Abstract
Staphylococcus aureus is an important pathogen in nosocomial pneumonia. Lipoteichoic acid (LTA) and peptidoglycan (PepG) are part of the staphylococcal cell wall. Here we show that LTA and PepG act in synergy to cause polymorphonuclear cell recruitment in the pulmonary compartment during S. aureus pneumonia.
Staphylococcus aureus is a common pathogen in hospital-acquired pneumonia (2). Peptidoglycan (PepG) and lipoteichoic acid (LTA) are components of the cell wall of gram-positive bacteria, including S. aureus. PepG is a large polymer that contains long sugar chains and is predominantly responsible for the protective and shape-maintaining properties of bacterial cell walls. LTAs are phosphate-containing polymers that mediate the attachment of certain bacteria to host cells. Both membrane components can stimulate the generation of proinflammatory cytokines and activate leukocytes in vitro (22, 25, 29, 31).
Recently, it was demonstrated that the intranasal administration of LTA or PepG from S. aureus to mice resulted in acute pulmonary inflammation characterized by an influx of polymorphonuclear cells (PMNs) into the alveolar compartment and local production of proinflammatory cytokines and chemokines (14). Interestingly, lung inflammation elicited by LTA or PepG appeared to be regulated by different mechanisms, considering that interleukin-6 (IL-6)-deficient mice displayed enhanced PMN recruitment after local instillation of LTA but a reduced PMN influx after exposure to PepG (14). Intravenous administration of LTA and PepG to rats has been found to induce synergistic systemic inflammation compared with the infusion of either bacterial product alone (6, 12). Together, this prompted us to examine the combined effects of LTA and PepG in the mouse lung.
BALB/c mice (Harlan Sprague Dawley Inc., Horst, The Netherlands; 8 weeks of age) were intranasally inoculated with saline (controls), S. aureus LTA (50 μg; Sigma, St. Louis, Mo.), S. aureus PepG (50 μg), or LTA and PepG (both 50 μg; final volume, 50 μl in normal saline) according to methods described previously (14). PepG was prepared from S. aureus according to the method of Peterson et al. (16) as described earlier (25). The amount of lipopolysaccharide (LPS) present in LTA and PepG was determined with the chromogenic Limulus amebocyte lysate assay (Chromogenix, Mölndal, Sweden) and was 4.15 pg of LPS/mg of LTA and below the detection limit (2.5 pg/ml), respectively. Hence, 100 μg of LTA (the highest dose used in our experiments) contained <1 pg of LPS. This LPS dose is not capable of eliciting an inflammatory reaction in the lung (data not shown). Each experimental group consisted of five mice. After 4 h, mice were anesthetized by intraperitoneal injection of fluanison and fentanyl citrate (Hypnorm; Janssen Pharmaceutica, Beerse, Belgium) and midazolam (Roche, Mijdecht, The Netherlands) and sacrificed by being bled from the vena cava inferior. Lungs were lavaged with two aliquots of 0.5 ml of saline via a catheter inserted into the trachea. The 4-h time point was chosen since it is representative for studying inflammatory responses to bacterial products in the lung (11, 14, 33). Total leukocyte counts were determined by using a hemocytometer. Numbers of alveolar macrophages (AMs), PMNs, and lymphocytes were calculated from these totals, by using cytospins from bronchoalveolar lavage (BAL) cells stained with Diff-Quick (Baxter, McGaw Park, Ill.). The BAL fluid (BALF) was centrifuged for 10 min at 750 × g and stored at −20°C. Cytokines and chemokines were measured in duplicate in BALF by specific enzyme-linked immunosorbent assays according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn.). The detection limits of these enzyme-linked immunosorbent assays were 31 pg/ml for tumor necrosis factor alpha (TNF-α), 8 pg/ml for keratinocyte chemoattractant (KC), and 46 pg/ml for macrophage inflammatory protein 2 (MIP-2). For histopathological investigations, lungs were removed and fixed in 4% paraformaldehyde in phosphate-buffered saline. After being embedded in paraffin, 4-μm-thick sections were stained with hematoxylin-eosin. The Animal Care and Use Committee of the University of Amsterdam, Amsterdam, The Netherlands, approved all experiments. All values are expressed as means ± standard errors of the means (SEM). Differences between groups were analyzed by the Mann-Whitney U test. To examine a possible synergistic effect of the individual bacterial components, P values were calculated by linear regression analysis. A P value of ≤0.05 was considered statistically significant.
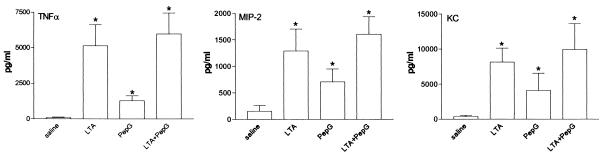
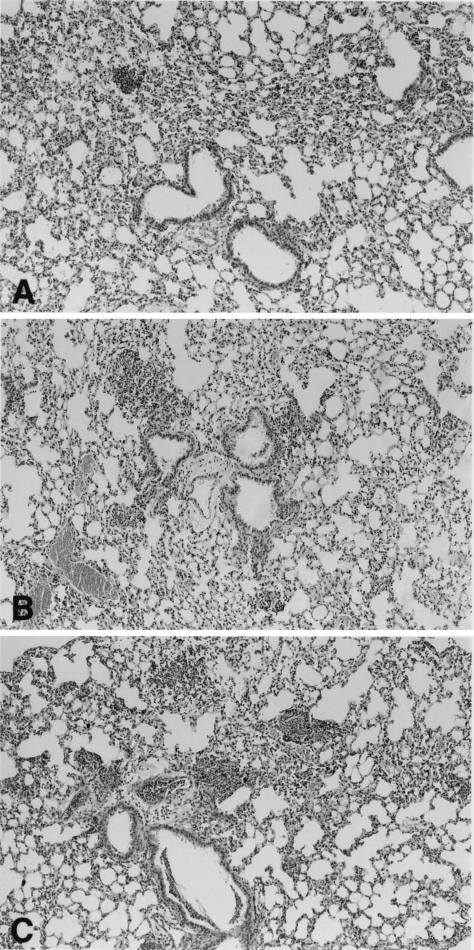
Inoculation with either LTA or PepG induced a profound increase in total leukocyte numbers in BALF, which was predominantly due to a rise in PMN numbers (P < 0.05 versus saline; Table 1). PepG, but not LTA, induced a modest increase in AMs in BALF (P < 0.05 versus saline). Interestingly, the combined administration of LTA and PepG resulted in a synergistic effect on PMN influx (P < 0.05 versus the effect of LTA and PepG together). Additionally, mice treated with LTA or PepG showed an increase in TNF-α, MIP-2, and KC production in BALF compared to BALF of saline-treated animals (Fig. 1). Although the concentrations of TNF-α, MIP-2, and KC in BALF were highest after simultaneous administration of LTA and PepG, the effect of LTA and PepG was not synergistic. Histopathological examination of lung tissue of mice administered LTA showed a dense granulocytic inflammatory infiltrate (Fig. 2A). After administration of PepG numerous well-defined collections of PMNs (abscesses) together with a slight interstitial inflammatory infiltrate were found (Fig. 2B). The combined administration of LTA and PepG resulted in an increased accumulation of leukocytes in small clusters (Fig. 2C).
TABLE 1.
Effect of the administration of LTA and PepG separately or combined ± on cell subsets in BALFa
| Inoculation | No. of cells (104)/mL
|
|||
|---|---|---|---|---|
| Leukocytes | AMs | PMNs | Lymphocytes | |
| Saline | 3.0 ± 1.0 | 2.9 ± 1.0 | 0.04 ± 0.04 | 0.07 ± 0.07 |
| LTA | 24.0 ± 2.8* | 2.2 ± 0.3 | 21.1 ± 1.3* | 0.7 ± 0.2 |
| PepG | 15.3 ± 1.3 | 8.4 ± 1.2* | 8.1 ± 1.4* | 0.7 ± 0.3 |
| LTA + PepG | 51.8 ± 5.1† | 10.1 ± 1.8 | 39.0 ± 4.4† | 1.4 ± 0.4 |
Mice were intransally inoculated with LTA (50 μg), PepG (50 μg), or a combination of these components and sacrificed after 4 h, after which the leukocyte influx in BALF was analyzed. Data are means and SEM of five mice. *, P < 0.05 compared to saline; †, P < 0.05 compared to the effect of the individual components together.
FIG. 1.
Effect of intranasal administration of LTA (50 μg), PepG (50 μg), or a combination of these components on the release of TNF-α, MIP-2, and KC in BALF. Data are means and SEM of five mice. *, P < 0.05 compared to saline.
FIG. 2.
Representative histologic sections of lungs from mice intranasally inoculated with 50 μg of LTA (A), 50 μg of PepG (B), or a combination of them (C) 4 h after inoculation. Original magnification, ×50.
Previous studies have documented synergistic abilities of intravenously administered LTA and PepG to induce septic shock and multiorgan failure in rats (6, 12). We here demonstrate that intrapulmonary delivery of LTA and PepG from S. aureus elicits recruitment of PMNs to mouse lungs in a synergistic way, whereas the induction of TNF-α, MIP-2, and KC by the combined administration of LTA and PepG is additive at best.
CXC chemokines with an ELR (Glu-Leu-Arg) motif near the N-terminal end play a pivotal role in the recruitment of PMNs to sites of infection and inflammation (15). MIP-2 and KC are the most prominent ELR-positive CXC chemokines in the mouse. Both chemokines have been found to contribute to the influx of PMNs to the alveolar compartment in various models of lung infection and inflammation (8, 18, 20, 26). Recently, our laboratory reported that administration of recombinant MIP-2 and KC to the cisterna magna of rats elicited leukocyte influx into cerebrospinal fluid in a synergistic way (34). It is therefore conceivable that the modestly elevated levels of MIP-2 and KC in BALF of mice treated with both LTA and PepG played a role in the synergistic effect of the two staphylococcal cell wall components on PMN recruitment. In addition, the locally elevated concentrations of TNF-α may have contributed to this response (27, 28).
It remains to be established why LTA and PepG did not synergistically induce TNF-α and CXC chemokines in mouse lungs in vivo. In human whole blood in vitro, LTA and PepG do induce the release of TNF-α in a synergistic way (our own unpublished data). Nonetheless, the clear synergism regarding PMN recruitment between LTA and PepG could mean that different receptors and signaling pathways are simultaneously activated by the two agents. Indeed, the signal transduction pathways that are used by PepG and LTA likely are at least in part different. Toll-like receptor 2 (TLR2) has been reported to be the signaling receptor for PepG from S. aureus (24, 30). Although TLR2 has also been implicated as a signal transducer for LTA from Bacillus subtilis, Streptococcus pyogenes, and Streptococcus sanguis (19), TLR4 has been found to be required for signal transduction by S. aureus LTA (7, 23, 24). The concept that synergism is caused by the use of different receptors is supported by several observations. Indeed, bacterial DNA, which signals via TLR9 (9), synergistically acts with LPS, which signals via TLR4, for induction of inflammatory cytokine production (5, 21). In addition, bacterial lipopeptides and lipoprotein, or mycoplasmal lipopeptides, all signaling via TLR2 (1, 4, 10), induced cytokine production in synergy with LPS (13, 17). Moreover, intravenously injected PepG synergized with LPS to cause systemic inflammation and organ injury in rats in vivo (32). Hence, the data from the present study support the concept that LTA and PepG act in synergy to cause pulmonary inflammation in the early phase of pneumonia caused by S. aureus. This synergy either may function as a safety mechanism for the host by triggering an adequate innate immune response (3) or on the other hand may cause tissue injury and lethal shock as observed during fulminant sepsis.
Acknowledgments
We thank Joost Daalhuisen and Ingvild Kop for expert technical assistance.
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1996. National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am. J. Infect. Control 24:380-388. [PubMed] [Google Scholar]
- 3.Beutler, E., T. Gelbart, and C. West. 2001. Synergy between TLR2 and TLR4: a safety mechanism. Blood Cells Mol. Dis. 27:728-730. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Cowdery, J. S., J. H. Chace, A. K. Yi, and A. M. Krieg. 1996. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156:4570-4575. [PubMed] [Google Scholar]
- 6.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dziarski, R., Q. Wang, K. Miyake, C. J. Kirschning, and D. Gupta. 2001. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J. Immunol. 166:1938-1944. [DOI] [PubMed] [Google Scholar]
- 8.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, L. L. Laichalk, D. C. McGillicuddy, and T. J. Standiford. 1996. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 173:159-165. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 11.Juffermans, N. P., A. Verbon, J. T. Belisle, P. J. Hill, P. Speelman, S. J. van Deventer, and T. van der Poll. 2000. Mycobacterial lipoarabinomannan induces an inflammatory response in the mouse lung. A role for interleukin-1. Am. J. Respir. Crit. Care Med. 162:486-489. [DOI] [PubMed] [Google Scholar]
- 12.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreutz, M., U. Ackermann, S. Hauschildt, S. W. Krause, D. Riedel, W. Bessler, and R. Andreesen. 1997. A comparative analysis of cytokine production and tolerance induction by bacterial lipopeptides, lipopolysaccharides and Staphylococcus aureus in human monocytes. Immunology 92:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leemans, J. C., M. J. Vervoordeldonk, S. Florquin, K. P. Van Kessel, and T. Van Der Poll. 2002. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am. J. Respir. Crit. Care Med. 165:1445-1450. [DOI] [PubMed] [Google Scholar]
- 15.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 16.Peterson, P. K., B. J. Wilkinson, Y. Kim, D. Schmeling, S. D. Douglas, P. G. Quie, and J. Verhoef. 1978. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J. Clin. Investig. 61:597-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Muhlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]
- 18.Schmal, H., T. P. Shanley, M. L. Jones, H. P. Friedl, and P. A. Ward. 1996. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 156:1963-1972. [PubMed] [Google Scholar]
- 19.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 20.Shanley, T. P., H. Schmal, R. L. Warner, E. Schmid, H. P. Friedl, and P. A. Ward. 1997. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J. Immunol. 158:3439-3448. [PubMed] [Google Scholar]
- 21.Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27:1671-1679. [DOI] [PubMed] [Google Scholar]
- 22.Standiford, T. J., D. A. Arenberg, J. M. Danforth, S. L. Kunkel, G. M. VanOtteren, and R. M. Strieter. 1994. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect. Immun. 62:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi, O., and S. Akira. 2001. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 25.Timmerman, C. P., E. Mattsson, L. Martinez-Martinez, L. De Graaf, J. A. Van Strijp, H. A. Verbrugh, J. Verhoef, and A. Fleer. 1993. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect. Immun. 61:4167-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, W. C., R. M. Strieter, J. M. Wilkowski, K. A. Bucknell, M. D. Burdick, S. A. Lira, and T. J. Standiford. 1998. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J. Immunol. 161:2435-2440. [PubMed] [Google Scholar]
- 27.Ulich, T. R., E. S. Yi, S. Yin, C. Smith, and D. Remick. 1994. Intratracheal administration of endotoxin and cytokines. VII. The soluble interleukin-1 receptor and the soluble tumor necrosis factor receptor II (p80) inhibit acute inflammation. Clin. Immunol. Immunopathol. 72:137-140. [DOI] [PubMed] [Google Scholar]
- 28.Ulich, T. R., S. Yin, D. G. Remick, D. Russell, S. P. Eisenberg, and T. Kohno. 1993. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am. J. Pathol. 142:1335-1338. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, J. E., P. F. Jorgensen, M. Almlof, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidemann, B., H. Brade, E. T. Rietschel, R. Dziarski, V. Bazil, S. Kusumoto, H. D. Flad, and A. J. Ulmer. 1994. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect. Immun. 62:4709-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray, G. M., S. J. Foster, C. J. Hinds, and C. Thiemermann. 2001. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of tumour necrosis factor-alpha, nitric oxide production, shock, and multiple organ injury/dysfunction in the rat. Shock 15:135-142. [DOI] [PubMed] [Google Scholar]
- 33.Xing, Z., J. Gauldie, G. Cox, H. Baumann, M. Jordana, X. F. Lei, and M. K. Achong. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwijnenburg, P., M. Machteld, M. J. Polfliet, S. Florquin, T. van den Berg, C. Dijkstra, S. van Deventer, J. Roord, T. van der Poll, and M. van Furth. 2003. CXC-chemokines KC and macrophage inflammatory protein-2 (MIP-2) synergistically induce leukocyte recruitment to the central nervous system in rats. Immunol. Lett. 85:1-4. [DOI] [PubMed] [Google Scholar]