Abstract
We describe a fluorescent covalent microsphere immunoassay (FCMIA) method for the simultaneous (multiplexed) measurement of immunoglobulin G (IgG) antibodies to 23 pneumococcal capsular polysaccharide (PnPS) serotypes present in the pneumococcal polysaccharide vaccine (PPV23) licensed by the Food and Drug Administration, i.e., PnPSs 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. In addition, the assay incorporates an internal control that allows for contemporaneous evaluation of the effectiveness of pneumococcal cell wall polysaccharide (C-PS) preadsorption and a second control of PnPS 25 (which is not present in any polysaccharide or conjugate vaccine), which can be used to evaluate interassay reproducibility (useful for pre- versus postvaccination studies). The FCMIA was standardized with U.S. reference antipneumococcal serotype standard serum 89S-2. Preadsorption of 89S-2 with each PnPS and C-PS yielded homologous inhibition for serotypes 1, 6B, 9N, 9V, 11A, 12F,14, 15B, 18C, 19A, 19F, 20, 22F, 25, and 33F; heterologous inhibition for serotypes 9V, 10A, 11A, 12F, 15B, 17F, 20, and 23F; and neither homologous nor heterologous inhibition for serotypes 2, 3, 4, and 5. The minimum detectable concentrations for the 24 multiplexed (PnPS and C-PS) FCMIAs ranged from 20 pg/ml for PnPS 3 to 600 pg/ml for PnPS 14. The PnPS FCMIA method has numerous benefits over enzyme-linked immunosorbent assays commonly used to measure anti-PnPS-specific IgG levels, including increased speed, smaller sample volumes, equivalent or better sensitivity, and increased dynamic range.
Two pneumococcal vaccines are available in the United States: the pneumococcal polysaccharide vaccine (PPV) PNEUMOVAX 23 (PPV23; Merck, West Point, Pa.), a 23-valent vaccine (containing pneumococcal capsular polysaccharide [PnPS] serotypes 1, 2, 3, 4, 5, 6B, 7, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F), and Prevnar (Wyeth, Philadelphia, Pa.), a 7-valent protein conjugate vaccine containing PnPSs 4, 6B, 9V, 14, 18C, 19F, and 23F and a nontoxic variant of diphtheria toxin (diphtheria CRM197 protein). Prevnar is designed primarily for use in infants, whose antibody response to most capsular polysaccharide serotypes is generally poor. More than 80% of healthy adults who receive PPV23 develop antibodies against the serotypes contained in the vaccine, usually within 2 to 3 weeks after vaccination. Older adults and persons with some chronic illnesses or immunodeficiency may not respond as well, if at all. Elevated antibody levels persist for at least 5 years in healthy adults but fall more quickly in persons with certain underlying illnesses (3).
Enzyme-linked immunosorbent assay (ELISA) is the method most often used for measuring anti-PnPS serotype antibody concentrations (4, 5, 14, 16, 17). ELISAs have been used to measure a plethora of analytes; however, each ELISA is directed against a single analyte. Measuring antibodies against all 23 PnPS serotypes present in PPV23 necessitates performing at least 23 individual ELISAs, as the dynamic ranges of the assays are such that repeat testing at different dilutions is usually necessary. In ELISAs, in order to quantitate serotype-specific immunoglobulin G (IgG), test serum is routinely preadsorbed with exogenous soluble pneumococcal cell wall polysaccharide (C-PS) and PnPS 22F (5). Purified PnPSs contain approximately 5% (by weight) covalently bound contaminating C-PS (20). Children and adults have naturally acquired antibodies to C-PS (7, 21), which are not opsonic and do not protect against pneumococcal infection (9, 10, 21). C-PS preadsorption reduces the non-serotype-specific binding present in nonpreadsorbed serum, allowing for more accurate measurement of PnPS-specific antibodies by ELISA (4, 5). Heterologous PnPS 22F is added to test serum in ELISAs to adsorb the antibodies to common epitopes in addition to those removed by C-PS preadsorption (5). In the present work we describe an assay that can be used to measure antibodies to the 23 different serotypes present in PPV23 simultaneously, and we also describe a method to evaluate C-PS preadsorption efficiency. In addition, the assay includes another control for intra-assay variability. This becomes important when performing pre- versus postvaccination anti-serotype IgG measurements. The sample requirements of the method are such that heel or finger punctures could be used to draw blood samples, or data could be obtained from eluted blood spots (1). This sample collection method is desirable compared to conventional phlebotomy when taking samples from infants and children.
MATERIALS AND METHODS
PnPSs.
Twenty-four purified PnPS serotypes, i.e., 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 25, and 33F, were purchased from the American Type Culture Collection (Manassas, Va.). C-PS was obtained from Staten Seruminstitut (Copenhagen, Denmark). Stock aliquots of each PnPS serotype (1 mg/ml) and C-PS (1 mg/ml) were prepared in 10 mM phosphate-buffered saline, pH 7.4 (PBS) (Sigma Chemical Co., St. Louis, Mo.).
Serum standard.
U.S. reference antipneumococcal serotype standard serum 89S-2 was provided by Carl Frasch, Center for Biologics Evaluation and Research, Food and Drug Administration (Bethesda, Md.). This reference antiserum was prepared from 17 adult donors immunized with a 23-valent PPV. The protocol and human serum samples used in these investigations were reviewed by the Human Subjects Review Board of the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC), and were determined to be exempt due to the anonymity of the samples. All chemicals used were reagent grade or the highest grade commercially available.
Microspheres.
Microspheres (xMAP; Luminex Corporation, Austin, Tex.) were 5.6 μm in diameter and composed of polystyrene, divinyl benzene, and methacrylic acid, which provided surface carboxylate functionality for covalent attachment of biomolecules. Internally, the microspheres were dyed with red- and infrared-emitting fluorochromes. By adjusting the concentrations of each fluorochrome, spectrally addressable microsphere sets were obtained. When the microsphere sets were mixed and analyzed with the Luminex100 instrument (Luminex), each set was identified and classified by a distinct fluorescence signature pattern. In this study, 25 microsphere sets were used for covalent coupling of PnPSs and C-PS. Coupling of each PnPS to microspheres required the oxidation of each polysaccharide and chemical modification of carboxyl functional groups on the microsphere surface to amine groups (Fig. 1). In separate 1.5-ml microcentrifuge tubes (Fisher Scientific, Pittsburgh, Pa.), 300 μl of a 1,000-μg/ml solution of each PnPS serotype and C-PS were treated with 65.5 μl of 10 mM sodium periodate (NaIO4) for 30 min at room temperature in the dark. The reactions were quenched with glycerol (Sigma), and the mixtures were dialyzed against PBS overnight at 4°C with 10,000-molecular-weight-cutoff dialysis cassettes (Pierce, Rockford, Ill.). The carboxyl functional groups on microsphere surfaces were modified by using adipic acid dihydrazide (ADH) (Aldrich, Milwaukee, Wis.). Into separate 1.5-ml microcentrifuge tubes (Fisher Scientific), 1.25 × 107 microspheres from each microsphere set were added. The microspheres were washed by adding 500 μl of 100 mM 2-(N-morpholino)-ethanesulfonic acid (MES) (pH 6.0) (Sigma), microcentrifuging at 10,000 × g for 1 min at room temperature, and aspirating the supernatant. The microsphere pellet was resuspended (all resuspensions were performed by sonication [minisonicator; Cole Parmer, Vernon Hills, Ill.] and gentle vortexing [VWR International, West Chester, Pa.]) in 1 ml of ADH (35 mg/ml in 100 mM MES [pH 6.0]) and 200 μl of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) (200 mg/ml in 100 mM MES [pH 6.0]) (Pierce). The tubes were rotated (Labquake; Barnstead/Thermolyne, Dubuque, Iowa) for 1 h at room temperature in the dark. The microspheres were then washed twice with 1 ml of 100 mM MES (pH 4.5) (Sigma) by microcentrifugation as described above, and the supernatants were discarded. Five hundred microliters of each dialysate was added to the pellet of ADH-modified microspheres, and the microsphere-dialysate mixture was incubated for 4 h at room temperature with rotation. The microspheres were again washed twice by microcentrifugation with PBS-Tween (PBS-T) (10 mM PBS, 0.05% Tween 20 [pH 7.4]) (Sigma) to remove any noncovalently bound polysaccharides and then blocked with blocking buffer (10 mM PBS, 1% bovine serum albumin, 0.05% NaN3) (Sigma). The concentration of each PnPS-coupled microsphere set was determined with a hemacytometer, and the microspheres were stored at 4°C in the dark. The 25-plex mixture, a pool of the 25 microsphere sets (23 PPV PnPSs, C-PS, and PnPS 25), was made where each set was at a concentration of approximately 105 microspheres/ml.
FIG. 1.
Periodate oxidation of PnPS (A), conjugation with ADH (B), and covalent coupling of PnPS to microsphere (C).
25-plex PnPS assay.
Fifty microliters of the 25-plex microsphere mixture were added to the wells of a 1.2-μm-pore-size filter membrane microtiter plate (catalog no. MABVN1250; Millipore Corp., Bedford, Mass.) and liquid aspirated by use of a vacuum manifold filtration system (catalog no. MAVM09601; Millipore). Standards, prepared from serum 89S-2, were diluted in human serum depleted of IgG, IgA, and IgM (GAM) (catalog no. S5393; Sigma) containing 100 μg of C-PS per ml, yielding final dilutions of 1:100, 1:316, 1:1,000, 1:3,160, 1:10,000, 1:31,600, 1:100,000, and 1:316,000. Standard curves were also prepared in blocking buffer and GAM with and without C-PS. Standards were diluted in GAM in order to investigate whether dilution with blocking buffer had an effect on the median fluorescence intensity (MFI) responses of the standard curves. There was no significant difference (P > 0.05) in MFI values when either GAM or blocking buffer was used as a diluent (data not shown). The diluted serum or diluent controls were added to the 24- or 25-plex microspheres (depending on whether the effect of C-PS was being investigated) in the wells of a 1.2-μm-pore-size filter membrane microtiter plate (catalog no. MABVN1250; Millipore) and left for 30 min at 37°C with shaking, and the liquid was aspirated by use of a vacuum manifold filtration system (catalog no. MAVM09601; Millipore). The microspheres were then washed three times with 200 μl of PBS-T; each wash followed by vacuum aspiration. Fifty microliters of R-phycoerythrin-conjugated anti-human IgG (clone HP6043, IgG2b; Biotrend International, Destin, Fla.) (1 μg/ml in blocking buffer) were added to the wells of the plate and incubated for 30 min at 37°C with shaking. After another PBS-T wash, the microspheres were resuspended in 100 μl of PBS-T. The Luminex100 system (Luminex) was programmed to inject 50 μl of the sample volume into the sample port at a rate of 60 μl/min to collect a minimum of 100 microspheres per set. An accessory X-Y (Luminex XYP) plate sampler was utilized to allow automated data collection and analysis directly from the 96-well plate. Acquisition software (Luminex version 2.1) was used to collect data. To investigate the effect of C-PS on serotype-specific binding to the 24 PnPS and C-PS microspheres, experiments were performed with serum diluted 1:100 in GAM with and without C-PS preadsorption. To assess specificity and antibody cross-reactivity, each PnPS was added singly as a competitor to different wells containing the 24-PnPS microsphere mix and serum 89S-2 added at a 1:100 dilution. Specifically, 25 μl of an 800-μg/ml solution of each PnPS was pipetted into a separate well containing the microsphere mix, and then 25 μl of a 1:50 dilution of serum 89S-2 which had been preincubated with 200 μg of C-PS per ml was added. This resulted in final concentrations of 400 μg/ml for competitor PnPS and 100 μg/ml for C-PS with a 1:100 dilution of serum 89S-2. After preadsorption for 1 h at 37°C, the multiplex assay was performed as described above. Positive inhibition was defined as an inhibited MFI less than or equal to the mean of the MFIs for all 24 uninhibited PnPSs minus 2 standard deviations (SD).
Data Analyses.
A four parameter logistic model (4-PL model) was used to fit the relationship between MFI and anti-PnPS IgG concentrations (11, 15) (SigmaPlot; SPSS, Inc., Chicago, Ill.). Duplicate results from individual samples were averaged. The minimum detectable concentrations (MDCs) for the 24 anti-PnPS and C-PS IgG multiplexed fluorescent covalent microsphere immunoassay (FCMIA) standard curves were calculated from the intersection of the asymptote of the regression's 95% confidence interval with the 4-PL fit of the standards data (11, 18). Statistical tests were performed with SigmaStat (SPSS). Nonparametric tests were used throughout, as no assumptions regarding the distribution of the data were made. P values of ≤0.05 were considered statistically significant.
RESULTS
Standard curve analysis.
Antibody concentrations for all 23 PnPSs and C-PS have been assigned to reference serum 89S-2 by using ELISA methods (4, 16, 17). The anti-PnPS 25 content of serum 89S-2 has not been elucidated, so we used the dilution of serum 89S-2 to develop a standard curve. These values were used to standardize the PnPS FCMIAs. A 4-PL model was used to fit the FCMIA data, as it has been shown to be superior to log-log and other fits for immunoassays, even when coefficients of determination (r2 values) are high (>0.97), extending the range of the assay and thus providing a more precise measurement of antibody concentration (15). Results for 24 individual PnPS standard curves versus anti-PnPS antibody levels in serum 89S-2 are shown in Fig. 2. The result of a plot of MFI versus the dilution of serum 89S-2 for PnPS 25 is shown in Fig. 3. The mean r2 value (± SD) for the 24 PnPS 4-PL fits was 0.994 ± 0.003 (P < 0.001), suggesting an excellent fit to the 4-PL model for all standard curves. The mean interassay coefficient of variation (CV) (± SD) for the parameters of the 4-PL fit of the 23 PnPS standard curves obtained from two independent preparations of PnPS microspheres was 4.9% ± 1.2%. For PnPS 25, the interassay CV (from two independent microsphere preparations) for the prediction of dilutions was 13.2%. Intra-assay CVs were less than the interassay CVs. Linear regressions of plots of predicted results from 4-PL fits versus expected concentrations from PnPS standard and C-PS dilutions (1:100 to 1:316,000) yielded mean linear r2 values (± SD) of 0.987 ± 0.006, suggesting linearity on dilution across the dynamic range of the assays. When the MDCs for the 25 multiplexed FCMIAs were evaluated, they were observed to range from 20 pg/ml for PnPS 3 to 600 pg/ml for PnPS 14 (Table 1).
FIG. 2.
4-PL logistic fit of 24 anti-PnPS and anti-C-PS IgG concentrations in U.S. reference pneumococcal antiserum 89S-2 versus MFI measured by FCMIA. Standard curves were prepared after serum preadsorption with 100 μg of C-PS per ml. The C-PS standard curve was obtained from a 25-plex standard curve run without serum preadsorption with C-PS.
FIG. 3.
4-PL logistic fit of anti-PnPS 25 MFI versus dilutions of serum 89S-2 measured by FCMIA.
TABLE 1.
MDCs for 23 anti-PnPS IgGs, interpolated from the intersection of the lower asymptote of the upper 95% confidence interval of the 4-PL logistic fit with the 4-PL predicted fit line, and for C-PSa
| PnPS or C-PS | MDC (pg/ml) |
|---|---|
| 1 | 300 |
| 2 | 200 |
| 3 | 20 |
| 4 | 200 |
| 5 | 200 |
| 6B | 500 |
| 7F | 200 |
| 8 | 200 |
| 9N | 200 |
| 9V | 200 |
| 10A | 200 |
| 11A | 200 |
| 12F | 50 |
| 14 | 600 |
| 15B | 300 |
| 17F | 300 |
| 18C | 300 |
| 19A | 400 |
| 19F | 200 |
| 20 | 300 |
| 22F | 300 |
| 23F | 200 |
| 33F | 300 |
| C-PS | 500 |
The C-PS MDC was obtained from a 25-plex standard curve run without serum preadsorption with C-PS.
C-PS preadsorption.
The effect of C-PS on the levels of anti-PnPS-specific IgGs in serum 89S-2 were compared before and after C-PS preadsorption by using a 25-plex microsphere mixture. The mean (± SD) reduction in anti-PnPS IgG concentrations by C-PS preadsorption for all 24 PnPSs was 20.2 ± 8.9%, while the anti-C-PS-specific IgG concentration was decreased by 82.3% (Fig. 4).
FIG. 4.
C-PS inhibition of multiplexed 24 anti-PnPS and C-PS IgGs (serum 89S-2). Assays were performed with and without C-PS preadsorption (100 μg/ml), and the results were compared. Inhibition is expressed as the percent reduction in MFI for each analyte when assays with and without C-PS preadsorption were compared.
Cross-reactivity and inhibition.
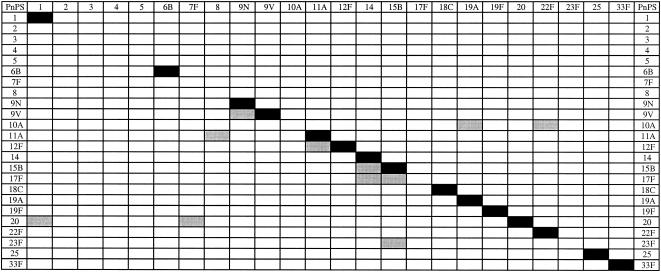
To assess the immunological specificities of the individual PnPS assays, we preadsorbed serum 89S-2 with 400 μg of each individual PnPS and ran the 24-plex PnPS assay. Using the criterion for inhibition of an inhibited MFI that was less than mean nonpreadsorbed MFI minus 2 SD for each PnPS, homologous inhibition was observed for serotypes 1, 6B, 9N, 9V, 11A, 12F,14, 15B, 18C, 19A, 19F, 20, 22F, 25, and 33F; heterologous inhibition was observed for serotypes 9V, 10A, 11A, 12F, 15B, 17F, 20, and 23F; and neither homologous nor heterologous inhibition was observed for serotypes 2, 3, 4, and 5 (Fig. 5).
FIG. 5.
Anti-PnPS IgG inhibition matrix for serum 89S-2. Dark and light shadings represent homologous and heterologous inhibition, respectively, by a specific PnPS according to the criterion described in the text.
DISCUSSION
The benefits of FCMIA technology over ELISA methodology for the measurement of PnPS as well as other antigens have been recently reviewed (2, 12, 13). Those investigators cited speed, the ability to multiplex, the ability to measure all antibody concentrations for all analytes with minimum sample dilutions, more desirable reaction kinetics of the liquid phase, and enhanced dynamic range as benefits of FCMIA versus ELISA. PnPS ELISAs are linear over a 10- to 12-fold dilution range (4, 8, 12), while the FCMIA presented here is linear over a 1:100 to 1:316,000 concentration range for all 24 PnPs and C-PS. In order to evaluate all possible component interactions and cross-reactivities of antibody responses from vaccines, analyses of all vaccine components are desirable (13). In the present 25-plex PnPS FCMIA, we measured all 23 components of PPV23 simultaneously as well as C-PS and a nonvaccine PnPS. Preadsorption of serum with C-PS allows for the measurement of PnPS-specific antibodies without the contribution of anti-C-PS antibodies (4, 5), which is desirable if accurate PnPS levels are to be measured. To measure the contribution of anti-C-PS-specific antibodies to observed anti-PnPS-specific antibody concentrations, we compared concentrations of anti-PnPS IgG and anti-C-PS IgG with and without C-PS preadsorption. Contemporaneous evaluation of C-PS antibody levels allows for the evaluation of the effectiveness of C-PS pretreatment. Individual serum samples differ in the levels of nonserotype antibodies (19) contained in them and would be expected to require different levels of inhibitors for maximum accuracy in the measurement of serotype-specific antibodies. Measuring anti-C-PS levels by ELISA with every serum tested would be overly tedious, so the effectiveness of C-PS preadsorption is assumed. The measurement of the effect of C-PS (or other inhibitor pretreatment) on anti-PnPS antibody levels with the FCMIA is trivial, necessitating the addition of one serum sample which was not preadsorbed with inhibitor. All PnPS serotype IgG levels were inhibited by C-PS preadsorption (mean, ∼20%), while the measurement of anti-C-PS IgG was inhibited by over 80% compared to nonpreadsorbed values (Fig. 4). This contemporaneous internal control would be difficult, if not impossible, to perform with ELISA methods and yields information on the specificity and accuracy of measured anti-PnPS antibody concentrations obtained with the FCMIA.
The results of our cross-inhibition studies showed homologous inhibition for serotypes 1, 6B, 9N, 9V, 11A, 12F,14, 15B, 18C, 19A, 19F, 20, 22F, 25, and 33F. We also observed heterologous inhibition with serotypes 9V, 10A, 11A, 12F, 15B, 17F, 20, and 23F. Neither homologous nor heterologous inhibition was observed for serotypes 2, 3, 4, and 5. Heterologous inhibition for numerous PnPS specificities has been demonstrated in C-PS-preadsorbed sera, both pre- and postvaccination (16). Preadsorption with PnPS 22F in addition to C-PS did increase the correlation between IgG ELISA results and the results of an opsonophagocytosis assay (5). These results suggest that PnPS 22F and C-PS should be utilized for test serum preadsorption and suggest the presence of a common epitope in addition to C-PS shared among several PnPS types and that antibodies to this common epitope are not absorbed by soluble C-PS but are removed by use of PnPS 22F as a second adsorbent. By convention, PnPS 22F preadsorption is not used in calibrating PnPS standard curves with reference sera.
The periodate oxidation and ADH coupling that we used to attach the PnPSs to the microspheres had the potential to modify serotype epitopes. In cross-inhibition studies we observed serotype-specific inhibition for all serotypes when pre- versus postadsorption antibody levels were compared. Fifteen of 24 serotypes exhibited positive homologous inhibition by our inhibition criteria. These data argue for the conservation of those PnPS epitopes during the periodate oxidation and ADH coupling procedures. However, for the PnPS serotypes which did not show homologous inhibition, it may be useful to titrate the level of periodate oxidation to minimize potential modification of epitopes and to preserve greater antigenicity of the polysaccharides.
Comparison of pre- versus postvaccination serotype-specific antibody levels by ELISA is commonly used to evaluate the effectiveness of PPV vaccination (4, 5, 16). Unless these anti-PnPS antibody levels are measured in the same assay, there is the possibility of inter- and intra-assay, random and nonrandom errors leading to confounding results. A method to minimize these potential confounders is to measure and compare antibody responses to a nonvaccine serotype in addition to serotypes present in a vaccine. PnPS 25 is not part of any present polysaccharide or conjugate vaccine, and therefore levels of antibody to it would not be expected change after treatment with vaccine preparations not containing PnPS 25. Serum 89S-2 contains anti-PnPS 25 IgG antibodies (Fig. 4), which do not appear to cross-react with other serotypes (Fig. 5) although it appears to contain contaminating C-PS (Fig. 4). The interassay CV for the PnPS 25 FCMIA was 13.8% (n = 2 separate microsphere preparations), a level within the guidelines for acceptable interassay precision (6). In general, these findings suggest that PnPS 25 may be able to serve as a control for intra-assay variability.
Microsphere-based assays for PnPS have been shown to correlate well with ELISA results for anti-PnPS antibody concentrations. A 14-plex anti-PnPS FCMIA has been shown to correlate well with anti-PnPS ELISA, with slopes of regression curves approximating 1.0 and regression coefficients of ∼0.90 for PnPSs 1, 4, 5, 6B, 9V, 14, 16C, 19F, and 23F (13). These data suggest that FCMIAs for anti-PnPS antibodies accurately reflect anti-PnPS levels measured by ELISA. At present we are investigating the correlation of anti-PnPSs measured by the 25-plex assay described in the present report with anti-PnPS-ELISA IgG results. These data will be reported elsewhere.
The prodigious amount of data acquired in performance of a 25-plex FCMIA, preparation of 25 4-PL standard curves, and interpolation of individual results from the 4-PL standard curves represents an extraordinary manual data reduction effort. To overcome these difficulties, commercial suppliers have offered proprietary data acquisition and reduction software for the Luminex100 system (e.g., StatLIA [Brendan Scientific], MasterPlex QT [MiraiBio/Hitachi, Alameda, Calif.], and Bio-Plex Manager software [Bio-Rad, Hercules, Calif.]) as solutions.
In conclusion, we describe a method to measure all 23 pneumococcal polysaccharide serotypes present in the PPV23 vaccine approved by the Food and Drug Administration (FDA). As the FDA-approved pneumococcal 7-valent conjugate vaccine (Prevnar) and other pneumococcal conjugate vaccines in clinical trials contain subsets of the PnPSs present in the 23-valent PPV (4), the FCMIA can be used in evaluating antibody levels from their use also. New pneumococcal vaccines will most certainly contain additional serotypes not present in the proposed or present FDA-approved PPV23 or conjugate vaccines, necessitating the measurement of numerous serotype-specific antibodies. This effort will become overly tedious, time-consuming, prone to error, and resource intensive unless alternative methodologies to ELISA are developed to evaluate vaccine-induced protective immunity. The present work describes a method to potentially measure numerous anti-PnPS IgGs simultaneously. To be useful as a replacement for ELISA methods, the FCMIA method must be validated against accepted ELISA methods (4, 5, 14, 16, 17), and its accuracy, sensitivity, specificity, and precision must be rigorously investigated.
From our data and the data of others (1, 2, 12, 13), the PnPS FCMIA method described in the present paper should have numerous benefits over PnPS ELISAs, including increased speed, smaller sample volumes, the ability to collect blood by heel or finger puncture or blood spot elution (desirable for infants), equivalent or better sensitivity, increased dynamic range allowing for all PnPSs to be measured in a minimal number of dilutions, the ability to evaluate C-PS preadsorption efficacy contemporaneously with anti-PnPS antibody measurements, and the inclusion of a control to evaluate interassay variation in pre- versus postvaccination studies. Because the PnPSs are covalently bound to the microspheres, in contrast to the electrostatic and vanDer Waals' forces which are the basis for ELISA plate protein absorption, FCMIA microspheres should be much more stable in storage and use.
Acknowledgments
This work was supported by an interagency agreement (Y02ES10189) between NIOSH and the National Institute of Environmental Health Sciences.
Mention of a product or company name does not constitute endorsement by NIOSH.
We thank George Carlone, Cheryl Elie, and Joseph Martinez (National Center for Infectious Diseases, CDC, Atlanta, Ga.); Dennis Lynch (NIOSH, CDC); and Robert Hamilton (Johns Hopkins University) for helpful comments on the paper.
REFERENCES
- 1.Bellisario, R., R. J. Colinas, and K. A. Pass. 2000. Simultaneous measurement of thyroxine and thyrotropin from newborn dried blood-spot specimens using a multiplexed fluorescent microsphere immunoassay. Clin. Chem. 46:1422-1424. [PubMed] [Google Scholar]
- 2.Biagini, R. E., D. M. Murphy, D. L. Sammons, J. P. Smith, C. A. Striley, and B. A. MacKenzie. 2002. Development of multiplexed fluorescence microbead covalent assays (FMCAs) for pesticide biomonitoring. Bull. Environ. Contam. Toxicol. 68:470-477. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Epidemiology and prevention of vaccine-preventable diseases, 7th ed. U.S. Department of Health and Human Services, Atlanta, Ga.
- 4.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson, R. H. 1998. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Technol. 17:469-526. [DOI] [PubMed] [Google Scholar]
- 7.Koskela, M. 1987. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr. Infect. Dis. J. 6:519-526. [DOI] [PubMed] [Google Scholar]
- 8.Messina, J. P., P. G. Hickox, M. L. Lepow, B. Pollara, and R. A. Venezia. 1985. Modification of a direct enzyme-linked immunosorbent assay for the detection of immunoglobulin G and M antibodies to pneumococcal capsular polysaccharide. J. Clin. Microbiol. 21:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musher, D. M., J. E. Groover, J. M. Rowland, D. A. Watson, J. B. Struewing, R. E. Baughn, and M. A. Mufson. 1993. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin. Infect. Dis. 17:66-73. [DOI] [PubMed] [Google Scholar]
- 10.Musher, D. M., D. A. Watson, and R. E. Baughn. 1990. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? J. Infect. Dis. 161:736-740. [DOI] [PubMed] [Google Scholar]
- 11.O'Connell, M., B. Belanger, and P. Haaland. 1993. Calibration and assay development using the four-parameter logistic model. Chemomet. Intell. Lab. Syst. 20:97-114. [Google Scholar]
- 12.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 13.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plikaytis, B. D., D. Goldblatt, C. E. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quataert, S., D. Martin, P. Anderson, G. S. Giebink, J. Henrichsen, M. Leinonen, D. M. Granoff, H. Russell, G. Siber, H. Faden, D. Barnes, and D. V. Madore. 2001. A multi-laboratory evaluation of an enzyme-linked immunoassay quantitating human antibodies to Streptococcus pneumoniae polysaccharides. Immunol. Invest. 30:191-207. [DOI] [PubMed] [Google Scholar]
- 17.Quataert, S. A., C. S. Kirch, L. J. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soininen, A., G. van den Dobbelsteen, L. Oomen, and H. Kayhty. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen, U. B., and J. Henrichsen. 1984. C-polysaccharide in a pneumococcal vaccine. Acta Pathol. Microbiol. Immunol. Scand. Sect. C 92:351-356. [DOI] [PubMed] [Google Scholar]
- 21.Vitharsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]