Abstract
Bovine tuberculosis in the United States has proven costly to cattle producers as well as to government regulatory agencies. While in vivo responsiveness to mycobacterial antigens is the current standard for the diagnosis of tuberculosis, in vitro assays are gaining acceptance, especially as ancillary or complementary tests. To evaluate in vitro indices of cellular sensitization, antigen-induced gamma interferon (IFN-γ), nitric oxide (NO), and tumor necrosis factor alpha (TNF-α) responses by blood mononuclear cells from Mycobacterium bovis-infected cattle were quantified and compared. Using an aerosol model of infection, two doses of each of two strains of M. bovis (95-1315 and HC-2045T) were used to induce a range of IFN-γ, NO, and TNF-α responses. Infection-specific increases in NO, but not in IFN-γ or TNF-α, were detected in nonstimulated cultures at 48 h, a finding that is indicative of nonspecific activation and spontaneous release of NO. The infective dose of M. bovis organisms also influenced responses. At 34 days postinfection, IFN-γ, NO, and TNF-α responses in antigen-stimulated cells from cattle receiving 105 CFU of M. bovis organisms were greater than responses of cells from cattle infected with 103 CFU of M. bovis organisms. The NO response, but not the IFN-γ and TNF-α responses, was influenced by infective strains of M. bovis. The TNF-α, NO, and IFN-γ responses followed similar kinetics, with strong positive associations among the three readouts. Overall, these findings indicate that NO and TNF-α, like IFN-γ, may prove useful as indices for the diagnosis of bovine tuberculosis.
First described by Robert Koch in 1891, the tuberculin skin reaction has been the principal means of tuberculosis diagnosis for both humans and domestic animals (23). For cattle, the caudal fold skin test (CFT) is the primary approved test for tuberculosis within the United States. The CFT relies on in vivo reactivity to Mycobacterium bovis purified protein derivative (PPDb) injected intradermally into a fold of skin at the base of the tail. Cattle classified as reactors or suspect with this test are often retested by using the comparative cervical skin test, in which PPDb is injected at one site and M. avium PPD (PPDa) is injected at a separate site. The comparative cervical skin test, while technically more challenging than the CFT, provides an added ability to distinguish M. avium (including M. avium subsp. paratuberculosis) responders from M. bovis responders. An in vitro method of tuberculosis diagnosis has also been developed (41) and approved for use in the United States as a complementary test (i.e., in conjunction with the skin test) (22). The in vitro assay detects gamma interferon (IFN-γ) produced differentially by peripheral blood mononuclear cells (PBMC) exposed to no antigen (i.e., background response), PPDa, PPDb, or mitogen (e.g., pokeweed mitogen, PWM) (40). The assay is particularly suitable for diagnostic laboratories, as whole blood cultures are used, thus circumventing the need for cumbersome cell separation techniques. More recently, recombinant antigens specific for virulent tubercle bacilli (e.g., ESAT-6, CFP-10, MPB-59, MPB-64, and MPB-70) have been evaluated for use in tests that discriminate among M. avium-exposed, M. bovis BCG-vaccinated, and tuberculous cattle (3, 4, 35). These antigens have demonstrated utility for both in vitro (i.e., IFN-γ test) and in vivo (i.e., skin test) use (12, 20, 27, 28, 34, 39). Despite these advances, there is still a need for convenient and inexpensive tests for bovine tuberculosis.
The proven, practical application of an IFN-γ-based assay for tuberculosis diagnosis is not surprising considering the robust cell-mediated response generated by tuberculosis complex mycobacteria. Indeed, IFN-γ is crucial for effective host defense during tuberculosis (8, 15, 18, 25, 30). Other readouts of mycobacterial immunity, especially cellular reactivity, may also have diagnostic application. Two essential components of tubercular host defense include nitric oxide (NO) and tumor necrosis factor alpha (TNF-α) (1, 13, 14, 16, 17, 21). Stimulation of inducible nitric oxide synthase in macrophages and subsequent generation of reactive nitrogen intermediates are potent mechanisms for mycobacterial killing (6, 7, 9, 13, 21). Mycobacterium-induced TNF-α and IFN-γ secretion by T cells and/or macrophages from infected individuals is responsible for an antimycobacterial defense mediated by reactive nitrogen intermediates (17, 33). TNF-α is also necessary for containment of the infection (i.e., granuloma formation). Mice deficient in TNF-α or TNF-α receptor are highly susceptible to fatal mycobacterial infections and fail to develop organized granulomas (11, 17, 19). NO and TNF-α, like IFN-γ, are readily produced by mycobacterium-induced PBMC from M. bovis-infected cattle (24, 38), thus demonstrating their potential as diagnostic readouts for M. bovis infection.
The objective of the present study was to quantify and compare mycobacterium-specific IFN-γ, NO, and TNF-α production by PBMC from M. bovis-infected cattle. An aerosol model of M. bovis infection using two dosages of each of two strains of M. bovis was used to initiate variable responses for comparisons. Isolated PBMC were used for recall stimulation studies because this population produces vigorous IFN-γ, NO, and TNF-α responses when stimulated with mycobacterial antigens. Responses were evaluated for effects of challenge dose and strain, kinetics, and associations.
Twenty crossbred cattle of approximately 9 months of age and obtained from herds with no history of tuberculosis were housed at the National Animal Disease Center, United States Department of Agriculture, Animal Research Service, Ames, Iowa, according to the Association for Assessment and Accreditation of Laboratory Animal Care International and institutional guidelines. At the initiation of the study, all animals were tested and confirmed negative for M. bovis and M. avium exposure by using a commercially available assay (Bovigam; CSL Limited, Parkville, Victoria, Australia) for detection of IFN-γ responses to in vitro mycobacterial antigen stimulation. The animals were housed in temperature- and humidity-controlled rooms (1 to 2 animals per room) within a biosafety level 3 confinement facility. Negative airflow exited the building through HEPA (high efficiency particulate air) filters, ensuring that air from the animal pens was pulled towards a central corridor and through HEPA filters before exiting the building. Airflow velocity was 10.4 air changes per h.
The strains of M. bovis used for the challenge inoculum were strain 95-1315 (United States Department of Agriculture Animal Plant and Health Inspection Service designation), originally isolated from a white-tailed deer in Michigan (31), and strain HC-2045T, originally isolated from a Holstein cow in Texas. Inoculum consisted of mid-log-phase M. bovis cells grown in Middlebrook's 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Mo.) as previously described (2). The challenge inoculum consisted of either ∼105 (n = 5 for each of the two strains) or ∼103 (n = 5 for each of the two strains) CFU in 2 ml of phosphate-buffered saline (PBS). The cattle were restrained, and the challenge inoculum was delivered by nebulization into a mask covering the animals' nostrils and mouths (26).
Nineteen of the twenty cattle challenged with M. bovis had typical tuberculous lesions with M. bovis organisms cultured from affected tissues. Restriction fragment length polymorphism patterns of M. bovis organisms isolated from tissues matched the challenge inoculum strain. Tracheobronchial and mediastinal lymph nodes and lungs were the most commonly affected tissues. Lung lesions were distributed diffusely among all lobes, consistent with aerosol exposure to droplet nuclei of <5 μm. Lesions were more severe and disseminated in cattle receiving the higher challenge dosage (i.e., 105 CFU), regardless of the challenge strain. Although it is difficult to determine the actual number of tuberculous lesions per animal, lesions were detected in more sites (i.e., organs, lymph nodes, lung lobes, etc.) in cattle receiving 105 CFU of strain HC-2045T than in those receiving 105 CFU of strain 95-1315. The numbers of lesion sites did not differ between challenge strains for cattle receiving 103 CFU. Detailed descriptions of gross, histologic, and bacteriologic findings are presented elsewhere (26).
PBMC were isolated from buffy coat fractions of peripheral blood collected in 2× acid citrate dextrose (5). The wells of 96-well round-bottom microtiter plates (Falcon; Becton Dickinson, Lincoln Park, N. J.) were seeded with 2 × 105 PBMC in a total volume of 200 μl per well. The medium was RPMI 1640 supplemented with 2 mM l-glutamine, 25 mM HEPES buffer, 100 units of penicillin per ml, 0.1 mg of streptomycin per ml, 1% nonessential amino acids (Sigma), 2% essential amino acids (Sigma), 1% sodium pyruvate (Sigma), 50 μM 2-mercaptoethanol (Sigma), and 10% (vol/vol) fetal bovine serum. The wells contained medium plus 5 μg per ml, of PPDb (CSL Limited) 5 μg per ml of PPDa (CSL Limited) 10 μg per ml, of M. bovis strain 95-1315 whole-cell sonicate (WCS) 10 μg per ml, of M. bovis strain HC-2045T WCS or 1 μg per ml, of PWM or medium alone (no stimulation) per ml. The WCS antigens were prepared from 4-week M. bovis cultures grown in Middlebrook's 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex. Bacilli were pelleted, sonicated in PBS, further disrupted with 0.1 to 0.15 mm glass beads (Biospec Products, Bartlesville, Okla.) in a bead beater (Biospec Products), and then placed on ice. The preparation was centrifuged, and the supernatant was harvested and filtered (0.22 μm). After incubation of PBMC cultures for 48 h at 37°C in 5% CO2, the supernatants were harvested and stored at −80°C until thawed for analysis.
Nitrite is the stable oxidation product of NO, and the amount of nitrite within culture supernatants is indicative of the amount of NO produced by cells in culture. Nitrite was measured by using the Griess reaction (29) performed in 96-well microtiter plates (Immunolon 2; Dynatech Laboratories, Inc., Chantilly, Va.). Nitrite concentrations in the supernatants were also determined by high-performance ion chromatography (HPIC). Briefly, macromolecules were separated from the aqueous portion of the sample by centrifugation through a 30,000-Da molecular mass cutoff filter. The microfiltrate was collected and injected directly into an ion-exchange high-pressure liquid chromatography system. A 4.1-by 250-mm strong anion-exchange column with a 10-μm inside diameter was used. The mobile phase consisted of an aqueous solution containing 5.28 g of NaH2PO4 · H2O, 43.46 g of Na2HPO4 · 7 H2O, 2.40 g of NaCl, and 100 ml of acetonitrile per liter. Nitrate was detected by absorbance at 214 nm; nitrite was detected by absorbance at 530 nm after a postanalytical column diazo-coupling reaction with an aqueous solution containing 100 ml of 85% o-phosphoric acid, 40.00 g of sulfanilamide, and 2.00 g of N-(1-naphthyl)ethylenediamine dihydrochloride per liter. Ions were quantitated against their respective standard curves.
A commercial enzyme-linked immunosorbent assay (ELISA)-based kit (Bovigam; CSL Limited) was used for determination of IFN-γ concentrations in culture supernatants. Duplicate samples for each individual treatment were analyzed. Each treatment represented three pooled replicate samples. TNF-α was measured by using a TNF-α capture ELISA (protocol and reagents were provided by L. Babiuk, Veterinary Infectious Diseases Organization, Saskatoon, Saskatchewan, Canada). The assays were performed in Immunolon II microtiter plates (Dynatech Laboratories, Inc., Chantilly, Va.). Reagents consisted of a capture antibody (mouse ascites anti-TNF-α, immunoglobulin G [IgG] fraction), a detection antibody, recombinant bovine TNF-α (IgG fraction), biotinylated goat anti-rabbit IgG (Zymed Laboratories, Inc., South San Francisco, Calif.), horseradish peroxidase-conjugated streptavidin-biotinylated complex (Amersham Corporation, Arlington Heights, Ill.), substrate (H2O2 at 0.1%, vol/vol), and dye (2,2′-azinodi-ethylbenzothiazoline-sulfonic acid). Internal standards of serially diluted rabbit anti-bovine TNF-α were prepared in PBS (pH 7.2, 0.01 M) with Tween 80 (0.1%, vol/vol) and gelatin (0.5%, vol/vol) (PBST-g). The positive and negative controls and test samples were also diluted serially in PBST-g. Capture antibody was diluted (1:1,000, vol/vol) in carbonate buffer (pH 9.6, 0.01 M), and detection antibody was diluted in PBST-g (1:1,500, vol/vol). Biotinylated goat anti-rabbit Ig was diluted 1:10,000, and horseradish peroxidase-conjugated streptavidin-biotinylated complex was diluted 1:2,000 in PBST without gelatin. Intervening washes were done with PBST without gelatin. Enzyme substrate and indicator were dye diluted in citrate buffer. All incubations were at room temperature with the exception of that of capture antibody in carbonate buffer, which was incubated at 4°C. The absorbance of the standards and test samples was read at 405 and 490 nm using an ELISA plate washer and reader (Dynatech MR7000). TNF-α concentrations (nanograms per milliliter) in the test samples were determined by comparing the absorbancy of the test samples with the absorbancy of standards within a linear curve fit.
The data were assessed for normality prior to statistical analysis. Arithmetic and log10-transformed data were analyzed as a split plot with repeated-measure analysis of variance (ANOVA) using Statview software (version 5.0; SAS Institute, Inc., Cary, N.C.). The statistical model included the effects of treatment (i.e., challenge strain and challenge dose), time (days relative to challenge), and the interaction of treatment and time on nitrite, IFN-γ, and TNF-α concentrations in supernatants from PBMC cultures. Fisher's protected-least significant difference test was applied when significant effects (P of <0.05) were detected by the model. Pearson's product-moment correlations were computed between nitrite production measured using Greiss and HPIC assays, as well as between concentrations of nitrite, IFN-γ, and TNF-α in culture supernatants.
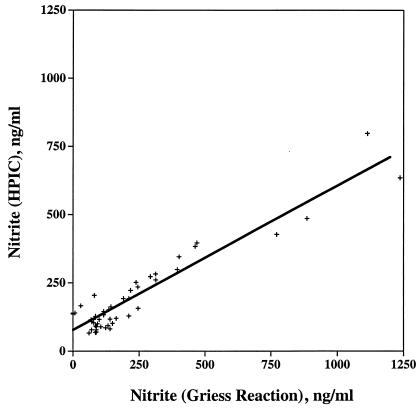
To validate the Griess reaction assay in our culture system, supernatants (49 samples) were evaluated for nitrite by HPIC at an accredited toxicology laboratory (i.e., University of Nebraska Veterinary Diagnostic Center) and by the Griess reaction (i.e., at the National Animal Disease Center). Samples included supernatants from PBMC cultures stimulated with medium alone, PPDa, PPDb, and PWM. Blood samples were collected at prechallenge (day 0) and at 34 days, 68 days, and 124 days postchallenge. For the analysis, supernatants representing a predicted wide range of responses were included. As demonstrated in Fig. 1, results from the two assays had a strong positive linear association (r = 0.94, P < 0.0001; y = 0.57x + 70.87), suggesting that both the Griess and HPIC assays generated similar results. The strongest associations between the Griess and HPIC assays were observed for samples from stimulated cultures (for PPDa, r = 0.92, y = 0.45x + 87; for PPDb, r = 0.96, y = 0.55x + 72; for PWM, r = 0.90, y = 0.48x + 95), which generated a wider range of responses than those from nonstimulated cultures (for medium alone, r = 0.18, y = 0.11x + 72.5), in which there was a minimal response.
FIG. 1.
Comparison of the results of the Griess reaction with those of HPIC for the measurement of nitrite in 48-h culture supernatants. The measurements (n = 49) utilized in this analysis were from four time points (prechallenge [day 0] and 34, 68, and 124 days postchallenge) and stimuli (nonstimulated [medium alone] and stimulated with PPDa, PPDb, or PWM). The symbols (+) represent individual responses, and the line was generated by linear regression analysis (r = 0.94, P < 0.0001; y = 0.57x + 70.87).
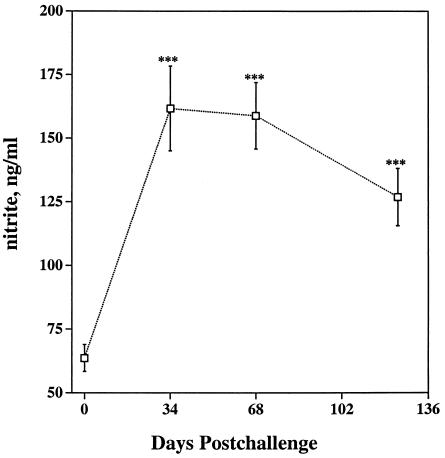
Nonspecific activation of circulating PBMC may alter spontaneous production of IFN-γ, nitrite, or TNF-α in nonstimulated cultures. Indeed, M. bovis BCG infection or intraperitoneal lipopolysaccharide injection of C3H/HeJ mice induces a five- to sixfold increase in serum nitrite or nitrate levels, resulting from increased production of NO by macrophages (32). Levels of IFN-γ in supernatants from 48-h nonstimulated PBMC cultures (0.07 ± 0.006 [mean ± standard error of the mean {SEM}] for samples throughout the study) were unaffected (P > 0.05) by time relative to infection or by the dosage or strain of M. bovis challenge (data not shown). Likewise, TNF-α levels in supernatants from nonstimulated cultures were unaffected (P > 0.05) by the dosage or strain of inoculum. However, less (P < 0.01) TNF-α was detected in supernatants from nonstimulated cultures collected at 68 days postchallenge (0.91 ± 0.05) than in those collected at 0 days (1.82 ± 0.25), 34 days (1.71 ± 0.21), and 124 days (2.15 ± 0.31) postchallenge, regardless of dosage or the strain of M. bovis. In contrast to results for IFN-γ and TNF-α, increases in nitrite relative to prechallenge levels were detected at 34 days, 68 days, and 124 days postchallenge (Fig. 2) (combined data including both dosages and strains; n = 20) in 48-h supernatants from nonstimulated cultures. Furthermore, increases (P < 0.05) in spontaneous nitrite production upon infection were detected for each of the groups (i.e., split by dose and strain; n = 5) when they were analyzed separately (data not shown). In general, spontaneous NO production increased upon infection, whereas minimal to no increases in spontaneous release of IFN-γ or TNF-α were observed. Similarly, NO within exhaled air (36) and NO produced spontaneously by PBMC (37) increases in humans infected with M. tuberculosis.
FIG. 2.
Spontaneous production of nitrite by nonstimulated PBMC during the experimental period. Nitrite production (means ± SEMs) in supernatants from 48-h nonstimulated cultures was measured by the Griess reaction. Asterisks (***) indicate that results for the postchallenge response differed (P < 0.001) from those for the prechallenge (day 0) response.
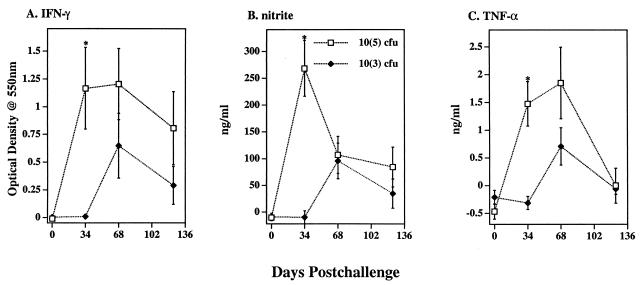
The challenge dosage of M. bovis administered influenced responses with greater (P < 0.01, n = 10; 34 days postchallenge) levels of IFN-γ, nitrite, and TNF-α detected in supernatants from PPDb-stimulated cultures from cattle receiving 105 CFU of M. bovis organisms than in those receiving 103 CFU of M. bovis organisms (Fig. 3). Dosage effects were not detected at 0 days, 68 days, or 124 days postchallenge. Increases (P < 0.01, n = 20) in IFN-γ, nitrite, and TNF-α levels, regardless of dose or strain of inoculum, were detected in supernatants from PPDb-stimulated cultures at 34 days and 68 days postchallenge in comparison to those of prechallenge responses (Table 1). PPDb-induced IFN-γ and nitrite levels were also increased (P < 0.05) at 124 days postchallenge in comparison to those of prechallenge responses (Table 1). IFN-γ, nitrite, and TNF-α responses to either PPDb or HC-2045T WCS exceeded (P < 0.05) the respective responses to either PPDa or 95-1315 WCS (Table 2), regardless of the challenge strain. In general, challenge dosage, duration of infection, and type of antigen used for stimulation affected IFN-γ, nitrite, and TNF-α responses similarly.
FIG. 3.
Effects of challenge dose and time relative to challenge on IFN-γ, nitrite, and TNF-α responses of cells in vitro. Blood mononuclear cells were isolated from cattle immediately prior to challenge (day 0) and at 34 days, 68 days, and 124 days after challenge with 103 (closed triangles, n = 10) or 105 (open boxes, n = 10) CFU of M. bovis strain 95-1315 organisms. The cells were cultured for 48 h with medium alone or with 5 μg of PPDb per ml. IFN-γ and TNF-α concentrations in culture supernatants were quantified by ELISA, and nitrite concentrations were quantified by the Griess reaction. Values are presented as mean (± SEM) responses to M. bovis PPDb stimulation minus the response to medium alone. An asterisk (*) indicates that the values for the responses for cattle receiving 105 CFU of PBMC differed (P < 0.05) from the values for the responses for cattle receiving 103 CFU of M. bovis organisms.
TABLE 1.
Longitudinal IFN-γ, nitrite, and TNF-α responses of blood mononuclear cells to stimulation with PPDba
| Prechallenge or day after challenge | Mean responses ± SEMb
|
||
|---|---|---|---|
| IFN-γ (OD) | Nitrite (ng/ml) | TNF-α (ng/ml) | |
| Prechallenge | 0 ± 0 A | −10 ± 5 A | −0.34 ± 0.09 A |
| 34 | 0.84 ± 0.26 B | 129 ± 41 C | 0.58 ± 0.29 B |
| 68 | 0.93 ± 0.22 B | 101 ± 24 BC | 1.28 ± 0.38 C |
| 124 | 0.55 ± 0.19 B | 59 ± 23 B | −0.03 ± 0.16 A |
Blood mononuclear cells were isolated immediately prior to challenge (day 0) and at 34 days, 68 days, and 124 days after challenge and were cultured for 48 h with medium alone or with 5 μg of PPDb per ml. IFN-γ and TNF-α concentrations were quantified by ELISA, and nitrite concentrations were quantified by the Griess reaction. Data were analyzed by repeated-measure ANOVA with P values of 0.001, 0.001, and <0.0001 for IFN-γ, nitrite, and TNF-α, respectively. Values followed by different letters are significantly different (P < 0.05).
Values represent mean responses (± SEM, n = 20) to PPDb stimulation minus responses to medium alone.
TABLE 2.
IFN-γ, nitrite, and TNF-α responses of blood mononuclear cells from cattle infected with M. bovis to soluble M. bovis antigensa
| Stimulant (concn) | Mean responses ± SEMb
|
||
|---|---|---|---|
| IFN-γ (OD) | Nitrite (ng/ml) | TNF-α (ng/ml) | |
| HC-2045T WCS (10 μg/ml) | 0.84 ± 0.24 A | 125 ± 39 A | 1.34 ± 0.38 A |
| 95-1315 WCS (10 μg/ml) | 0.12 ± 0.04 B | 45 ± 20 B | 0.21 ± 0.09 B |
| PPDa (5 μg/ml) | 0.24 ± 0.06 B | 41 ± 20 B | 0.15 ± 0.11 B |
| PPDb (5 μg/ml) | 0.93 ± 0.22 A | 101 ± 24 A | 1.28 ± 0.38 A |
| PWM (1 μg/ml) | 2.91 ± 0.29 | 137 ± 21 | 2.60 ± 0.30 |
Blood mononuclear cells were isolated from cattle 68 days postchallenge. IFN-γ and TNF-α concentrations in culture supernatants were quantified by ELISA, and nitrite concentrations were quantified by the Griess reaction. Values followed by different letters are significantly different (P < 0.05).
Values represent mean (± SEM, n = 20) responses to stimulation (i.e., antigen or PWM) minus the response to medium alone. Responses to PWM are presented to indicate a general responsiveness of the cell population to polyclonal stimulation (i.e., a positive control), and these responses were not compared to the responses of antigen-stimulated cells.
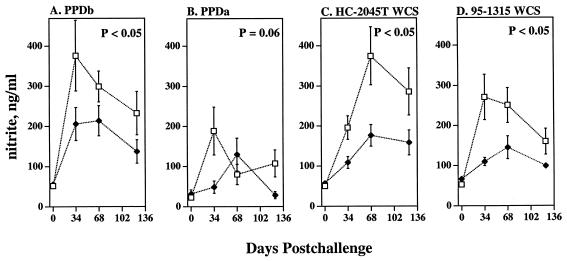
The antigen-specific IFN-γ and TNF-α responses of cattle infected with strain HC-2045T did not differ (P > 0.05, repeated-measure ANOVA; 0 to 124 days postchallenge; n = 10) from those of cattle infected with strain 95-1315. In contrast, nitrite responses to PPDb, PPDa, HC-2045T WCS, and 95-1315 WCS of PBMC from HC-2045T-infected cattle exceeded (P < 0.05) those of 95-1315-infected cattle (Fig. 4). While clear differences in disease severity among animals receiving equivalent doses of the two strains were difficult to determine, it did appear that cattle receiving the HC-2045T strain had slightly more severe cases of the disease than did cattle receiving the 95-1315 strain. Lesions were detected at more sites in cattle receiving 105 CFU of HC-2045T than in cattle receiving 105 CFU of 95-1315, likely impacting the nitrite response.
FIG. 4.
Effects of challenge strain on nitrite responses. Blood mononuclear cells collected immediately before challenge (day 0) and at 0 days, 34 days, 68 days, or 124 days after being challenged with M. bovis strain 95-1315 (closed triangles, n = 10) or M. bovis strain HC-2045T (open boxes, n = 10) were cultured with 5 μg of PPDb per ml, 5 μg of PPDa per ml, 10 μg of M. bovis strain 95-1315 WCS per ml, or 10 μg of M. bovis strain HC-2045T WCS per ml. Nitrate concentrations in 48-h culture supernatants were quantified by the Griess reaction. The P values for the effects of the strains are indicated on each graph.
Both IFN-γ and TNF-α are known to induce NO production (10, 17). Associations among these three responses by cattle PBMC to M. bovis antigens, however, are not clear. To evaluate these associations, differential responses to mycobacterial antigens of all M. bovis-infected cattle (n = 20) at 0 days, 34 days, 68 days, and 124 days postchallenge were evaluated by linear regression analysis (Table 3). In general, strong positive associations between all three readouts (i.e., IFN-γ, nitrite, and TNF-α) were detected with antigen-specific responses. Poor to no associations were detected with these responses to no stimulation or to PWM.
TABLE 3.
Association between IFN-γ, nitrite, and TNF-α responses by blood mononuclear cells from M. bovis-infected cattle to antigens and mitogena
| Stimulant (concn) | Correlation coefficient (r)b
|
||
|---|---|---|---|
| IFN-γ vs nitrite | IFN-γ vs TNF-α | Nitrite vs TNF-α | |
| Medium alone (no stimulant) | 0.36** | NS | NS |
| HC-2045T WCS (10 μg/ml) | 0.54**** | 0.61**** | 0.47**** |
| 95-1315 WCS (10 μg/ml) | 0.67**** | 0.35** | 0.24* |
| PPDb (5 μg/ml) | 0.69**** | 0.73**** | 0.54**** |
| PPDa (5 μg/ml) | 0.46**** | 0.41**** | 0.34** |
| PWM (1 μg/ml) | 0.37*** | NS | 0.24* |
Blood mononuclear cells were isolated from cattle immediately prior to challenge (day 0) and at 34 days, 68 days, and 124 days after challenge and cultured for 48 h in medium alone or medium plus antigen or mitogen. Supernatant IFN-γ and TNF-α concentrations were quantified by ELISA, and nitrite concentrations were quantified by the Griess reaction. NS, not significant.
Asterisks indicate significant correlatoins at a P of < 0.05 (*), < 0.01 (**), < 0.001 (***), or < 0.0001 (****).
In vitro-based cellular immune assays are gaining wide acceptance for use in tuberculosis diagnosis. Of relevance to cattle, an IFN-γ assay (in conjunction with skin testing) was recently approved for use in tuberculosis diagnosis. Other readouts of bovine cellular immune responsiveness (e.g., TNF-α and NO), however, have not been critically analyzed or compared to the IFN-γ response. In the present study, TNF-α and NO responses upon M. bovis infection followed similar kinetics, as did IFN-γ responses (Fig. 3 and 4; Table 1). The relative magnitude of each of these responses to variable antigens was consistent (Tables 1 and 2), and recall IFN-γ, TNF-α, and NO responses to crude M. bovis soluble antigens were clearly associated (Table 3). Thus, evaluation of TNF-α and NO responses, like that of IFN-γ responses, may prove useful for diagnosis of bovine tuberculosis. The nonspecific production of nitrite in PBMC cultures from infected cattle (Fig. 2) may be problematic for development of a useful NO-based diagnostic assay of infection. However, the NO response to M. bovis antigens generally exceeded the response to medium alone, thereby providing antigen specificity (Tables 1 and 2). As with the IFN-γ assay, adaptation of NO and TNF-α assays to a whole-blood format and usage of recombinant antigens will be necessary to enhance the practicality and specificity of these assays.
Acknowledgments
We thank Theresa Rahner, Rebecca Lyon, Jody Mentele, and Ryan Cook for technical assistance and Nate Horman, Larry Wright, Doug Ewing, Don Robinson, Norm Lyon, and Wayne Varland for animal care.
REFERENCES
- 1.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 2.Bolin, C. A., D. L. Whipple, K. V. Khanna, J. M. Risdahl, P. K. Peterson, and T. W. Molitor. 1997. Infection of swine with Mycobacterium bovis as a model of human tuberculosis. J. Infect. Dis. 176:1559-1566. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 4.Buddle B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, J. L., and M. E. Kehrli. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukoc. Biol. 59:90-99. [DOI] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 78:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 10.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 11.Ehlers S., J. Benini, S. Kutsch, R. Endres, E. T. Rietschel, and K. Pfeffer. 1999. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 67:3571-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flesch, I. E. A., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flesch, I. E. A., and S. H. E. Kaufmann. 1990. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect. Immun. 58:2675-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flesch, I., and S. H. Kaufmann. 1987. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:4408-4413. [PubMed] [Google Scholar]
- 16.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 17.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 18.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 20.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massengill C. E. 2002. Report of the committee on tuberculosis. United States Animal Health Association Annual Meeting. Pat Campbell and Associates, Richmond, Va.
- 23.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 24.Nonnecke, B. J., W. R. Waters, M. R. Foote, R. M. Fowler, R. L. Horst, and B. L. Miller. Interferon-γ and TNF-α secretion by mononuclear leukocytes from peripheral blood of young and adult cattle vaccinated with Mycobacterium bovis BCG: modulation by 1,25-dihydroxyvitamin D3. Int. J. Vitam. Nutr. Res., in press. [DOI] [PubMed]
- 25.Ottenhof, T. H., D. Kumararatne, and J. L Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 26.Palmer, M. V., W. R. Waters, and D. L. Whipple. 2003. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 82:275-282. [DOI] [PubMed] [Google Scholar]
- 27.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 28.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 29.Rajaraman, V., B. J. Nonnecke, S. T. Franklin, D. C. Hammell, and R. L. Horst. 1998. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy Sci. 81:3278-3285. [DOI] [PubMed] [Google Scholar]
- 30.Rook, G. A., J. Steele, M. Ainsworth, and B. R. Champion. 1986. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology 59:333-338. [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr, D. J., and M. A. Marletta. 1985. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA 82:7738-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 34.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, C. H., C. Y. Liu, H. C. Lin, C. T. Yu, K. F. Chung, and H. P. Kuo. 1998. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 11:809-815. [DOI] [PubMed] [Google Scholar]
- 37.Wang, C. H., H. C. Lin, C. Y. Liu, K. H. Huang, T. T. Huang, C. T. Yu, and H. P. Kuo. 2001. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int. J. Tuberc. Lung Dis. 5:283-291. [PubMed] [Google Scholar]
- 38.Waters, W. R., B. J. Nonnecke, T. E. Rahner, M. V. Palmer, D. L. Whipple, and R. L. Horst. 2001. Modulation of Mycobacterium bovis-specific responses of bovine peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin. Diagn. Lab. Immunol. 8:1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilcke, J. T., B. N. Jensen, P. Ravn, A. B. Andersen, and K. Haslov. 1996. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber. Lung Dis. 77:250-256. [DOI] [PubMed] [Google Scholar]
- 40.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creepeer, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 41.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]