Clemens, and S. Beggs. 1990. Effects of gastrointestinal candidiasis, antibiotics, dietary arabinitol, and cortisone acetate on levels of the Candida metabolite d-arabinitol in rat serum and urine. Infect. Immun. 58:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu, T., K. Wright, S. F. Hurst, and C. J. Morrison. 2000. Enhanced extracellular production of aspartyl proteinase, a virulence factor, by Candida albicans isolates following growth in subinhibitory concentrations of fluconazole. Antimicrob. Agents Chemother. 44:1200-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamamoto, T., K. Nohara, K. Uchida, and H. Yamaguichi. 1992. Purification and characterization of secretory proteinase of Candida albicans. Microbiol. Immunol. 36:637-641. [DOI] [PubMed] [Google Scholar]