Abstract
Fungal infections in the critically ill patient are difficult to diagnose and are associated with a high mortality rate. A major obstacle to managing fungal infection is the lack of a reliable clinical assay that will rapidly identify patients with fungal sepsis. Glucans are polymers of glucose that are found in the cell wall of fungi and certain bacteria. Glucans are also released from the fungal cell wall into the extracellular milieu. Several studies have reported that detection of fungal glucan in serum or plasma is useful in the diagnosis of mycoses. However, recent studies have questioned the clinical utility of this assay. In this study, we examined serum glucan levels in intensive care unit (ICU) patients and attempt to correlate serum glucan levels with the presence of fungal infection. Following attainment of informed consent, serum was harvested from 46 ICU patients with confirmed fungal infections, confirmed bacterial infections, or no evidence of infection. Sera from eight healthy volunteers served as control. Serum glucan was assayed with a glucan-specific Limulus assay. Serum glucan levels were increased (69.6 ± 17 pg/ml; P < 0.001) in ICU patients versus the normal (11.5 ± 1.3 pg/ml) and noninfected ICU (27.4 ± 17 pg/ml) controls. However, serum glucan levels were not different in patients with confirmed fungal infections versus those with confirmed bacterial infections. Thus, serum glucan levels did not show a correlation with the presence of fungal infections and do not appear to be specific for fungal infections. However, the assay may be useful as a negative predictor of infection.
Mycoses due to all sources now represent 10 to 15% of all nosocomial infections (10). The diagnosis of fungal infections is a significant clinical challenge particularly in the critically ill or immunocompromised patient because the signs and symptoms of fungal disease are often nonspecific. Fungal infection can be difficult to distinguish from colonization, and the presence of immunosuppression not only predisposes the patient to opportunistic fungal infections, but it may also prevent the patient from displaying typical signs and symptoms of an infectious process (11). A current strategy for the management of patients with suspected fungal infection is to empirically administer antifungal therapy (11). Clearly, this is not the most desirable therapeutic approach. What is needed are rapid and specific clinical assays for the diagnosis of fungal infections. Stevens (11) has recently reviewed the current status of diagnosing fungal sepsis. While many tests are currently under development or are in clinical trials, we are still dependent upon antemortem clinical microbiology or postmortem autopsy to definitively diagnose fungal infections.
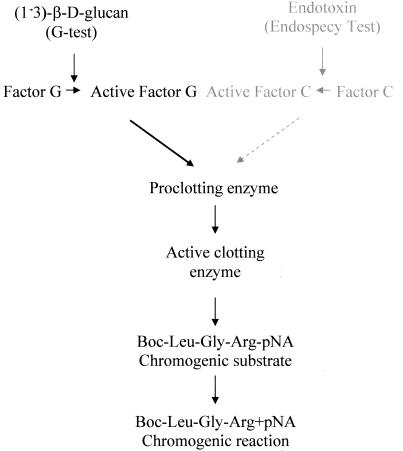
Glucans are (1→3)-β-d-linked polymers of glucose that are part of the outer cell wall of saprophytic and pathogenic fungi as well as certain bacteria (12). Glucans are also released or actively secreted from the cell wall as exopolymers (1, 5, 7). Of greater significance, glucans are reported to be released into the blood of patients with fungal infections (1, 5, 7). Since glucans are evolutionarily conserved in microorganisms and are not produced by higher species, including humans, the presence of fungal cell wall glucan in the blood is presumed to be indicative of the presence of a pathogen. A Japanese firm (Seigakaku, Tokyo) has developed a test for the broad-spectrum detection of fungal infections which is based on the detection and measurement of (1→3)-β-d-glucan in patient plasma or serum. The assay is based on the ability of Limulus polyphemus hemolymph to clot in response to lipopolysaccharide (LPS) or glucan (9) (Fig. 1). Upon contact with LPS or (1→3)-β-d-glucan, Limulus amebocytes degranulate, releasing zymogens that become active serine proteases. The G test is specific for (1→3)-β-d-glucan because the amebocyte lysate is processed to remove Factor C, making the lysate specific for the glucan pathway (5).
FIG. 1.
Diagram of the (1→3)-β-d-glucan and LPS Limulus assay. The assay is based on the ability of L. polyphemus hemolymph to clot in response to glucan or LPS. Upon contact with (1→3)-β-d-glucan or LPS, Limulus amebocytes degranulate and release zymogens that become active serine proteases. Glucan is recognized by Factor G, while LPS is recognized by Factor C. The G test is specific for (1→3)-β-d-glucan because the amebocyte lysate is processed to remove Factor C, thus making the lysate specific for the glucan pathway. In the assay, the clotting enzyme cleaves pNA from the chromogenic substrate peptide. The free pNA is measured at 405 nm in a kinetic microplate assay.
Yoshida (2, 13), Kohno (4), Mori (5) and colleagues have reported on the utility of the glucan-specific assay for the clinical diagnosis of mycotic infections. However, Obayashi (8), Kami (3), and coworkers have presented data which indicates that the glucan-specific assay has a positive predictive value of only 59%, but its negative predictive value is 97%. This suggests that the assay may be of greater value in ruling out a fungal infection. Virtually all of the clinical studies done with the G test have emanated from Japan (2-4, 8, 13), where the assay was originally developed. Recently, the G test has become available in the United States. We employed the glucan-specific Limulus assay to detect and quantify (1→3)-β-d-glucan levels in the sera of a cross-section of patients in our intensive care units (ICU). The purpose of the study was to (i) determine whether the assay was specific for fungal infections in critically ill ICU patients, and (ii) determine the feasibility of detecting and quantifying serum glucan levels in a clinically relevant patient population and laboratory setting.
MATERIALS AND METHODS
Description of patients.
This was a prospective study. Following attainment of informed consent, venous blood was obtained from 46 ICU patients at the Johnson City Medical Center Hospital, Holston Valley Hospital, or the James H. Quillen Veterans Affairs Medical Center. Each patient sample was assigned a number. The samples were analyzed by laboratory personnel who were not involved in the collection of the sera. During analysis, the samples were identified by the patient number. At the completion of the analysis, the biostatistician and attending physician (JD) stratified the patient groupings. A description of the patient populations is shown in Table 1.
TABLE 1.
Description of ICU patients and control subjects enrolled in the serum glucan study
| Patient group | No. of patients in group | No. male/no. female | Mean age (yr) ± SEM | No. (%) positive for fungi | No. (%) positive for bacteria | Mortality at dayc:
|
|
|---|---|---|---|---|---|---|---|
| 30 | 90 | ||||||
| Control subjects | 8 | 5/3 | 39 ± 4.4 | NDa | ND | 0 (0) | 0 (0) |
| Control ICU patients | 7 | 7/0 | 63.3 ± 2.7b | ND | ND | 0 (0) | 0 (0) |
| ICU patients with infections | 46 | 29/17 | 59 ± 2.6b | 2.6 (56.5) | 11 (23.9) | 11 (23.9) | 15 (32.6) |
ND, no data.
P < 0.05.
Values are numbers of persons that were dead on the given day. Values in parentheses are percentages.
Control sera were obtained after informed consent from normal volunteers in our laboratory. All protocols and procedures were reviewed and approved by the Institutional Review Board at East Tennessee State University and the Research and Development Committee at the James H. Quillen VAMC. All of the individuals involved in this study received Institutional Review Board training and certification.
Collection, storage, and handling of patient sera.
The patient sera were harvested by venipuncture in 10-ml Vacutainers (without coagulants). Samples were allowed to clot for ∼1 h. The serum was harvested by aspiration and stored in a liquid nitrogen dewar in sterile, pyrogen-free 1.5-ml Cryovials (Nalgene) until assayed.
Determination of (1→3)-β-d-glucan in serum.
The G-test kit was purchased from Associates of Cape Cod/Seigakaku (East Falmouth, Mass.). Patient serum were assayed for (1→3)-β-d-glucan according to the manufacturer's instructions. Briefly, serum samples were thawed and brought to room temperature. Assay reagents were prepared immediately prior to the running of the assay. In the assay, the clotting enzyme cleaves p-nitroaniline (pNA) from the chromogenic substrate peptide. The free pNA is measured in a kinetic microplate assay (Fig. 1). The samples were analyzed at 405 and 490 nm on a Molecular Devices SpectraMax 340 (Sunnyvale, Calif.) spectrometer maintained at 37°C.
Data analysis.
Continuous variables (serum glucan [picograms per milliliter]) were summarized by the mean, standard deviation, and standard error of the mean for control and patient groups. To assess the effect of one or more grouping factors (infection classification, antifungal treatment) on serum glucan, analysis of variance and multiple comparison procedures (least significant difference and Tukey tests) were used. Linear regression analysis was used to relate serum glucan to continuous variables of interest (white blood cell count, peak body temperature, etc). Categorical variables (mortality subgroups) were summarized by the number and corresponding percentage for each category level. Chi-square analysis was used to assess differences in categorical variates with respect to relevant study factors. A probability level of 0.05 or smaller was used to indicate statistical significance.
RESULTS
Stratification of serum glucan levels in ICU patients and normal controls.
We were able to detect and quantify glucan in the serum of all subjects tested (Table 2). All of the control subjects showed serum glucan levels of >20 pg/ml. We established 20 pg/ml of glucan as the cut off level, i.e., a result of <20pg/ml was considered a negative test. Eleven of the 46 ICU patients (23.9%) showed serum glucan levels of ≤20 pg/ml. Twenty-five of 46 ICU patients (54.3%) showed serum glucan levels between 20 and 100 pg/ml. The remaining 10 ICU patients had serum glucan levels greater than 100 pg/ml (Table 2).
TABLE 2.
Stratification of serum glucan levels in ICU patients and control subjects
| Patient group | No. | No. (%) positive by G test | No. (%) of G test-positive subjects with a serum glucan level (pg/ml) of:
|
||
|---|---|---|---|---|---|
| 1-20 | 20-100 | <100 | |||
| Control subjects | 8 | 8 (100) | 8 (100) | 0 (0) | 0 (0) |
| Control ICU patients | 7 | 7 (100) | 5 (71.4) | 1 (14.3) | 1 (14.3) |
| ICU patients with infections | 46 | 46 (100) | 11 (23.9) | 25 (54.3) | 10 (21.7) |
Serum glucan levels were increased in ICU patients with infections versus levels in noninfected ICU patients.
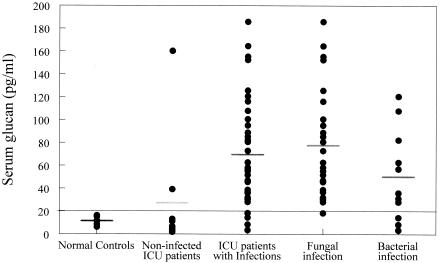
Serum glucan levels were significantly increased in ICU patients with confirmed infections compared to those in normal controls and ICU patients without infections (Fig. 2). Four of the 37 patients in the infected group had serum glucan levels of ≤20 pg/ml. Serum glucan levels in ICU patients with infections from any source (fungal or bacterial) were increased by 500% (P < 0.05) compared to the normal controls and 154% compared to the noninfected ICU patients (Fig. 2). It is important to note that seven of the nine ICU patients without infections showed serum glucan levels of ≤20 pg/ml and thus fell below the cutoff level. However, two of the nine patients in that group showed glucan levels of 39.3 and 160.3 pg/ml, respectively.
FIG. 2.
Serum glucan levels were significantly increased in ICU patients with confirmed infections compared to levels in normal controls and ICU patients without infections. However, assaying serum glucan levels did not appear to be specific for the presence of fungal infections, since glucan levels were increased in ICU patients with either fungal or bacterial infections. A 20-pg/ml cutoff was established based on the normal control values. The data are presented as individual patient values. The bars show the mean value for each group.
Serum glucan levels were not specific for fungal infections in ICU patients.
We observed that serum glucan levels were elevated in ICU patients with either fungal or bacterial infections (Fig. 2). There was no significant difference in the serum glucan levels between patients with confirmed fungal infections and those with bacterial infections.
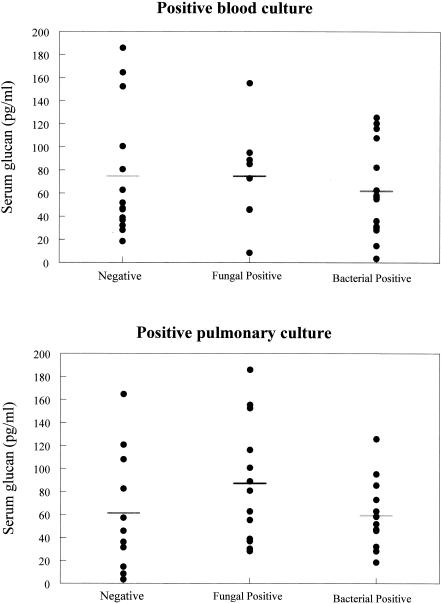
Serum glucan levels did not correlate with positive blood or pulmonary cultures in ICU patients with infections.
We compared serum glucan levels in ICU patients with positive blood or pulmonary cultures and those in ICU patients who did not show positive blood or pulmonary cultures (Fig. 3). Serum glucan levels were not significantly different in patients with positive blood or pulmonary cultures regardless of microbial etiology (i.e., fungal or bacterial) (Fig. 3).
FIG. 3.
A positive blood or pulmonary culture in ICU patients with fungal or bacterial infections did not correlate with serum glucan levels. Serum glucan levels in ICU patients with no evidence of fungemia, bacteremia, or pulmonary infection were equivalent to levels in ICU patients with positive blood cultures. The data are presented as individual patient values. The bars show the mean value for each group.
Serum glucan levels did not correlate with age, gender, mortality, comorbidities, antifungal therapy, or polymicrobial infection.
We observed that serum glucan levels did not appear to correlate with age, gender, acute or chronic mortality, diabetes, febrile response, hemodialysis, hypotension status, malignancy, renal function, trauma, total parenteral nutrition (NADP), or wound infection (data not shown). We subgrouped those patients who received antifungal therapy prior to the time we obtained sera. There was a trend toward lower serum glucan levels in patients who had previously received antifungal chemotherapy, but no definitive correlation could be shown. We also compared patients with polymicrobial infections versus those with a single infectious etiology and found no significant difference in serum glucan levels (data not shown).
DISCUSSION
In the present study, we were able to detect and quantify serum glucan levels in all subjects tested. Normal controls consistently showed serum glucan levels that were ≤20 pg/ml. This compares well with previous reports of glucan levels in normal individuals (13). Approximately 88% of ICU patients with infections of fungal or bacterial etiology showed serum glucan levels greater than 20 pg/ml, which we arbitrarily designated a positive test. Contrary to previously published results (5, 13), we were not able to distinguish between patients with fungal infections and those with bacterial infections using the G-test approach. Patients with confirmed infections, regardless of microbial etiology, showed elevated serum glucan levels. Seven of nine ICU patients (77.8%) who did not have an infection showed serum glucan levels below the cutoff of 20 pg/ml. Stevens (11) has recently reviewed the G test for the diagnosis of fungal infections. He noted that the G test has a negative predictive value of 97%, but the positive predictive value was much less favorable for this assay (59%) (11). Overall, our data tend to support the conclusions of Stevens. We observed a negative predictive value of ∼78%. The positive predictive value for infections from any source was 89%, but we could not distinguish between patients with fungal versus bacterial infections. Thus, the assay does not appear to be specific for fungal infections. Consequently, the assay may have value as a negative predictor of infections.
Our data bring up several interesting questions. Glucans are primarily found in the cell wall of fungi (12). However, there are certain bacteria, such as Alcaligenes and Streptococcus, which produce glucan or glucan-like polymers (12). We detected elevated serum glucan levels in patients with a variety of gram-negative or gram-positive infections. Some of the ICU patients had streptococcal infections, but this was a minority of patients. It is not clear whether the bacteria found in the ICU patients produced glucan or glucan-like polymers. If they do not, then we must consider the possibility that the G test assay cross-reacts with other microbial carbohydrates that might also be released from the cell wall. Additional studies are required to identify the precise nature of the ligand(s) in serum that are being recognized by the G test Limulus lysate. We also observed that there was no significant difference in the serum glucan levels of patients with positive blood cultures versus those with negative blood cultures. One would expect that the presence of microbes in the blood might result in evaluated serum glucan levels, but that does not appear to be the case. Yoshida et al. (13) have also observed that serum glucan levels were elevated in infected patients in the presence or absence of positive blood cultures. This suggests that the assay can readily detect a localized, as well as a systemic, infection through the release of cell wall glucans.
There are caveats to the present study that might be considered in the design of future trials. We obtained a single sample from each patient for assay of serum glucan. This was part of our study design because it was our experience that recruiting patients to a study of this type was significantly easier if enrollment entailed a single blood draw. Yoshida et al. (13) have reported that serum glucan levels in patients with fungal infections are episodic. This may be due to clearance of the carbohydrate by the renal system and/or uptake by the mononuclear phagocyte system (6). Therefore, it might be advantageous to obtain a temporal profile of serum glucan levels. This was a small clinical trial with a limited patient enrollment. We attempted to critically evaluate the data for a wide range of parameters. Some of the patient subgroups did not have sufficient numbers to draw meaningful conclusions. Increasing the number of patients evaluated might strengthen future studies.
In conclusion, we were able to confirm the presence of (1→3)-β-d-glucan in serum samples from ICU patients with infections. The assay is rapid, simple and straightforward to use. However, we could not distinguish between patients with fungal infections versus those with bacterial infections or polymicrobial infections. Thus, the G test does not appear to be specific for fungal infections and may be of limited value as a positive predictor of fungal infection. Our data also suggest that the assay may not be specific for fungal glucans. Additional studies are required to confirm the specificity of the assay. The majority of ICU patients without confirmed infections showed serum glucan levels that were equivalent to the normal control values, suggesting that the G test may have value as a negative predictor of infection.
Acknowledgments
This material is based on work supported by the Office of Research and Development, Department of Veterans Affairs, Merit Review grant to I.W.B. This work was also supported, in part, by Public Health Service grants GM53522 from the National Institute of General Medical Sciences, AI45829 from the National Institute of Allergy and Immunology, and AT00501 from the National Center for Complementary and Alternative Medicine to D.W.
REFERENCES
- 1.Hiyoshi, M., S. Tagawa, S. Hashimoto, C. Sakamoto, and N. Tatsumi. 1999. Evaluation of a new laboratory test measuring plasma (1→3)-β-D-glucan in the diagnosis of Candida deep mycosis: comparison with a serologic test. Kansenshogaku Zasshi 73:1-6. [DOI] [PubMed] [Google Scholar]
- 2.Iwama, A., M. Yoshida, A. Miwa, T. Obayashi, S. Sakamoto, and Y. Miura. 1993. Improved survival from fungaemia in patients with haematological malignancies: analysis of risk factors for death and usefulness of early antifungal therapy. Eur. J. Haematol. 51:156-160. [DOI] [PubMed] [Google Scholar]
- 3.Kami, M., Y. Tanaka, Y. Kanda, S. Ogawa, T. Masumoto, K. Ohtomo, T. Matsumura, T. Saito, U. Machida, T. Kashima, and H. Hirai. 2000. Computer tomographic scan of the chest, latex agglutination test and plasma (1→3)-β-D-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745-752. [PubMed] [Google Scholar]
- 4.Kohno, S., K. Mitsutake, S. Maesaki, A. Yasuoka, T. Miyazaki, M. Kaku, H. Koga, and Hara K. 1993. An evaluation of serodiagnostic tests in patients with candidemia: beta-glucan, mannan, candida antigen by Cand-Tec and D-arabinitol. Microbiol. Immunol. 37:207-212. [DOI] [PubMed] [Google Scholar]
- 5.Mori, T., H. Ikemoto, M. Matsumura, M. Yoshida, K. Inada, S. Endo, A. Ito, S. Watanabe, H. Yamaguchi, M. Mitsuya, M. Kodama, T. Tani, T. Yokota, T. Kobayashi, J. Kambayashi, T. Nakamura, T. Masaoka, H. Teshima, T. Yoshinaga, S. Kohno, K. Hara, and S. Miyazaki. 1997. Evaluation of plasma (1→3)-beta-D-glucan measurement by the kinetic turbidimetric Limulus test, for the clinical diagnosis of mycotic infections. Eur. J. Clin. Chem. Biochem. 35:553-560. [PubMed] [Google Scholar]
- 6.Mueller, A., P. J. Rice, H. E. Ensley, P. S. Coogan, J. H. Kalbfleisch, J. L. Kelley, E. J. Love, C. A. Portera, T. Ha, I. W. Browder, and D. L. Williams. 1996. Receptor binding and internalization of water-soluble (1→3)-beta-D-glucan biologic response modifier in two monocyte/macrophage cell lines. J. Immunol. 156:3418-3425. [PubMed] [Google Scholar]
- 7.Nakamura, Y., S. Tazawa, and M. Tsutiya. 1998. The clinical significance of plasma (1→3)-beta-D-glucan measurement by the kinetic turbidimetric Limulus test for fungal febrile episodes. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 9:33-39. [PubMed] [Google Scholar]
- 8.Obayashi, T., M. Yoshida, T. Mori, H. Goto, A. Yasuoka, H. Iwasaki, H. Teshima, S. Kohno, A. Horiuchi, A. Ito, al. et. 1995. Plasma (1→3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17-20. [DOI] [PubMed] [Google Scholar]
- 9.Saito, H., Y. Yoshioka, and N. Uehara. 1991. Relationship between conformation and biological response for (1→3)-β-D-glucan in the activation of coagulation Factor G from Limulus amebocyte lysate and host-mediated antitumor activity. Demonstration of single-helix conformation as a stimulant. Carbohydr. Res. 217:181-190. [DOI] [PubMed] [Google Scholar]
- 10.Shelton, B. K. 2000. Opportunistic fungal infections in the critically ill. Crit. Care Nurs. Clin. North Am. 12:323-340. [PubMed] [Google Scholar]
- 11.Stevens, D. A. 2002. Diagnosis of fungal infections: current status. J. Antimicrob. Chemother. 49:11-19. [DOI] [PubMed] [Google Scholar]
- 12.Stone, B. A., and A. E. Clarke. 1992. Chemistry and Biology of (1→3)-β-d-Glucan. La Trobe University Press, Melbourne, Australia.
- 13.Yoshida, M., T. Obayashi, A. Iwama, M. Ito, S. Tsunoda, T. Suzuki, K. Muroi, M. Ohta, S. Sakamoto, and Y. Miura. 1997. Detection of plasma (1→3)-beta-D-glucan in patients with Fusarium, Trichosporon, Saccharomyces and Acremonium fungaemias. J. Med. Vet. Mycol. 35:371-374. [PubMed] [Google Scholar]