Abstract
For years, anti-Leishmania immunoglobulin G (IgG) antibodies have been detected in the sera of dogs living in areas of leishmaniasis endemicity. They have also been found in the aqueous humor and cerebrospinal fluid. In contrast, a review of the literature failed to identify the detection of anti-Leishmania antibodies in urine samples from dogs with leishmaniasis. Ninety-five dog urine samples were examined for the presence of anti-Leishmania antibodies by using a protein A enzyme-linked immunosorbent assay (ELISA). Twenty additional urine samples were collected from healthy dogs as controls. An IgG2 ELISA was performed on 26 urine samples found positive by the protein A ELISA. Twenty-three urine samples found positive to anti-Leishmania antibodies were tested for the local production of anti-Leishmania antibodies in the urinary tract by means of the urine antibody coefficient. Ten urine samples (and the corresponding serum samples) were compared by Western blot (WB) analysis. Thirty-five out of the 95 urine samples were found positive, 57 were found negative, and 3 were found inconclusive for antibody detection by the protein A ELISA. A high correlation between protein A and IgG2 levels was found in positive urine samples. Anti-Leishmania antibodies were present in the urine of dogs that had leishmaniasis, urinary protein/creatinine (U P/C) ratios of greater than one, and normal urinary sediment. A statistically significant correlation was observed between the U P/C ratios and the levels of anti-Leishmania antibodies in positive urine samples. In general, WB analysis and the urine antibody coefficient suggested that the presence of anti-Leishmania antibodies in urine was the consequence of an impairment of filtration of the glomerular barrier. However, in some dogs, WB analysis could be interpreted as suggesting that the presence of anti-Leishmania antibodies was caused, to a lesser extent, by local antibody production in the urinary tract. Antibody detection in urine could be a noninvasive method for leishmaniasis diagnosis and prognosis in dogs with glomerulonephropathies.
Canine leishmaniasis, caused by the protozoan parasite Leishmania infantum, is a severe systemic disease highly prevalent in the Mediterranean basin. Clinical manifestations of the disease include nonpruritic skin lesions, such as exfoliative dermatitis and ulcerations; local or generalized lymphadenopathy; loss of weight; poor appetite; ocular lesions; epistaxis; lameness; renal failure; and diarrhea (8, 12, 21, 36). Dogs with leishmaniasis have high anti-Leishmania immunoglobulin G (IgG) antibody levels in sera (23), and clinicopathological findings include anemia, hypoalbuminemia, hyperglobulinemia, hypercreatinemia, and proteinuria (22).
In the vast majority of cases, the fatal course of canine leishmaniasis is due to renal involvement (5, 32). The major renal lesion in canine (32) and human (7, 11) leishmaniasis is glomerulonephritis. However, interstitial nephritis, tubular nephropathy, and glomerular amyloidosis in conjunction with glomerulonephritis have also been found in dogs with leishmaniasis (30). The pathogenesis of renal lesions in both human (7, 40) and canine (38) visceral leishmaniasis is mainly attributed to immune complex deposition and subsequent glomerular injury. There have been reports of immunoglobulins and immune complex deposition in the glomerular capillaries and mesangial matrix of human (7) and canine (28, 32) patients with leishmaniasis. Moreover, immune complexes have been found in the sera of human (19) and canine (26, 32) patients.
Because of the importance of glomerular injury in canine leishmaniasis, several tests, such as protein/creatinine (U P/C) ratios (31) and enzymuria (30), have been used to detect early renal damage in canine leishmaniasis to establish a prognosis and appropriate treatment. A recent study demonstrated that a large number of dogs with leishmaniasis (46%) have U P/C ratios of greater than one (22).
For years, anti-Leishmania IgG antibodies have been detected in the sera of dogs living in areas of leishmaniasis endemicity (6). They have also been found, but less frequently, in the aqueous humor (14) and cerebrospinal fluid (39). Moreover, investigators have detected anti-Leishmania antibodies in the urine of human patients with visceral leishmaniasis (20) and in the urine of Leishmania donovani-infected hamsters in association with glomerulonephritis (34). Nevertheless, a review of the literature failed to identify the detection of anti-Leishmania antibodies in urine samples from dogs with leishmaniasis.
We hypothesized that in dogs with Leishmania-associated glomerular injury, anti-Leishmania antibodies can pass through the glomerular barrier and therefore that urine from these dogs may contain anti-Leishmania antibodies. The first objective of this study was to investigate the presence of anti-Leishmania antibodies in the urine of dogs with leishmaniasis. The second objective was to determine whether the presence of anti-Leishmania antibodies in the urine was caused by an impairment of the charge and/or size selectivity of the glomerular capillary wall or whether the antibodies were locally produced in the urinary tract.
MATERIALS AND METHODS
Urine and serum samples.
Ninety-five dog urine samples collected from patients examined for a variety of diseases or disorders at the Veterinary Teaching Hospital of the Universitat Autònoma de Barcelona (VTH-UAB) between the years 2000 and 2002 were obtained from the sample bank kept at the Veterinarian Clinical Biochemistry Service of the Universitat Autònoma de Barcelona.
The urine samples were analyzed at the Veterinarian Clinical Biochemistry Service for U P/C ratios. For the 95 urine samples, we were able to obtain clinicopathological data for 50 dogs from complete records maintained at the VTH-UAB (30 dogs with leishmaniasis and 20 dogs with other disorders). Partial information for 6 of the remaining 45 dogs was obtained from a database developed at the Serological Diagnostic Laboratory of Leishmaniasis of the Universitat Autònoma de Barcelona. For urine samples from 39 dogs, we had no information at all.
The 95 dog urine samples were examined for the presence of anti-Leishmania antibodies by a protein A enzyme-linked immunosorbent assay (ELISA). Twenty urine samples were collected from healthy dogs as controls.
In order to ensure the detection of Leishmania-specific IgG by the protein A ELISA, 26 urine samples positive for protein A were screened for the presence of anti-Leishmania IgG2 antibodies. Twenty-three urine samples had anti-Leishmania antibodies, and their corresponding serum samples were used to assess the local production of anti-Leishmania antibodies in the urinary tract. Ten urine samples (and the corresponding serum samples) were used for immunoblot analysis.
All urine and serum samples used in this study were stored frozen at −20°C until assayed.
ELISA for the detection of anti-Leishmania antibodies in urine samples.
All urine samples were tested for the presence of anti-Leishmania antibodies by the protein A ELISA. An ELISA that had been used for dog serum samples was adapted for urine samples (33). Microtiter plates were coated with 20 μg of L. infantum antigen ml−1 in 0.1 ml of coating buffer (0.1 M carbonate-bicarbonate [pH 9.6]) and incubated overnight at 4°C. One hundred microliters of dog urine, diluted 1:100 in phosphate-buffered saline (PBS)-0.05% Tween 20 (PBST)-1% dried skim milk (PBST-M), was incubated in each well for 1 h at 37°C. After three washes with PBST and one wash with PBS, 100 μl of protein A (1:5,000 dilution in PBST-M) conjugated to horseradish peroxidase (HRPO) (Sigma, St. Louis, Mo.) was added to each well. The plates were incubated for 1 h at 37°C and then rewashed. The substrate solution (ortho-phenylenediamine [0.4 mg/ml] [Sigma]-H2O2 [0.4 μl/ml] in 0.1 M phosphate-citrate buffer [pH 5.0]) was added at 200 μl/well and developed for 20 min at 24°C. The reaction was stopped with 50 μl of 3 M H2SO4. Absorbance values were read at 492 nm with an automatic microELISA reader (Anthos 2001; Anthos Labtec Instruments GmbH, Wales, Austria).
An anti-dog IgG2-HRPO conjugate (1:2,500 dilution in PBST-M) (Bethyl Laboratories, Montgomery, Ala.) was also used with some of the urine samples to verify that the results were mainly due to IgG. The Bethyl anti-dog antisera specific to IgG2 react with the four fractions of IgG so that the anti-dog IgG2- HRPO conjugate can be considered a reagent that measures total IgG (27).
The reaction was quantified as units relative to a positive urine sample used as a calibrator and arbitrarily set at 100 U. The cutoff values were established at 10 U for protein A and 16 U for IgG2 (mean and 4 standard deviations for 20 urine samples from healthy dogs). Negative results were established at 6 U for protein A and 10 U for IgG2 (mean and 2 standard deviations for 20 urine samples from healthy dogs). Uncertain results were established between positive and negative ELISA results for both conjugates.
All determinations included one serum sample from a sick dog with confirmed infection as a positive control.
Demonstration of the local production of anti-Leishmania antibodies in the urinary tract.
Twenty-three urine samples found positive for anti-Leishmania antibodies were tested for local production in the urinary tract. The testing was performed as follows.
An ELISA to detect anti-Leishmania antibodies was performed with urine and serum samples from the same animal as described before but with some modifications. To assess the relative concentrations of antibodies in the two samples, the quantification was expressed as a titer (maximal dilution at which the sample gave the same optical density as the negative control). A 1:15,000 dilution of protein A-HRPO was used for urine and serum samples from each dog. Urine and serum samples from each dog were assessed on the same microtiter plate.
Total IgG amounts in both serum and urine samples from the same dog were measured by a sandwich ELISA. The ELISA was performed according to the manufacturer's instructions (Dog IgG ELISA; Bethyl Laboratories) but with some modifications. Briefly, microtiter plates were coated with affinity-purified sheep anti-dog IgG at 10 μg ml−1 in 0.1 M carbonate-bicarbonate buffer and incubated overnight at 4°C. Serum and urine samples were diluted 1:28,000 and 1:1,786, respectively, in PBST-M and were additionally diluted twofold four times for each tested sample. One hundred microliters of diluted dog serum or urine sample was incubated in each well for 1 h at 37°C. Reference serum dilutions were used to construct the standard curve. After three washes with PBST and one wash with PBS, 100 μl of sheep anti-dog IgG (1:80,000) conjugated to HRPO was added to each well and incubated for 1 h at 37°C. The substrate solution contained ortho-phenylenediamine. The reaction was stopped with 3 M H2SO4. Absorbance values were read at 492 nm with an automatic microELISA reader. Urine samples from eight healthy dogs were also tested.
The results for titers and total amounts of IgG in urine and serum samples from each dog were used to calculate the urine antibody coefficient (C) with the following formula adapted from previous studies of feline (24) and human (10) toxoplasmosis: C = (anti-Leishmania antibody titer for urine × total IgG concentration in serum)/(anti-Leishmania antibody titer for serum × total IgG concentration in urine).
Test results were interpreted as follows (10, 24): a C of <1 did not support the local production of antibody, a C of between 1 and 8 was suggestive of the local production of antibody, and a C of >8 was definitive evidence of the local production of antibody.
Immunoblot analysis.
Western blot (WB) analysis was performed as described elsewhere (1) to confirm the positive results found for urine samples and to identify and compare the parasite antigens recognized by urine and serum samples from dogs with leishmaniasis. Immunoblotting was performed for 10 protein A ELISA-positive urine samples and for four protein A ELISA-negative urine samples. Urine and serum samples were collected on the same day from each patient. Antigen for immunoblot analysis was obtained from cultures of promastigotes in the logarithmic growth phase in Schneider's medium. Promastigotes were washed, counted, adjusted to a concentration of 3 × 108/ml in sample buffer (0.5 M Tris-HCl [pH 6.8], 0.01 M EDTA, 5% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 0.0125% bromophenol blue), and boiled for 5 min. Electrophoresis was performed with 0.1% sodium dodecyl sulfate-13% polyacrylamide gels. Polypeptides were transblotted onto nitrocellulose sheets (Millipore, Bedford, Mass.). Immunoblot analysis was carried out with serum samples at a 1:100 dilution and urine samples at a 1:25 dilution in 20 mM Tris-0.13 mM NaCl (pH 7.6) containing 0.05% Tween 20 and 1% dry skim milk. Protein A-HRPO and anti-dog IgG2-HRPO were used at 1:1,000 dilutions. Color was developed with 4-chloro-1-naphthol (Sigma) and H2O2, and the reaction was stopped with tap water. The relative molecular weights of the detected bands were calculated with Quantity One software (version 4.1.1; Bio-Rad, Hercules, Calif.).
Statistical analysis.
The Student t test of independence and the Pearson coefficient r analysis were performed by using the SPSS program.
RESULTS
Detection of anti-Leishmania antibodies in urine samples by ELISA.
Of the 95 urine samples tested, 35 were found positive, 57 were found negative, and 3 were found inconclusive for antibody detection by the protein A ELISA. For the 35 positive urine samples, we had records from the VTH-UAB for 20 dogs (20 out of 30 dogs with leishmaniasis), and we had a diagnosis of leishmaniasis for six more dogs from the Serological Diagnostic Laboratory of Leishmaniasis of the Universitat Autònoma de Barcelona. The remaining nine dogs with positive urine samples were from the 39 dogs for which we had no information at all.
The levels of anti-Leishmania antibodies in positive urine samples ranged from 10.9 to 268.1 ELISA units, with a mean and standard deviation of 84.3 ± 81.5 ELISA units. The levels of anti-Leishmania antibodies in negative urine samples ranged from 0 to 5.6 ELISA units, with a mean and standard deviation of 1.5 ± 1.5 ELISA units. The levels of anti-Leishmania antibodies in inconclusive urine samples ranged from 8.3 to 10 ELISA units, with a mean and standard deviation of 8.9 ± 0.9 ELISA units.
The IgG2 ELISA was performed for 26 out of 35 urine samples found positive by the protein A ELISA. All 26 urine samples were also found positive by the IgG2 ELISA. The levels of anti-Leishmania IgG2 in urine samples ranged from 22.6 to 324.0 ELISA units, with a mean and standard deviation of 149.0 ± 105.0 ELISA units. The levels of anti-Leishmania antibodies detected by the protein A ELISA in the 26 urine samples ranged from 13.6 to 300.0 ELISA units, with a mean and standard deviation of 103.0 ± 87.6 ELISA units. A high correlation between protein A and IgG2 ELISA results (r = 0.9, P < 0.001) was found for the 26 positive urine samples.
Clinicopathological data for dogs.
The records kept at the VTH-UAB showed that 30 urine samples were from leishmaniasis patients and that 20 were from patients with other disorders.
All 30 leishmaniasis patients showed high anti-Leishmania antibody levels in sera. Twenty out of these 30 leishmaniasis patients were also positive for antibody detection in urine (designated group 1), while the remaining 10 were negative for antibody detection in urine and positive for antibody detection in sera (designated group 2). All 20 patients with other disorders were negative for antibody detection in both urine and sera.
Dogs with leishmaniasis and with antibodies in urine had nonspecific clinical signs related to glomerulonephritis or renal involvement, such as loss of weight, lethargy, anorexia, polydipsia-polyuria, and vomiting. Dogs with leishmaniasis and without antibodies in urine had nonspecific clinical signs of leishmaniasis, such as loss of weight, cutaneous and ocular lesions, and lymphadenopathy.
The results of serum and urine analyses for dogs previously diagnosed with leishmaniasis are recorded in Table 1. Serum analysis showed that in both leishmaniasis groups, the altered parameters were higher concentrations of gamma globulin and beta globulin and a lower concentration of albumin. However, dogs in group 1 had a statistically significant higher concentration of gamma globulin and a lower concentration of albumin than those in group 2. In addition, dogs in group 1 had an altered albumin/globulin (A/G) ratio and a significantly lower A/G ratio than dogs in group 2. In the urine analysis, dogs in group 1 had a U P/C ratio of greater than one and normal urinary sediment. Dogs in group 1 had a statistically significant higher U P/C ratio than dogs in group 2. In summary, as shown in Table 1, dogs with antibodies in urine had clinicopathological abnormalities related to glomerulonephritis or renal involvement, such as hypoalbuminemia, hyperglobulinemia, and proteinuria (U P/C ratio of greater than one).
TABLE 1.
Clinicopathological data for 30 leishmaniasis patientsa
| Type of analysis | Parameter | Value for the following group of dogs with leishmaniasis:
|
Normal range | t test value | |
|---|---|---|---|---|---|
| 1 (n = 20) | 2 (n = 10) | ||||
| Serum | Urea (mg/dl) | 57.4 ± 46.0 | 53.6 ± 23.9 | 21.4-59.9 | NS |
| Creatinine (mg/dl) | 1.2 ± 0.8 | 1.2 ± 0.6 | 0.5-1.5 | NS | |
| Total protein (g/dl) | 8 ± 1.6b | 7.6 ± 1.3b | 5.4-7.1 | NS | |
| Gamma globulin (g/dl) | 3.4 ± 1.5b | 2.1 ± 1b | 0.5-1.3 | P < 0.05 | |
| Beta globulin (g/dl) | 2 ± 0.4b | 1.9 ± 0.5b | 0.9-1.6 | NS | |
| Alpha 2 globulin (g/dl) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.3-1.1 | NS | |
| Alpha 1 globulin (g/dl) | 0.3 ± 0.08 | 0.4 ± 0.1 | 0.2-0.5 | P < 0.01 | |
| Albumin (g/dl) | 1.6 ± 0.4b | 2.3 ± 0.4 | 2.6-3.3 | P < 0.001 | |
| A/G ratio | 0.26 ± 0.11b | 0.5 ± 0.2 | >0.45 | P < 0.001 | |
| Urine | U P/C ratio | 5.8 ± 5b | 0.7 ± 0.6 | <1 | P < 0.01 |
| Density (g/ml) | 1,031 ± 8.8 | 1,030 ± 9.8 | >1,020 | NS | |
| pH | 7 ± 0.6 | ND | |||
Data are reported as means and standard deviations. NS, not significant; ND, not determined.
Altered parameter.
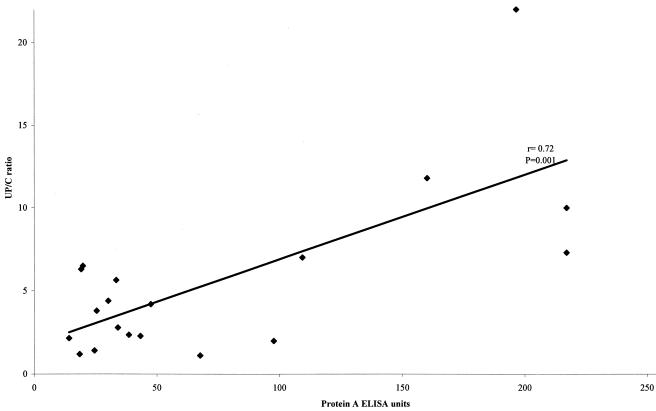
A statistically significant correlation between U P/C ratios and levels of anti-Leishmania antibodies was found for the 20 positive urine samples (r = 0.72, P < 0.0001). The results are shown in Fig. 1.
FIG. 1.
Relationship between U P/C ratios and anti-Leishmania antibody levels, as determined by the protein A ELISA, for 20 positive urine samples.
Local production of anti-Leishmania antibodies in the urinary tract.
Twenty-three urine samples positive for anti-Leishmania antibodies were tested for local production in the urinary tract, and only one had a C of greater than 1 (1.036). As expected, the anti-Leishmania antibody titers and concentrations of IgG in the urine samples were much lower than the anti-Leishmania antibody titers and concentrations of IgG in the serum samples for all dogs (Table 2).
TABLE 2.
Antibody detection and C for 23 dogs with leishmaniasis
| Parameter | Anti-Leishmania antibody titer in:
|
IgG concn (mg/ml) in:
|
C | ||
|---|---|---|---|---|---|
| Urine | Serum | Urine | Serum | ||
| Mean | 3,791 | 799,165 | 0.707 | 46.618 | 0.397 |
| SD | 5,956 | 616,092 | 0.913 | 25.485 | 0.258 |
The IgG concentration in the urine of eight healthy dogs showed a mean and standard deviation of 0.0026 ± 0.0036 mg/ml. This IgG concentration in the urine of healthy dogs was significantly lower than that in the urine of dogs with leishmaniasis (P value determined by the t test, 0.05).
Immunoblot analysis.
All positive samples (urine and serum samples) recognized numerous polypeptide fractions of the L. infantum antigen with masses ranging from 12 to 85 kDa, with most bands at 16 to 69 kDa. The most frequent polypeptide fractions observed in both urine and serum samples were those of 14, 16, 20, 30, 31, 48, and 69 kDa. The immunoblot analysis did not reveal any polypeptide fractions of the L. infantum antigen in ELISA-negative urine samples.
The band pattern revealed by urine from dogs with high levels of specific anti-Leishmania antibodies detected by the ELISA was more intense than the pattern revealed by urine from dogs with low levels of anti-Leishmania antibodies detected by the ELISA.
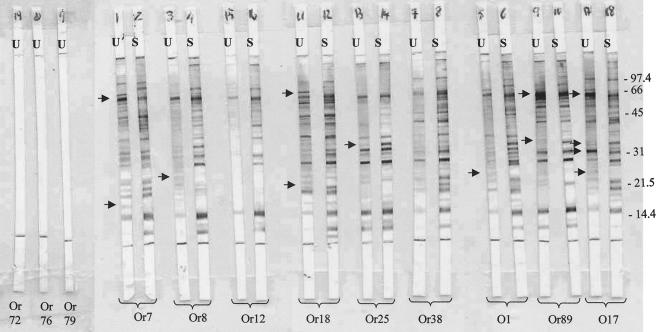
In general, when the patterns obtained by immunoblotting for the urine and serum samples from the same dog were compared, we found that the number of bands revealed by serum antibodies and the intensity of those bands were higher than those of bands revealed by urine antibodies. The patterns of bands revealed by protein A and IgG2 conjugates showed slight differences. When the anti-IgG2 conjugate was used, some differences were observed in the patterns of bands revealed by urine and serum antibodies over the molecular mass range. In some cases, urine antibodies revealed bands that were not observed with the corresponding serum antibodies, and in other cases, some bands revealed by urine antibodies showed a higher intensity than the homologous bands in the serum strips (Fig. 2). When the protein A conjugate was used, some additional differences were observed, mainly in the region of low molecular mass (less than 30 kDa).
FIG. 2.
Results of WB analysis with an IgG2-HRPO conjugate. U, urine; S, serum. Or7, Or8, Or12, Or18, Or25, Or38, O1, Or89, and O17 represent positive dogs. Or72, Or76, and Or79 represent negative controls. The urine from dogs Or7, Or8, and Or12 had low levels of Leishmania-specific IgG detected by the ELISA. The urine from dogs Or18, Or25, and Or38 had medium levels of Leishmania-specific IgG. The urine from dogs O1, Or89, and O17 had high levels of Leishmania-specific IgG. Arrows indicate idiotype differences between serum and urine samples from the same dog. Numbers at right are kilodaltons.
DISCUSSION
Immunoglobulins have been found in the urine of human patients with glomerular disease in a variety of disorders (4, 3, 25, 29). Moreover, several infectious diseases are diagnosed by the finding of specific antibodies in urine, such as Helicobacter pylori infection (18), filariasis (17), or schistosomiasis (35). The presence of anti-Leishmania antibodies in the urine of human patients with visceral leishmaniasis (16, 20) and in the urine of L. donovani-infected hamsters (34) has also been described. In contrast, there is a lack of information about immunoglobulins in the urine of healthy and sick dogs. In this study, we report the presence of anti-Leishmania antibodies in the urine of dogs with leishmaniasis. In addition, we describe, for the first time, the detection of total IgG concentrations in the urine of healthy dogs and dogs with leishmaniasis.
One of the common features of canine leishmaniasis is the development of hyperglobulinemia, and the elevation occurs in the IgG fraction. Dogs with leishmaniasis have high Leishmania-specific IgG levels in sera (23). The diagnosis of canine leishmaniasis can be performed by serological tests, such as the dot ELISA (13), the ELISA (33), and WB (1), with protein A as the detection probe to detect Leishmania-specific immunoglobulins. The reactivity of protein A with immunoglobulins from dogs is quite strong. Protein A reacts with all subclasses of IgG and partially reacts with dog IgA and IgM (15). To verify that the results found for urine by the ELISA and WB were mainly due to the detection of IgG, an anti-dog IgG2- HRPO conjugate was also used with some of the urine samples. Previous studies demonstrated that the anti-dog IgG2- HRPO conjugate used (Bethyl) reacts with all subclasses of IgG (27) and that the levels of anti-Leishmania antibodies detected with this conjugate correlate with those detected with an anti-dog IgG-HRPO conjugate (37).
The correlation found between protein A and IgG2 ELISA units in positive urine samples was high. Therefore, we could consider that the anti-Leishmania antibodies found in urine were predominantly IgG. Immunofluorescence studies of pathological kidneys from dogs with leishmaniasis have described IgG and other immunoglobulins, such as IgM and IgA, in the affected glomeruli (28). Further studies are needed to determine the importance of other immunoglobulins in dogs with leishmaniasis.
For clinical veterinarians, it is very important to check the renal status of dogs with leishmaniasis in order to provide appropriate prognosis and treatment. The U P/C ratio has proven useful for detecting proteinuria as an index of renal failure in dogs (41). Furthermore, it has been demonstrated that the total urinary protein level and the U P/C ratio have better diagnostic value than a serum biochemical renal profile for detecting early renal lesions in canine leishmaniasis (31). For these reasons, clinician veterinarian practitioners use the U P/C ratio to detect proteinuria in dogs with leishmaniasis both at diagnosis and during follow-up treatment. However, few extensive studies are available concerning U P/C ratios in dogs with leishmaniasis at diagnosis and during follow-up treatment (22). A recent study demonstrated that a large number of dogs with leishmaniasis (21 out of 45) had U P/C ratios of greater than one. After 4 months of allopurinol treatment, most of the proteinuric dogs (15 of 17) not only did not experience any further deterioration of their renal function but also showed a decrease in (8 of 15) or even a disappearance of (4 of 15) proteinuria at the end of the trial (22). This previous study demonstrated the usefulness of assessing proteinuria at the time of diagnosis and during treatment to obtain an accurate prognosis for a patient with leishmaniasis. However, infection and marked blood contamination of urine samples could result in an abnormal U P/C ratio in the absence of glomerular disease. Consequently, the U P/C ratio alone does not distinguish between the types of proteinuria. Total serum protein, albumin, and urinary sediment evaluations would complete the screening profile to differentiate prerenal, renal, or postrenal proteinuria (2). Therefore, techniques that evaluate the renal status of dogs with leishmaniasis are of great value.
As we expected, dogs that had anti-Leishmania antibodies in their urine had U P/C ratios of greater than one and urinary sediment findings compatible with renal disease. Moreover, we found a statistically significant correlation between U P/C ratios and anti-Leishmania antibody levels in urine. In contrast, dogs that had leishmaniasis demonstrated by high anti-Leishmania antibody levels in sera but that were nonproteinuric were found negative for anti-Leishmania antibodies in urine. Because the detection of anti-Leishmania antibodies in urine is a more specific test than the U P/C ratio, it might be a useful tool for establishing appropriate prognosis and treatment. Further studies are needed to investigate the usefulness of detection of anti-Leishmania antibodies in urine samples for the follow-up of treated dogs with leishmaniasis.
Due to the clinical and retrospective points of view of this study, we did not perform histopathological studies of the kidneys from any of the dogs. However, for 23 dogs, we tried to predict whether the presence of anti-Leishmania antibodies in the urine was caused by an inability of the glomeruli to properly filter or by local production in the urinary tract. In the majority of cases (22 out of 23), C was suggestive of passage from plasma through the glomerular barrier. None of the dogs had a C of greater than eight, which is considered strong evidence of local production (10). Therefore, our results compared favorably with those of previous histopathological studies. Because the major renal lesion in canine leishmaniasis is glomerulonephritis (32), most dogs appear to have glomerular injury. This nonselective proteinuria can explain the finding of IgG in the urine. Nonselective proteinuria in human patients has been defined as increased urinary excretion of high-molecular-weight plasma proteins, such as IgG, and this finding is a good indicator of renal disease progression (3).
Studies that have compared the WB patterns in different samples from patients with Toxoplasma gondii infections (9) have considered the detection of specific oligoclonal IgG bands in cerebrospinal fluid to be indicative of a local immune response. In the present study, slight differences were observed in the patterns of polypeptide fractions in the Leishmania antigens recognized by urine and serum samples from dogs with leishmaniasis. The passage of IgM through the glomerular barrier may be restricted due to its high molecular weight, thus resulting in differences in the patterns revealed by urine and serum samples when protein A is used. Nevertheless, this restriction will not be evident when only the IgG2 isotype is investigated. In this situation, if all specific antibodies present in urine were of blood origin, the proportions of the different idiotypes in serum and urine would be the same. Consequently, the findings observed suggest that the presence of Leishmania-specific antibodies in urine is mainly caused by the passage into the urinary tract of blood-derived immunoglobulins and, to a lesser extent, by the local production of antibodies. This local production of anti-Leishmania antibodies in the urinary tract might be explained by the presence of a predominant tubulointerstitial nephritis lesion in dogs (32) or by lesions in other urinary or genital organs, such as the bladder, urethra, or prostate.
In conclusion, this is the first study that describes the detection of anti-Leishmania IgG antibodies in urine samples from dogs with leishmaniasis. Our results showed that urine antibodies were found only in proteinuric dogs. Further studies are needed to evaluate the meaning and usefulness of the presence of anti-Leishmania IgG antibodies in urine. However, the findings suggest that anti-Leishmania antibody detection in urine is a more specific and more reliable noninvasive method for leishmaniasis diagnosis and prognosis than is the U P/C ratio for dogs with glomerulonephropathies.
Acknowledgments
We are grateful to the patients and veterinarians from VTH-UAB and to the Veterinarian Clinical Biochemistry Service of the Universitat Autònoma de Barcelona for collaboration in this study.
REFERENCES
- 1.Aisa, M. J., S. Castillejo, M. Gállego, R. Fisa, C. Riera, M. de Colmenares, S. Torras, X. Roura, J. Sentis, and M. Portús. 1998. Diagnostic potential of western blot analysis of sera from dogs with leishmaniasis in endemic areas and significance of the pattern. Am. J. Trop. Med. Hyg. 58:154-159. [DOI] [PubMed] [Google Scholar]
- 2.Bagley, R. S., S. A. Center, R. M. Lewis, S. Shin, S. A. Dougherty, J. F. Randolph, and H. Erb. 1991. The effect of experimental cystitis and iatrogenic blood contamination on the urine protein/creatine ratio in the dog. J. Vet. Intern. Med. 5:66-70. [DOI] [PubMed] [Google Scholar]
- 3.Bakoush, O., A. Grubb, B. Rippe, and J. Tencer. 2001. Urine excretion of protein HC in proteinuric glomerular diseases correlates to urine IgG but not to albuminuria. Kidney Int. 60:1904-1909. [DOI] [PubMed] [Google Scholar]
- 4.Bakoush, O., J. Tencer, J. Tapia, B. Rippe, and O. Torffvit. 2002. Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Kidney Int. 61:203-208. [DOI] [PubMed] [Google Scholar]
- 5.Benderitter, T., P. Casanova, L. Nashkidachvili, and M. Quilici. 1988. Glomerulonephritis in dogs with canine leishmaniasis. Ann. Trop. Med. Parasitol. 82:335-341. [DOI] [PubMed] [Google Scholar]
- 6.Bettini, S., and L. Gradoni. 1986. Canine leishmaniasis in the Mediterranean area and its implications for human leishmaniasis. Insect Sci. Appl. 7:241-245. [Google Scholar]
- 7.Brito, T., S. Hashino-Shimizu, V. AmatoNato, I. S. Duarte, and D. O. Penna. 1975. Glomerular involvement in human Kala-azar. A light immunofluorescent and electron microscope study based on kidney biopsies. Am. J. Trop. Med. Hyg. 24:9-18. [PubMed] [Google Scholar]
- 8.Ciaramella, P., G. Oliva, R. de Luna, L. Gradoni, R. Ambrosio, L. Cortese, A. Scalone, and A. Persechino. 1997. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet. Rec. 141:539-543. [DOI] [PubMed] [Google Scholar]
- 9.Contini, C., E. Fainardi, R. Cultrera, R. Canipari, F. Peyron, S. Delia, E. Paolino, and E. Granieri. 1998. Advanced laboratory techniques for diagnosing Toxoplasma gondii encephalitis in AIDS patients: significance of intrathecal production and comparison with PCR and ECL-Western blotting. J. Neuroimmunol. 92:29-37. [DOI] [PubMed] [Google Scholar]
- 10.Desmonts, G. 1966. Definitive serological diagnosis of ocular toxoplasmosis. Arch. Ophthalmol. 76:839-851. [DOI] [PubMed] [Google Scholar]
- 11.Dutra, M., R. Martinelli, E. M. de Carvalho, L. E. Rodrigues, E. Brito, and H. Rocha. 1985. Renal involvement in visceral leishmaniasis. Am. J. Kidney Dis. 6:22-27. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer, L., R. Rabanal, D. Fondevila, J. A. Ramos, and M. Domingo. 1988. Skin lesions in canine leishmaniasis. J. Small Anim. Pract. 29:381-388. [Google Scholar]
- 13.Fisa, R., M. Gállego, C. Riera, M. J. Aisa, D. Valls, T. Serra, M. de Colmenares, S. Castillejo, and M. Portús. 1997. Serologic diagnosis of canine leishmaniasis by dot-ELISA. J. Vet. Diagn. Investig. 9:50-55. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Alonso, M., A. Blanco, D. Reina, F. J. Serrano, C. Alonso, and C. G. Nieto. 1996. Immunopathology of the uveitis in canine leishmaniasis. Parasite Immunol. 18:617-623. [DOI] [PubMed] [Google Scholar]
- 15.Goudswaard, J., J. A. van der Donk, A. Noordzij, R. H. van Dam, and J. P. Vaerman. 1978. Protein A reactivity of various mammalian immunoglobulins. Scand. J. Immunol. 8:21-28. [DOI] [PubMed] [Google Scholar]
- 16.Islam, M. Z., M. Itoh, S. M. Shamsuzzaman, R. Mirza, F. Matin, I. Ahmed, A. K. Shamsuzzaman Choudhury, M. A. Hossain, X. G. Qiu, N. Begam, M. Furuya, J. L. Leafasia, Y. Hashiguchi, and E. Kimura. 2002. Diagnosis of visceral leishmaniasis by enzyme-linked immunosorbent assay using urine samples. Clin. Diagn. Lab. Immunol. 9:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh, M., M. V. Weerasooriya, G. Qiu, N. K. Gunawardena, M. T. Anantaphruti, S. Tesana, P. Rattanaxay, Y. Fujimaki, and E. Kimura. 2001. Sensitive and specific enzyme-linked immunosorbent assay for the diagnosis of Wuchereria bancrofti infection in urine samples. Am. J. Trop. Med. Hyg. 65:362-365. [DOI] [PubMed] [Google Scholar]
- 18.Kato, S., T. Tachikawa, K. Ozawa, M. Konno, M. Okuda, T. Fujisawa, Y. Nakazato, H. Tajiri, and K. Linuma. 2001. Urine-based enzyme-linked immunosorbent assay for the detection of Helicobacter pylori infection in children. Pediatrics 107:E87. [DOI] [PubMed] [Google Scholar]
- 19.Kharazmi, A., H. R. Rezai, M. Fani, and N. C. Behforouz. 1982. Evidence for the presence of circulating immune complexes in serum and C3b and C3d on red cells of kala-azar patients. Trans. R. Soc. Trop. Med. Hyg. 76:793-796. [DOI] [PubMed] [Google Scholar]
- 20.Kohanteb, J., S. M. Ardehali, and H. R. Rezai. 1987. Detection of Leishmania donovani soluble antigen and antibody in the urine of visceral leishmaniasis patients. Trans. R. Soc. Trop. Med. Hyg. 81:578-580. [DOI] [PubMed] [Google Scholar]
- 21.Koutinas, A. F., Z. S. Polizopoulou, M. N. Saridomichelakis, D. Argyriadis, A. Fytianou, and K. G. Plevraki. 1999. Clinical considerations on canine visceral leishmaniasis in Greece: a retrospective study of 158 cases (1989-1996). J. Am. Anim. Hosp. Assoc. 35:376-383. [DOI] [PubMed] [Google Scholar]
- 22.Koutinas, A. F., M. N. Saridomichelakis, M. E. Mylonakis, L. Leontides, Z. Polizopoulou, C. Billinis, D. Argyriadis, N. Diakou, and O. Papadopoulos. 2001. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniasis. Vet. Parasitol. 98:247-261. [DOI] [PubMed] [Google Scholar]
- 23.Lanotte, G., J. A. Rioux, J. Perieres, and Y. Vollhardt. 1979. Ecologie des leishmaniose dans le sud de la France. 10. Les formes evolutives de la leishmaniose viscerale canine. Elaboration d'une typologie bio-clinique à finalité épidémiologique. Ann. Parasitol. Hum. Comp. 54:227-295. [PubMed] [Google Scholar]
- 24.Lappin, M. R., S. M. Roberts, M. G. Davidson, C. C. Powell, and J. S. Reif. 1992. Enzyme-linked immunosorbent assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats. Am. Vet. Med. Assoc. 201:1010-1016. [PubMed] [Google Scholar]
- 25.Lee, T. M., S. F. Su, and C. H. Tsai. 2002. Effect of pravastatin on proteinuria in patients with well-controlled hypertension. Hypertension 40:67-73. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, R., R. Lucena, M. Novales, P. J. Ginel, E. Martin, and J. M. Molleda. 1996. Circulating immune complexes and renal function in canine leishmaniasis. Zentbl. Vetmed. Reihe B 43:469-474. [DOI] [PubMed] [Google Scholar]
- 27.Mazza, G., W. P. Duffus, C. J. Elson, C. R. Stokes, A. D. Wilson, and A. H. Whiting. 1993. The separation and identification by monoclonal antibodies of dog IgG fractions. J. Immunol. Methods 161:193-203. [DOI] [PubMed] [Google Scholar]
- 28.Nieto, C. G., I. Navarrete, M. A. Habela, F. Serrano, and E. Redondo. 1992. Pathological changes in kidneys of dogs with natural Leishmania infection. Vet. Parasitol. 45:33-47. [DOI] [PubMed] [Google Scholar]
- 29.Norden, A. G., M. Lapsley, P. J. Lee, C. D. Pusey, S. J. Scheinman, F. W. Tam, R. V. Thakker, R. J. Unwin, and O. Wrong. 2001. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 60:1885-1892. [DOI] [PubMed] [Google Scholar]
- 30.Palacio, J., F. Liste, and M. Gascon. 1997. Enzymuria as an index of renal damage in canine leishmaniasis. Vet. Rec. 140:477-480. [DOI] [PubMed] [Google Scholar]
- 31.Palacio, J., F. Liste, and M. Gascón. 1995. Urinary protein/creatinine ration in the evaluation of renal failure in canine leishmaniasis. Vet. Rec. 137:567-568. [DOI] [PubMed] [Google Scholar]
- 32.Poli, A., F. Abramo, F. Mancianti, M. Nigro, S. Pieri, and A. Bionda. 1991. Renal involvement in canine leishmaniasis. A light-microscopic, immunohistochemical and electron-microscopic study. Nephron 57:444-452. [DOI] [PubMed] [Google Scholar]
- 33.Riera, C., J. E. Valladares, M. Gállego, M. J. Aisa, S. Castillejo, R. Fisa, N. Ribas, J. Carrió, J. Alberola, and M. Arboix. 1999. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet. Parasitol. 84:33-47. [DOI] [PubMed] [Google Scholar]
- 34.Sartori, A., A. V. De Oliveira, M. C. Roque-Barreira, M. A. Rossi, and A. Campos-Neto. 1987. Immune complex glomerulonephritis in experimental kala-azar. Parasite Immunol. 9:93-103. [DOI] [PubMed] [Google Scholar]
- 35.Shaker, Z. A., M. A. Kaddah, S. B. Hanallah, and M. I. El-Khodary. 1998. Production of monoclonal antibodies against target schistosomal antigen secreted in the urine of Schistosoma mansoni-infected patients. Int. J. Parasitol. 28:1893-1901. [DOI] [PubMed] [Google Scholar]
- 36.Slappendel, R. J. 1988. Canine leishmaniasis: a review based on 95 cases in the Netherlands. Vet. Q. 10:1-16. [DOI] [PubMed] [Google Scholar]
- 37.Solano-Gallego, L., C. Riera, X. Roura, L. Iniesta, M. Gállego, J. E. Valladares, R. Fisa, S. Castillejo, J. Alberola, L. Ferrer, M. Arboix, and M. Portús. 2001. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet. Parasitol. 96:265-276. [DOI] [PubMed] [Google Scholar]
- 38.Tafuri, W. L., M. S. Michalick, M. Dias, O. Genaro, V. H. Leite, A. J. Barbosa, E. A. Bambirra, C. A. da Costa, M. N. Melo, and M. W. Mayrink. 1989. Optical and electron microscopic study of the kidney of dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi. Rev. Inst. Med. Trop. Sao Paulo 31:139-145. [DOI] [PubMed] [Google Scholar]
- 39.Vinuelas, J., M. Garcia-Alonso, L. Ferrando, I. Navarrete, I. Molano, C. Miron, J. Carcelen, C. Alonso, and C. G. Nieto. 2001. Meningeal leishmaniasis induced by Leishmania infantum in naturally infected dogs. Vet. Parasitol. 101:23-27. [DOI] [PubMed] [Google Scholar]
- 40.Weisinger, J. R., A. Pinto, G. A. Velazquez, I. Bronstein, J. J. Dessene, J. F. Duque, J. Montenegro, F. Tapanes, and A. R. de Rousse. 1978. Clinical and histological kidney involvement in human kala-azar. Am. J. Trop. Med. Hyg. 27:357-359. [DOI] [PubMed] [Google Scholar]
- 41.White, J. V., N. B. Olivier, K. Reimann, and C. Johnson. 1984. Use of protein-to-creatinine ratio in a single urine specimen for quantitative estimation of canine proteinuria. J. Am. Vet. Med. Assoc. 185:882-885. [PubMed] [Google Scholar]