Abstract
We evaluated industrially prepared Western blot strips designed to avoid the cross-reactions observed with indirect immunofluorescence and enzyme-linked immunosorbent assays used for the serodiagnosis of trichinellosis. The antigen preparations were crude extracts of Trichinella spiralis. The Western blot profile characteristic of trichinellosis was characterized by comparing 60 sera from patients infected by Trichinella to 11 sera from healthy subjects, 51 sera from patients with other proven parasitic diseases (cysticercosis, schistosomiasis, strongyloidosis, fascioliasis, toxocariasis, liver amebiasis, anisakiasis, filariasis, toxoplasmosis, hydatidosis, or malaria), and 23 sera from patients with autoantibodies. Specific 43- to 44-kDa and 64-kDa bands were obtained with all of the sera from 51 patients with acute trichinellosis, in 4 out of 9 patients at the early stages of the disease, and in only 1 control patient, who had suspected anisakiasis and in whom trichinellosis could not be ruled out by muscle biopsy.
Trichinellosis is a widespread zoonosis (12) acquired by ingestion of undercooked meat (e.g., pork and horse meat) containing infective larvae of Trichinella parasites. Most clinical symptoms are nonspecific, consisting mainly of fever, facial edema, and myalgia. These symptoms are associated with biological signs, such as high eosinophil counts and increased muscle enzyme levels (e.g., creatine phosphokinase and aldolase). However, these signs and symptoms mimic those of conditions such as influenza, dermatomyositis, and other parasitoses, such as toxocariasis and distomatosis. Serologic tests are thus important in the diagnosis of trichinellosis (11).
Many antibody assay techniques have been developed (e.g., indirect immunofluorescence [IIF] and enzyme-linked immunosorbent assay [ELISA]), as reviewed by Gomez-Priego et al. (15). However, while these techniques are sensitive, they are also subject to cross-reactions that make the interpretation of weakly positive results difficult. Indeed, such faint reactions can correspond to several clinical situations, such as recently acquired trichinellosis, cross-reaction with parasites other than Trichinella spp., and interference by autoantibodies. In a national survey involving all medical parasitology departments in metropolitan France, 136 of the 4,700 sera tested were found positive by ELISA or IIF, but only 42 corresponded to real cases of trichinellosis (13). Several investigators have reported the usefulness of Western blot analysis for investigating such cross-reactions (9, 30), but no industrial kit was available until recently. The present study describes the development and specificity of industrially produced Western blot strips made with crude Trichinella antigens.
MATERIALS AND METHODS
Patients and sera.
Sixty serum samples from patients with trichinellosis were used to identify specific Trichinella antigens. These samples were from patients who had presented with a clinical suspicion of trichinellosis. The diagnosis of trichinellosis was confirmed for all of these patients by the presence of a high fever associated with myalgia, facial edema, a high level of eosinophilia, and the notion of ingestion of undercooked meat. All of these patients had positive ELISA or IIF results for trichinellosis. The patients were infected at various foci by a variety of Trichinella species: Trichinella spiralis (48 patients), T. britovi (3 patients), T. murrelli (2 patients), T. nativa (1 patient), T. nelsoni (2 patients), and T. pseudospiralis (4 patients). All samples from patients infected by T. spiralis were obtained during a horse meat-related outbreak which occurred in 1998 in southern France (16). Nine samples from these 48 patients infected by T. spiralis were obtained early in the disease; the patients had signs and symptoms suggestive of trichinellosis but negative ELISA results. In subsequent samples, ELISA results were positive. The other samples were from patients infected in other outbreaks reported in France and whose clinical and epidemiological data have been described elsewhere (2, 13, 14, 24, 28).
Sera used to assess cross-reactivity were selected from 11 healthy individuals and 51 patients with other proven parasitic diseases confirmed by blood smears, stool examinations, positive specific serologic or histologic test results, echography, or CT scanning: Taenia solium neurocysticercosis (5 patients), schistosomiasis (7 patients with Schistosoma mansoni infection), fascioliasis (5 patients), filariasis (6 patients), hydatidosis (3 patients), strongyloidosis (4 patients), toxocariasis (11 patients), liver amebiasis (2 patients), anisakiasis (4 patients), toxoplasmosis (2 patients), and malaria (2 patients). Twenty-three sera from patients with autoimmune diseases were used to assess the risk of nonspecific reactions related to systemic disorders, including anti-mitochondrial type 2 antibodies (1 case), anti-stomach cell antibodies (2 cases), rheumatoid factor (10 cases), and antinuclear antibodies (10 cases). All sera were stored at −20°C until use.
ELISA.
All sera (cases and controls) were tested for anti-Trichinella antibodies (immunoglobulin G) by using a commercial ELISA based on excretory or secretory (ES) antigens of Trichinella muscle larvae and peroxidase-labeled protein A (ELISA Trichinella; Biotrin International, Lyon, France). A test was considered positive when the optical density value exceeded 0.3. Negative, low-positive, and high-positive controls included in the kit were tested at each assay. The negative control was from healthy individuals, and the positive controls were from infected rabbits.
Antigens.
Western blot antigens were obtained from purified larvae collected after HCl-pepsin digestion of T. spiralis-infected mouse muscle. The strain (MFEL/FR/93/ISS406) was obtained from a cat infected during a horse meat-related outbreak which occurred in France in 1993. Larvae were washed three times in phosphate-buffered saline and then resuspended in Tris-EDTA buffer, containing 0.25 M sucrose, 1 mM EDTA, 40 mM Tris, and protease inhibitors (leupeptin, 0.5 μg/ml; phenylmethylsulfonyl fluoride, 170 μg/ml; and pepstatin 0.7 μg/ml). The suspension was homogenized at 4°C for 1 h, submitted to three cycles of thawing and freezing in liquid nitrogen, and sonicated (six times for 1 min each time). The crude extract was centrifuged at 100,000 × g for 1 h, and the supernatant was filtered (0.22-μm-pore size) and stored at −80°C. Protein content was quantified with a bicinchoninic acid-based method.
Immunoblotting.
The antigens were delivered to LDBIO Diagnostics (Lyon, France), which produced the Western blot strips. Briefly, a 50-μg antigen solution was electrophoresed in 13% acrylamide slab gels with the discontinuous sodium dodecyl sulfate buffer system described by Laemmli (20), with slight modifications. A mixture of biotinylated and prestained proteins (myosin, β-galactosidase, phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, trypsin inhibitor, lysozyme, and aprotinin) obtained from Bio-Rad Laboratories (Hercules, Calif.) was used as a molecular weight standard. The gels were run until the 30-kDa trypsin inhibitor (prestained blue) reached the bottom of the gels. Proteins were then transferred to nitrocellulose sheets as described by Towbin et al. (31), with slight modifications. The nitrocellulose sheets were coated with Tris-NaCl (pH 7.4) containing 5% nonfat milk. The blots were washed twice with Tris-NaCl, dried, and cut into 4-mm-wide strips.
The Western blot assay was performed with the strips and reagents provided with the kit according to the manufacturer's instructions. Briefly, the strips were incubated with sera diluted 1:50 in Tris-NaCl sample buffer for 90 min. After a washing step with Tris-NaCl washing buffer, the strips were incubated with an anti-human immunoglobulin G- alkaline phosphatase conjugate for 60 min. After another washing step, the protein fractions recognized by the sera were revealed by the corresponding substrate-chromogenic solution containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. The reaction was stopped by washing the strips with distilled water. The strips were dried and glued to paper for reading and storage. Positive and negative controls were tested in each assay.
Repeatability, reproducibility, and interference.
Repeatability and reproducibility were assessed by testing three times in the same assay four sera (three positive and one negative) with the same reagent, by testing the same four sera in four different assays with the same reagent, and by testing two times in the same assay eight sera (six positive and two negative) with reagents from two different batches. Interference was analyzed by testing sera containing hemoglobin (n = 2), lipids (n = 2), or bilirubin (n = 2).
Statistical analysis.
The 95% confidence limits (CL) of the proportion of positive results and the specificity of the test were calculated by a simplified method: CL = {1.96 × √[Pu × (1 − Pu)]}/sample population; in this situation, Pu is the proportion of positive results or the specificity of the test).
RESULTS
Western blot profiles obtained with sera from 60 patients with trichinellosis yielded bands of 37, 38, 39, 43, 44, 46, 49, 64, 96, 110, and 127 kDa. The frequency of each band is reported in Table 1 The 43-, 44-, 64-, 96-, and 127-kDa bands were present in every case, independent of the species of Trichinella (Fig. 1) Two samples from healthy subjects recognized the 127-kDa antigen. No bands were recognized by serum samples from patients with amebiasis, strongyloidosis, hydatidosis, malaria, or toxoplasmosis, while serum samples from patients with Anisakis infection, bilharziasis, cysticercosis, fascioliasis, filariasis, or toxocariasis yielded weak reactions: 20 recognized the 127-kDa band, 16 recognized the 96-kDa band, 10 recognized the 110-kDa band, 4 recognized the 64-kDa band, 2 recognized the 43-kDa band, and 1 each recognized either the 44- or the 46-kDa band. Among the patients with autoimmune disorders, 11 samples recognized the 127-kDa band, 10 recognized the 110-kDa band, and 2 recognized the 43-kDa band. Globally, bands were recognized by 18% (95% CL, 0 to 40) of negative controls, 51% (95% CL, 37.3 to 64.7) of samples from patients with other parasitoses, and 65% (95% CL, 45.5 to 84.5) of samples from patients with autoimmune disorders.
TABLE 1.
Frequencies of different antigenic fractions reacting with sera from patients with Trichinella infections and other health disorders
| Sample source | No. of samples
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Recognizing the following band (kDa):
|
Positive in the ELISA | ||||||||||||
| 37 | 38 | 39 | 43 | 44 | 46 | 49 | 64 | 96 | 110 | 127 | 43-44 + 64 | |||
| Trichinellosis | 51 | 17 | 20 | 18 | 51 | 51 | 30 | 30 | 51 | 51 | 48 | 51 | 51 | 51 |
| Early sera | 9 | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 0 | |||||
| Negative controls | 11 | 2 | 0 | 0 | ||||||||||
| Amebiasis | 2 | 0 | 1 | |||||||||||
| Malaria | 2 | 0 | 0 | |||||||||||
| Toxoplasmosis | 2 | 0 | 0 | |||||||||||
| Strongyloidosis | 4 | 0 | 1 | |||||||||||
| Anisakiasis | 4 | 1 | 1 | 2 | 4 | 1 | 4 | 1 | 2 | |||||
| Bilharziasis | 7 | 1 | 1 | 1 | 3 | 0 | 2 | |||||||
| Cysticercosis | 5 | 1 | 3 | 3 | 2 | 0 | 0 | |||||||
| Distomatosis | 5 | 3 | 1 | 3 | 0 | 5 | ||||||||
| Filariasis | 6 | 1 | 4 | 2 | 4 | 0 | 5 | |||||||
| Hydatidosis | 3 | 0 | 0 | |||||||||||
| Toxocariasis | 11 | 1 | 1 | 2 | 4 | 0 | 4 | |||||||
| Anti-DNA antibody | 10 | 1 | 4 | 4 | 0 | 1 | ||||||||
| Rheumatoid factor | 10 | 1 | 6 | 7 | 0 | 0 | ||||||||
| Antimitochondrial antibody | 1 | 0 | 0 | |||||||||||
| Antistomach antibody | 2 | 0 | 0 | |||||||||||
| Total | 85 | 4 | 1 | 1 | 4 | 16 | 20 | 33 | 1 | 21 | ||||
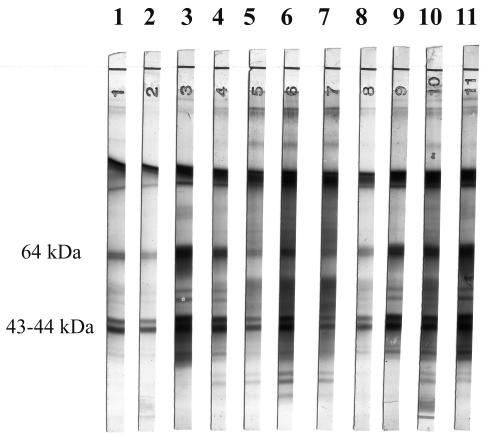
FIG. 1.
Western blot profiles obtained with sera from patients infected by various species of Trichinella: lanes 1 and 2, T. spiralis; lanes 3 and 4, T. nelsoni; lane 5, T. nativa; lanes 6 and 7, T. pseudospiralis; lanes 8 and 9, T. murrelli; and lanes 10 and 11, T. britovi.
These data suggested that the 127-, 110-, and 96-kDa bands, observed in 33, 20, and 16 controls, respectively, had poor specificities. In contrast, the 43- to 44-kDa and 64-kDa bands were reactive with all of the serum samples from the 51 patients with acute trichinellosis but with only 1 of the 85 control serum samples, which was from a patient with a diagnosis of anisakiasis, yielding a 98.8% specificity (95% CL, 96.5 to 100).
Nine sera were collected early in the course of the infection from patients with clinical signs of trichinellosis but with a negative ELISA result. The diagnosis of trichinellosis was confirmed by subsequent positive results in serologic tests performed a few days later. For four of these patients, Western blotting of early sera yielded the 43- to 44-kDa and 64-kDa bands, thus allowing an earlier serologic diagnosis than with the ELISA.
The ELISA yielded no false-positive results with the negative controls, but 4.3% (95% CL, 0 to 12.6%) and 39.2% (95% CL, 25.8 to 52.6) false-positive results were observed with sera from patients with autoimmune disorders and other parasitoses, respectively. The global specificity of the ELISA was 75.3% (95% CL, 66.2 to 84.4). The repeatability and reproducibility of Western blotting were perfect. In addition, no interference was observed with hemoglobin, bilirubin, or lipids.
DISCUSSION
It was previously reported that false-positive IIF reactions can occur in the course of various parasitoses and other disorders and that Western blotting can distinguish true infections from cross-reactions (30). However, our home-made Western blots were largely inferior to industrially prepared blots, as the latter yielded a larger number of sharper bands. The highly complex antigenic composition of the genus Trichinella was confirmed, as we identified at least 11 antigenic fractions. It is difficult to compare our results to the results of previous studies with human sera, owing to the use of different antigenic preparations. Alcantara and Correa (1) detected eight distinctive bands from 38 to 104 kDa in a crude extract of T. spiralis. Thirteen bands ranging from 14 to 66 kDa were identified by Nunez et al. (26). The bands at approximately 55, 36, 29, and 14 kDa proved to be specific for T. spiralis. In a study reported by Chapa-Ruiz et al. (8), the most frequently recognized antigens had molecular masses of 96, 67, 63, 55, and 47 kDa. An antigenic fraction containing peptides of 43 and 47 kDa was isolated by elution from polyacrylamide gel slabs and was used as the antigen in an ELISA in comparison with a total soluble crude extract. This purified fraction improved the diagnostic specificity and sensitivity of the ELISA for human trichinellosis. Mahannop et al. (22) identified specific 94-, 67-, 63-, and 39-kDa components.
Most authors consider that the major antigenic fraction of Trichinella (TSL1) has a molecular mass of 45 kDa (8, 10, 17), and the 43- to 44-kDa doublet identified in our study could well correspond to this fraction. We chose as a specific profile the 43- to 44-kDa doublet associated with the 64-kDa band, as it was observed in 100% of the 51 patients with acute trichinellosis, in 4 out of 9 patients at the early stages of the disease, and in only 1 (1%) of 85 control subjects; this control subject had suspected anisakiasis. On the basis of the results shown in Table 1, one should consider why we did not choose only the 44-kDa band as a sole criterion, as this band resulted in only one false-positive reaction, whereas the 43- and 64-kDa bands resulted in four false-positive reactions. Distinguishing the 44-kDa band from the 43-kDa band was sometimes difficult (particularly when there was a high titer of antibodies); therefore, recognizing a doublet was easier. We added to the pattern the 64-kDa band because it was present in all samples from confirmed cases, because it did not decrease the specificity, and because we predicted that adding a third band could decrease the risk of false-positive results. In contrast, the ELISA based on ES antigens was positive for 21 of the 85 control subjects (24.7%; 95% CL, 15.6 to 33.8). Therefore, as previously reported by others, the performance of commercial ELISA kits should be improved by the use of purified antigens (8, 18, 22).
Cross-reactive antigens from different species of helminths have been known since the initial studies of Capron et al. (6) and explain the similar antigenic bands observed in our study with sera from patients infected by different helminth species (Table 1). More specifically, shared antigens have been described for Trichinella and several other parasitic worms, including nematodes (Trichuris spp.) and trematodes (Fasciola hepatica and Schistosoma mansoni or S. mekongi) (4, 7, 18, 21, 29). Mahannop et al. (23) reported cross-reactions of crude antigens prepared from T. spiralis larvae with sera from patients with capillariasis, gnathostomiasis, opisthorchiasis, strongyloidiasis, and hookworm infection. Cross-reactions have also been observed with sera from patients with angiostrongyliasis (25). Linder et al. (21) reported that anti-gp50 monoclonal antibodies against schistosomes and anti-gp50-positive sera from patients with schistosomiasis reacted with hypodermis and Trichinella stichocytes. However, in our study with sera from other parasitoses, only one patient, with suspected anisakiasis (chronic abdominal pain, notion of ingestion of undercooked fish, and IIF positivity with frozen Anisakis sections), exhibited the 43- to 44-kDa doublet and the 64-kDa band. However, a muscle biopsy, which could have confirmed the diagnosis of trichinellosis, was not performed for ethical reasons. Of note, this patient developed primary biliary cirrhosis a few months later. Bands were also detected with sera from patients with autoimmune disorders (anti-DNA antibodies or rheumatoid factor). Such false-positive results in serologic tests for Trichinella in patients with connective tissue diseases have already been reported (30). However, none of the patients with autoimmune disorders displayed both the 43- to 44-kDa doublet and the 64-kDa band. In contrast, both bands were found in all of the Trichinella-infected patients, regardless of the Trichinella species involved. Other bands showed slight species differences, but these bands could not be considered species specific, owing to the small number of sera tested.
The genus Trichinella is now composed of 11 species or genotypes, namely, T. spiralis, T. nativa, T. britovi, T. pseudospiralis, T. murrelli, T. nelsoni, T6, T8, T9, T. papuae, and T. zimbabwensis. None of our samples was from patients infected by the latter five species or genotypes, which have never been isolated from humans and are localized to very small areas. Dupouy-Camet et al. (10) and other investigators have observed differences in Western blot profiles of sera from humans and animals infected by the same species. Pozio et al. (27) described antigenic differences between T. britovi and T. spiralis assessed with human sera. Bolas-Fernandez and Wakelin (5) found differences in T. spiralis, T. pseudospiralis, and T. murrelli by using mouse sera. However, Kapel et al. tested sera from pigs infected by T. spiralis, T. nativa, and T. britovi and did not find any such differences (19). Additional work is needed in order to identify from Western blot patterns the species involved in an infection. Finally, it is important to note that the Western blot kit tested here detected specific anti-Trichinella antibodies in patients who had recently been infected before an ELISA based on ES antigens.
In conclusion, Western blotting is a useful tool for the differential diagnosis of trichinellosis, a disease that necessitates specific treatment. It can be used to investigate ELISA and IIF cross-reactions, which can be observed with certain connective tissue diseases or helminth infections that can mimic the clinical manifestations of trichinellosis. Importantly, compared to the ELISA, Western blotting could be suitable for early diagnosis, thus allowing early treatment, which is known to reduce the complications of trichinellosis (11). The kit tested here is now commercially available, and its sensitivity and specificity have been compared with those of other serologic methods (3). The value of this kit during outbreaks remains to be determined.
Acknowledgments
We thank Jean François Magnaval and Paulette Recco (Toulouse, France), Martine Gari-Toussaint (Nice, France), Bernard Faugères (Marseille, France), Sandrine Houzé and Jean Pierre Nozais (Paris, France), and Sun Xin (Bengbu, China) for providing some of the sera used in this study. We thank David Young for revising the manuscript.
This work was supported by ADERMEPT and the Centre National de Référence des Trichinella.
REFERENCES
- 1.Alcantara, P., and D. Correa. 1993. Human humoral immune responses against Trichinella spiralis. Int. J. Parasitol. 23:657-660. [DOI] [PubMed] [Google Scholar]
- 2.Ancelle, T., J. Dupouy-Camet, M. E. Bougnoux, V. Fourestie, H. Petit, G. Mougeot, J. P. Nozais, and J. Lapierre. 1988. Two outbreaks of trichinosis caused by horsemeat in France in 1985. Am. J. Epidemiol. 127:1302-1311. [DOI] [PubMed] [Google Scholar]
- 3.Andiva, S., H. Yera, S. Haeghebaert, C. Tourte-Schaefer, J. F. Magnaval, and J. Dupouy-Camet. 2002. Comparative evaluation of a latex agglutination test, two ELISA tests and a Western blot test for the serodiagnosis of human trichinellosis. Ann. Biol. Clin. (Paris) 60:79-83. [PubMed] [Google Scholar]
- 4.Aronstein, W. S., S. A. Lewis, A. P. Norden, J. P. Dalton, and M. Strand. 1986. Molecular identity of a major antigen of Schistosoma mansoni which cross-reacts with Trichinella spiralis and Fasciola hepatica. Parasitology 92:133-151. [DOI] [PubMed] [Google Scholar]
- 5.Bolas-Fernandez, F., and D. Wakelin. 1990. Infectivity, antigenicity and host responses to isolates of the genus Trichinella. Parasitology 100:491-497. [DOI] [PubMed] [Google Scholar]
- 6.Capron, A., J. Biguet, A. Vernes, and D. Afchain. 1968. Structure antigénique des helminthes: aspects immunologiques des relations hôte-parasite. Pathol. Biol. 16:121-138. [PubMed] [Google Scholar]
- 7.Chan, S. W., and R. C. Ko. 1988. Comparison between standard ELISA and dot-ELISA for serodiagnosis of human trichinosis. Trans. R. Soc. Trop. Med. Hyg. 82:892-894. [DOI] [PubMed] [Google Scholar]
- 8.Chapa-Ruiz, M. R., M. R. Salinas-Tobon, D. J. Aguilar-Alvarez, and R. Martinez-Maranon. 1992. Recognition of Trichinella spiralis muscle larvae antigens by sera from human infected with this parasite and its potential use in diagnosis. Rev. Latinoam. Microbiol. 34:95-99. [PubMed] [Google Scholar]
- 9.De-la-Rosa, J. L., P. Alcantara, and D. Correa. 1995. Investigation of cross-reactions against Trichinella spiralis antigens by enzyme-linked immunosorbent assay and enzyme-linked immunoelectrotransfer blot assay in patients with various diseases. Clin. Diagn. Lab. Immunol. 2:122-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupouy-Camet, J., M. E. Bougnoux, T. Ancelle, R. Fagard, and J. Lapierre. 1988. Antigenic characteristics of two strains of Trichinella spiralis isolated during the horsemeat-related outbreaks of 1985 in France. Parasitol. Res. 75:79-80. [DOI] [PubMed] [Google Scholar]
- 11.Dupouy-Camet, J., W. Kociecka, F. Bruschi, F. Bolas-Fernandez, and E. Pozio. 2002. Opinion on the diagnosis and treatment of human trichinellosis. Exp. Opin. Pharmacother. 3:1117-1130. [DOI] [PubMed] [Google Scholar]
- 12.Dupouy-Camet, J. 2000. Trichinellosis: a worldwide zoonosis. Vet. Parasitol. 93:191-200. [DOI] [PubMed] [Google Scholar]
- 13.Dupouy-Camet, J., S. Allegretti, and T. P. Truong. 1998. Enquête sur l'incidence de la trichinellose en France (1994-1995). Bull. Epidemiol. Hebdo. 28:122-123. [Google Scholar]
- 14.Gari-Toussaint, M., E. Bernard, and J. F. Quaranta. 1994. First report in France of an outbreak of human trichinellosis due to Trichinella britovi, p. 465-468. In W. C. Campbell, E. Pozio, and F. Bruschi (ed.), Trichinellosis. Istituto Superiore di Sanita Press, Rome, Italy.
- 15.Gomez-Priego, A., L. Crecencio-Rosales, and J. L. De La Rosa. 2000. Serological evaluation of thin-layer immunoassay-enzyme-linked immunosorbent assay for antibody detection in human trichinellosis. Clin. Diagn. Lab. Immunol. 7:810-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeghebaert, S., M. Servat, C. Duchen, J. C. Minet, A. E. Agrech, I. Thiese, C. Leclerc, V. Vaillant, C. Hemery, E. Maillot, C. Soulé, E. Pozio, P. Massip, J. F. Magnaval, and J. C. Desenclos. 1998. Epidémie de trichinellose région midi-Pyrénées, Janvier-Mars 1998. Bull. Epidemiol. Hebdo. 28:121-122. [Google Scholar]
- 17.Homan, W. L., A. C. Derksen, and F. van Knapen. 1992. Identification of diagnostic antigens from Trichinella spiralis. Parasitol. Res. 78:112-119. [DOI] [PubMed] [Google Scholar]
- 18.Ittiprasert, W., P. Butraporn, V. Kitikoon, K. Klongkamnuankarn, K. Pholsena, V. Vanisaveth, Y. Sakolvaree, M. Chongsa-Nguan, P. Tapchaisri, Y. Mahakunkijcharoen, H. Kurazono, H. Hayashi, and W. Chaicumpa. 2000. Differential diagnosis of schistosomiasis mekongi and trichinellosis in human. Parasitol. Int. 49:209-218. [DOI] [PubMed] [Google Scholar]
- 19.Kapel, C. M. O., P. Webster, P. Lind, E. Pozio, S. A. Henriksen, K. D. A. Murrell, and P. Nansen. 1998. Trichinella spiralis, T. britovi, T. nativa: infectivity, larval distribution in muscle and antibody response after experimental infection of pigs. Parasitol. Res. 84:264-271. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Linder, E., C. Thors, L. Lundin, I. Ljungstrom, S. Farah, H. Hagi, and F. Dias. 1992. Schistosome antigen gp50 is responsible for serological cross-reactivity with Trichinella spiralis. J. Parasitol. 78:999-1005. [PubMed] [Google Scholar]
- 22.Mahannop, P., W. Chaicumpa, P. Setasuban, N. Morakote, and P. Tapchaisri. 1992. Immunodiagnosis of human trichinellosis using excretory-secretory (ES) antigen. J. Helminthol. 66:297-304. [DOI] [PubMed] [Google Scholar]
- 23.Mahannop, P., P. Setasuban, N. Morakote, P. Tapchaisri, and W. Chaicumpa. 1995. Immunodiagnosis of human trichinellosis and identification of specific antigen for Trichinella spiralis. Int. J. Parasitol. 25:87-94. [DOI] [PubMed] [Google Scholar]
- 24.Nozais, J. P., V. Mannevy, and M. Danis. 1996. Deux cas de trichinose après ingestion de viande d'ours blanc au Groenland. Med. Mal. Infect. 26:732-733. [Google Scholar]
- 25.Nuamtanong, S. 1996. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J. Trop. Med. Public Health 27:291-296. [PubMed] [Google Scholar]
- 26.Nunez, G. G., S. L. Malmassari, S. N. Costantino, and S. M. Venturiello. 2000. Immunoelectrotransfer blot assay in acute and chronic human trichinellosis. J. Parasitol. 86:1121-1124. [DOI] [PubMed] [Google Scholar]
- 27.Pozio, E., P. Varese, M. A. Morales, G. P. Croppo, D. Pelliccia, and F. Bruschi. 1993. Comparison of human trichinellosis caused by Trichinella spiralis and by Trichinella britovi. Am. J. Trop. Med. Hyg. 48:568-575. [DOI] [PubMed] [Google Scholar]
- 28.Ranque, S., B. Faugere, E. Pozio, G. La Rosa, A. Tamburrini, J. F. Pellissier, and P. Brouqui. 2000. Trichinella pseudospiralis outbreak in France. Emerg. Infect. Dis. 6:543-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roach, T. I., D. Wakelin, K. J. Else, and D. A. Bundy. 1988. Antigenic cross-reactivity between the human whipworm, Trichuris trichiura, and the mouse trichuroids Trichuris muris and Trichinella spiralis. Parasite Immunol. 10:279-291. [DOI] [PubMed] [Google Scholar]
- 30.Robert, F., B. Weil, N. Kassis, and J. Dupouy-Camet. 1996. Investigation of immunofluorescence cross-reactions against Trichinella spiralis by Western blot (immunoblot) analysis. Clin. Diagn. Lab. Immunol. 3:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]