Abstract
Like obese humans, Zucker diabetic fatty (ZDF) rats exhibit early β cell compensation for insulin resistance (4-fold β cell hyperplasia) followed by decompensation (>50% loss of β cells). In prediabetic and diabetic ZDF islets, apoptosis measured by DNA laddering is increased 3- and >7-fold, respectively, compared with lean ZDF controls. Ceramide, a fatty acid-containing messenger in cytokine-induced apoptosis, was significantly increased (P < 0.01) in prediabetic and diabetic islets. Free fatty acids (FFAs) in plasma are high (>1 mM) in prediabetic and diabetic ZDF rats; therefore, we cultured prediabetic islets in 1 mM FFA. DNA laddering rose to 19.6% vs. 4.6% in lean control islets, preceded by an 82% increase in ceramide. C2-Ceramide without FFA induced DNA laddering, but fumonisin B1, a ceramide synthetase inhibitor, completely blocked FFA-induced DNA laddering in cultured ZDF islets. [3H]Palmitate incorporation in [3H]ceramide in ZDF islets was twice that of controls, but [3H]palmitate oxidation was 77% less. Triacsin C, an inhibitor of fatty acyl-CoA synthetase, and troglitazone, an enhancer of FFA oxidation in ZDF islets, both blocked DNA laddering. These agents also reduced inducible nitric oxide (NO) synthase mRNA and NO production, which are involved in FFA-induced apoptosis. In ZDF obesity, β cell apoptosis is induced by increased FFA via de novo ceramide formation and increased NO production.
The mechanism by which obesity, now the most common American disease (1), leads to non-insulin-dependent diabetes mellitus (NIDDM), probably the second most common American disease, is unknown. It is generally agreed that insulin resistance is an invariable accompaniment of obesity but that normoglycemia is maintained by compensatory hyperinsulinemia until the pancreatic β cells become unable to meet the increased demand for insulin, at which point NIDDM begins. The mechanism by which β cells become unable to meet rising insulin demand has never been elucidated, primarily because of the unavailability of human pancreatic islets for appropriate study. However, post-mortem studies in patients with NIDDM indicate that the β cell mass is reduced (2).
Zucker Diabetic Fatty (ZDF) rats have provided a useful replica of the human phenotype of adipogenic NIDDM in which to study the islets in the obese prediabetic (7 weeks of age) and obese diabetic (14 weeks of age) stages of the disease (3). Such studies implicate fat deposition in islets as the cause of the β cell decompensation, so-called “lipotoxicity” (4, 5). Excess fat in β cells and other nonadipocytes in this form of obesity is ascribed to the high plasma levels of free fatty acids (FFAs) (4, 5), coupled with a greatly enhanced capacity for lipogenesis (6). There is compelling in vitro evidence that the modest 5- to 10-fold increase in islet fat content that occurs in vivo in the prediabetic phase of the disease causes the compensatory β cell hyperplasia and hyperinsulinemia; a further increase in islet fat to ∼50 times normal reverses the foregoing compensatory changes and causes β cell dysfunction, a reduction in the number of β cells, and diabetes (7, 8). In other words, the surfeit of fat in islets is associated with a dose-related biphasic effect, initially enhancing insulin output by stimulating hyperplasia (7, 8) but subsequently reversing these compensatory changes when the fat content rises to extremely high levels (7, 8).
We have reported that the β cell decompensation in this form of diabetes may involve exaggerated induction by FFA of inducible nitric oxide synthase (iNOS) and excess nitric oxide (NO) generation (9). Because intracellular NO is an important mediator of programmed cell death (10–12), it seemed possible that the loss of the β cells observed late in the course of adipogenic NIDDM (13) might be the result of NO-induced apoptosis. Indeed, apoptosis has been reported in fat-laden hepatocytes (14). Moreover, ceramide, a key component of the signal transduction pathway for apoptosis (15, 16), contains long-chain fatty acids. Further, the susceptibility of β cells to apoptotic stimuli such as interleukin 1β is tightly correlated to islet fat content; measures that deplete islet fat, such as hyperleptinemia, provide striking protection against interleukin 1β cytotoxicity (17, 18), perhaps by depleting the fatty acid source for ceramide synthesis. We therefore suspected that the β cell lipotoxicity in obesity might involve ceramide- and/or NO-mediated apoptosis. This study was designed to test this hypothesis.
MATERIALS AND METHODS
Animals.
Lean wild-type (+/+) male ZDF rats and obese homozygous (fa/fa) male ZDF rats were bred in our laboratory from [ZDF/Drt-fa(F10)] rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis).
Islet Isolation and Culture.
Pancreatic islets were isolated by the method of Naber et al. (19) with modifications (7). Isolated islets were cultured as described (7, 9). In some experiments, islets were cultured with or without 1 mM long-chain FFAs (2:1 oleate/palmitate; Sigma) in the absence and presence of 15 μM fumonisin B1, 15 μM C2-ceramide, 0.5 mM aminoguanidine (Sigma), 10 μM triacsin C (Biomol, Plymouth Meeting, PA), and 10 μM troglitazone (Sankyo).
DNA Fragmentation Assay.
DNA fragmentation was assayed by a modification of the method of Duke and Sellins (20). Groups of freshly isolated or cultured islets were washed twice with ice-cold PBS and suspended in 100 μl of lysis buffer (10 mM Tris⋅HCl/10 mM EDTA/0.5% Triton X-100, pH 8.0), vortex-mixed, sonicated, and incubated on ice for 20 min. After centrifugation for 20 min at 4°C (14,000 × g), the supernatant containing fragmented (soluble) DNA was transferred to another tube. Lysis buffer (100 μl) was added to the pellet containing insoluble DNA. Both samples were treated with RNase A (0.5 mg/ml) for 1 hr at 37°C and then with proteinase K (Sigma, 0.4 mg/ml) for 1 hr at 37°C. After adding 20 μl of 5 M NaCl and 120 μl of isopropanol, the samples were incubated overnight at −20°C, and the DNA concentrations were measured by the method of Hopcroft et al. (21). Fragmented DNA was calculated as 100% × soluble DNA/(soluble + insoluble DNA). The soluble fraction of DNA was determined by electrophoresis on 1.5% agarose gel and has a ladder-like appearance.
Ceramide Determination.
Ceramide concentrations were measured in freshly isolated or cultured islets by a modification of the diacylglycerol kinase assay (22, 23). Islets were washed twice with ice-cold PBS and lipids were extracted by the method of Bligh and Dyer (24). The dried lipid was solubilized in 20 μl of detergent solution [7.5% octyl β-d-glucopyranoside/5 mM cardiolipin in 1 mM diethylenetriamine pentaacetic acid (DETAPAC)]. After adding 50 μl of assay buffer (100 mM imidazole hydrochloride, pH 6.6/100 mM NaCl/25 mM MgCl2/2 mM EGTA), 20 μl of 10 mM DTT, and 10 μl of 1:1 diluted diacylglycerol kinase (Calbiochem), the reaction was started by adding 10 μl of 10 mM [γ-32P]ATP (specific activity, 30,000–40,000 cpm/nmol) prepared in 100 mM imidazole/1 mM DETAPAC, pH 6.6. After mixing, the reaction was continued for 45 min at 27°C. Lipids were extracted again and the lower chloroform phase was washed twice with 2 ml of 1% perchloric acid (PCA), and dried under N2. The sample was resuspended in 80 μl of chloroform, spotted onto a Silica Gel 60 (Merck) HPTLC plate, and developed with chloroform/acetone/methanol/acetic acid/water (10:4:3:2:1). The radioactive spot corresponding to ceramide-1-phosphate was quantified by using the Molecular Imager (Bio-Rad).
De Novo Synthesis of [3H]Ceramide and Oxidation from [3H]Palmitate.
Freshly isolated islets were labeled for 0.5–4 hr with [3H]palmitate (Amersham) in the absence and presence of the ceramide synthase inhibitor fumonisin B1 (Sigma). Lipids were extracted as described above. Lipid extracts dissolved in 80 μl of chloroform were spotted onto high-performance thin-layer chromatography plate and developed with CHCl3/CH2OH/7 M NH4OH/H2O (85:15:0.5:0.5). The radioactivity in the spot corresponding to [3H]palmitate was counted as described (5, 6). [3H]Palmitate oxidation were determined as [3H]H2O production in medium as described (6, 7).
Semiquantitation of iNOS mRNA by Reverse Transcription-Coupled PCR.
iNOS mRNA expression was analyzed in cultured islets by using reverse transcription-coupled PCR as described in detail (9). Briefly, total RNA was extracted by using a TRIzol isolation kit (Life Technologies) and treated with RNase-free DNase. First-strand cDNA was obtained by using a first-strand cDNA synthesis kit (CLONTHECH). iNOS and β-actin genes were amplified by PCR as described (9). The products were electrophoresed on a 1.2% agarose gel. After transferring to Hybond-N Nylon membrane (Amersham), DNA samples were hybridized with [32P]ATP-labeled specific probes and analyzed in the Molecular Imager (Bio-Rad).
Nitrite Determination.
NO formation was determined as nitrite by a modification of the method of Green et al. (25) with modifications (9).
RESULTS
Measurements of DNA Laddering and Ceramide in Prediabetic and Diabetic ZDF Islets.
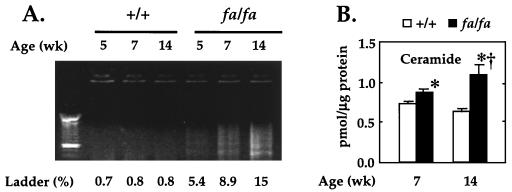
Our initial goal was to determine whether apoptosis was, in fact, the cause of the previously observed loss of β cells that coincided with the onset of diabetes in ZDF rats. We therefore isolated islets from obese ZDF rats in the early prediabetic stage (5 weeks of age), in the late prediabetic stage (7 weeks), and about 4 weeks after the onset of diabetes (14 weeks) and determined whether DNA fragmentation, an index of apoptosis, was present. As shown in Fig. 1A, there was a more than 7-fold increase in DNA ladder formation in freshly isolated islets from 5-, 7-, and 14-week-old ZDF rats, whereas none was detected in lean wild-type controls (Fig. 1A). Moreover, in islets of wild-type rats apoptosis did not increase with age, whereas it increased almost 3-fold in those of the obese rats. Thus, in ZDF islets, there was evidence of a high level of apoptosis in islet cells prior to the onset of diabetes and a further increase thereafter.
Figure 1.
(A) DNA fragmentation in freshly isolated islets from 5 (preobese)-, 7 (obese and prediabetic)-, and 14 (diabetic)-week-old fa/fa ZDF rats and lean +/+ controls. Ladder (%), fragmented DNA percent; lane 1, 100-bp DNA size marker (Boeringer Mannheim). (B) Ceramide content in pancreatic islets of obese fa/fa ZDF (solid bars) and lean +/+ controls (open bars). Values are the mean ± SEM of three or four experiments. ∗, P < 0.05 vs. +/+; †, P < 0.05 vs. 7 week.
To determine whether ceramide, a fatty acid-containing messenger for certain cytokines in the apoptosis pathway, might also be involved in the apoptosis of ZDF rats, it was measured in freshly isolated islets from 7-week-old obese, prediabetic, and 14-week-old obese diabetic fa/fa ZDF rats and in age-matched lean wild-type controls. As shown in Fig. 1B, the levels of ceramide were slightly but significantly higher (P < 0.01) in fa/fa rats than in +/+ controls at 7 weeks of age and they increased further at 14 weeks.
Effect of FFA on Ceramide and DNA Laddering.
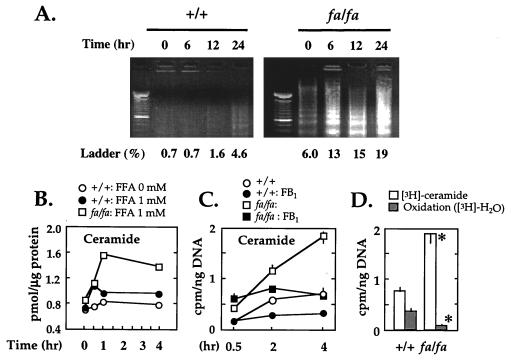
To determine whether an increase in ceramide and DNA laddering are induced by FFA, we measured, at various times, the effect of FFA on ceramide levels on and DNA fragmentation in cultured islets isolated from prediabetic fa/fa ZDF rats and wild-type controls. In islets from the former rats cultured in 1 mM FFA, DNA laddering rose from 6% at 0 time to 19% at 24 hr, whereas in wild-type controls, it rose from 0.7% to only 4.6% at 24 hr (Fig. 2A). The peak 82% increase in ceramide in fa/fa ZDF rats took place within 1 hr (Fig. 2B), before the increase in DNA fragmentation had occurred (data not shown).
Figure 2.
(A) Effect of FFA on DNA fragmentation in pancreatic islets from lean wild-type (+/+) and obese prediabetic homozygous (fa/fa) ZDF rats. Pancreatic islets were isolated from 7-week-old rats and cultured with 1 mM FFA as indicated. Ladder %, fragmented DNA percent. (B) Effect of FFA on ceramide content in pancreatic islets of ZDF rats. Islets from lean wild-type (•) and obese homozygous (□) ZDF rats were cultured with 0 or 1 mM FFA at the indicated times, and ceramide contents were determined. (C) De novo synthesis of [3H]ceramide from [3H]palmitate in islets of wild type (+/+) and fa/fa ZDF rats in the absence and presence of the ceramide synthase inhibitor fumonisin B1 (FB1). Rats were 7 weeks of age. Values are the mean ± SEM of triplicate experiments. (D) Comparison of [3H]ceramide and [3H]H2O to assess relative rates of de novo ceramide synthesis vs. oxidation of [3H]palmitate by isolated islets of +/+ and fa/fa rats. Values are the mean ± SEM of triplicate experiments. ∗, P < 0.05 vs. +/+.
Source of FFA-Induced Increase in Ceramide and Relation to FFA Oxidation.
Ceramide can be generated either by degradation of sphingomyelin or by ceramide synthase-catalyzed condensation of fatty acyl-CoA and sphinganine (26). To determine whether the increase in ceramide reflected de novo synthesis from FFA and to provide support for the diacylglyceride kinase assay for ceramide (27), we incubated islets in 1 mM [3H]palmitate and measured [3H]ceramide. In addition we measured [3H]H2O as an index of FFA oxidation. In wild-type islets, [3H]ceramide increased 3-fold within 2 hr. In islets from fa/fa rats, [3H]ceramide was at least twice that of wild-type rats at all time points (Fig. 2C), whereas [3H]palmitate oxidation in islets of fa/fa rats was only 23% of that of wild-type rats (Fig. 2D). Because palmitoyl CoA is rate-limiting for ceramide synthesis in other systems (28), the reduction in FFA oxidation may have contributed increased substrate for ceramide synthesis. However, the increase in [3H]ceramide was greater than could be explained by even a complete block in oxidation (Fig. 2D), suggesting an intrinsic increase in ceramide synthase activity.
Effect of C2-Ceramide and Ceramide Synthase Inhibition on FFA-Induced Apoptosis.
If ceramide is, in fact, the cause of FFA-induced apoptosis in β cells, exogenous ceramide should induce it, and inhibition of ceramide synthesis should prevent it. In islets from prediabetic obese fa/fa ZDF rats cultured in 15 μM C2-ceramide without FFA, DNA fragmentation rose 2-fold (Fig. 3). By contrast, in culture medium containing 1 mM FFA, the presence of 50 μM fumonisin B1 (the ceramide synthase inhibitor) almost completely prevented the FFA-induced increase in apoptosis (Fig. 3). These results point strongly to ceramide as the mediator of FFA-induced apoptosis in these islets.
Figure 3.
Effect of exogenous ceramide and of blockade of ceramide synthesis on DNA fragmentation in islets from obese fa/fa ZDF rats. Islets isolated from 7-week-old obese prediabetic fa/fa ZDF rats were cultured for 24 hr in medium containing 15 μM C2-ceramide (C2-Cer) without FFA or 1 mM FFA plus 50 μM fumonisin B1 (FB1), an inhibitor of ceramide synthetase. Lane 1, 100-bp DNA size marker (Boeringer Mannheim); Ladder (%), fragmented DNA percent.
Role of NO in FFA-Induced Apoptosis.
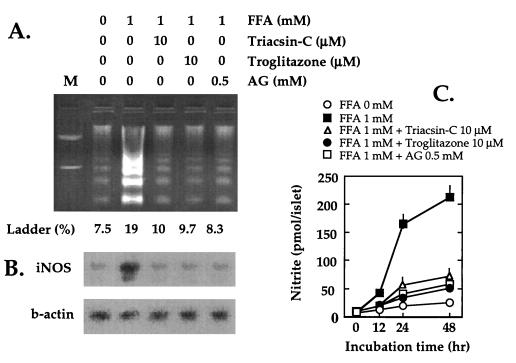
We have observed an exaggeration of the normal FFA-induced increase of iNOS mRNA and NO production in fat-laden islets of fa/fa rats (9). Because inhibitors of iNOS prevent the diabetic phenotype in islets of fa/fa rats, we have hypothesized that FFA-induced β cell dysfunction and damage is mediated by NO (9). If so, any maneuver that inhibits iNOS expression in islets should block DNA fragmentation and prevent the diabetes. Aminoguanidine is an iNOS inhibitor (29) that profoundly reduces NO production in vitro and effectively prevents β cell loss and diabetes when administered to ZDF rats (9). In ZDF islets cultured in 1 mM FFA plus aminoguanidine, iNOS expression and NO production were decreased and DNA fragmentation was lowered substantially (Fig. 4).
Figure 4.
(A) Inhibitory effect of triacsin C, troglitazone, and aminoguanidine on FFA-induced DNA fragmentation in islets from obese fa/fa ZDF rats. Islets isolated from 7-week-old fa/fa ZDF rats were cultured for 24 hr at 0 or 1 mM FFA with 10 μM triacsin C, 10 μM troglitazone, or 0.5 mM aminoguanidine. M, 100-bp DNA size marker. (B) Inhibitory effect of triacsin C, troglitazone, and aminoguanidine on FFA-induced iNOS mRNA induction. (C) FFA-induced NO production in islets of obese fa/fa ZDF rats. Effect of triacsin C, troglitazone, and aminoguanidine on islets cultured as described in A except for 48 hr.
Effects of Fatty Acyl-CoA Blockade on DNA Fragmentation and NO.
For evidence that excess fatty acyl-CoA causes DNA fragmentation via increased NO production, we blocked fatty acyl-CoA synthetase by adding 10 μM triacsin C to culture medium containing 1 mM FFA (Fig. 4). Triacsin C has been shown to inhibit palmitate esterification in INS-1 cells and islets (ref. 30 and P. A. Antinozzi, M. Prentki, L. Segall, J. D. McGarry, and C. B. Newgard, personal communication). Both NO production and iNOS mRNA were reduced and DNA fragmentation was concomitantly lowered in the triacsin C-treated cells.
Effect of Reducing Islet Fat Content on DNA Fragmentation.
Finally, to determine whether a reduction of islet triglycerides would rescue β cells from apoptosis, we cultured islets in 10 μM troglitazone, which has recently been shown to reduce the fat content of ZDF islets by increasing fatty acid oxidation (17, 18). Troglitazone in the culture medium effectively blocked the FFA-induced increase in iNOS mRNA, NO production, and apoptosis (Fig. 4).
DISCUSSION
The foregoing results indicate that apoptosis is increased in islets of fa/fa ZDF rats that are progressing through the prediabetic and diabetic stages of their disease. They suggest that the apoptotic effect of FFA on β cells of fa/fa ZDF rats is somehow related to the high fat content of their islets and that this greatly exaggerates the apoptotic effect of fatty acyl-CoA. In addition, they strongly implicate ceramide as a mediator of the FFA-induced apoptosis. FFA, a precursor of ceramide, raised ceramide levels in fat-laden ZDF islets via a greater rate of de novo biosynthesis, as indicated by increased incorporation of [3H]palmitate into [3H]ceramide in the fa/fa islets. The reduction in fatty acid oxidation in islets of fa/fa ZDF rats may have contributed to their high ceramide levels by providing increased substrate for ceramide formation; indeed, it has been shown in another cell type that etomoxir, an inhibitor of mitochondrial β oxidation, increases ceramide synthesis (28). However, because even a complete blockade of oxidation could not account for the striking increase in de novo ceramide formation, an underlying increase in ceramide synthase activity seems likely.
The role of ceramide in the apoptosis was supported by the demonstration that C2-ceramide caused DNA fragmentation in the absence of FFA while blockade of ceramide synthesis by fumonisin B1 prevented FFA-induced DNA fragmentation in the presence of FFA. It is not clear, however, whether ceramide mediates the FFA effect on NO or whether the FFA-induced increase in iNOS represents a separate ceramide-independent avenue for apoptosis.
In summary, we have demonstrated in islets of obese ZDF rats a pathway of lipotoxicity leading to diabetes. Elevated levels of circulating FFA (4) and lipoproteins transport to islets of obese ZDF rats far more FFA than can be oxidized. Because fa/fa islets exhibit a markedly increased lipogenic capacity and a decreased oxidative capacity (6), unused FFA in islets are esterified and over time an excessive quantity of fat is deposited (6). This is associated with an increase in ceramide, iNOS expression, and NO production, which cause apoptosis.
Because islets of obese humans are not available for assessment of their fat content, one cannot assume that this mechanism necessarily explains obesity-associated diabetes in humans. Despite the similarities of the rodent and human syndromes, it is unlikely that fat can accumulate as rapidly in islets of obese humans as it does in ZDF rats. Perhaps slower and more variable accumulation of ectopic fat stores in obese humans accounts for the later onset and more variable incidence of their diabetes. Even though the lipotoxic etiology of human adipogenic diabetes cannot be directly proven, the fact that troglitazone, an agent that reduces islet fat in ZDF rats (17, 18) and prevents their diabetes (31–33), is equally efficacious in the human form of the disease is consistent with a common etiology. It is therefore possible that prophylactic interventions that reduce fat accumulation and NO production in islets will prevent the anticipated epidemic of obesity-associated NIDDM in the U.S.
Acknowledgments
We thank Drs. Daniel Foster and Christopher Newgard for critical review of the paper, Xiaodong Wang and Michael Brown for important suggestions, Dr. Hua-Min Wang for technical help, and Ms. Tess Perico for secretarial support. We are particularly grateful to Drs. Ruedi Aebersold and Julian Watts for their technical advice. This work was supported by the National Institutes of Health (DK02700-37), National Institutes of Health/Juvenile Diabetes Foundation Interdisciplinary Research Program, and Department of Veterans Affairs Institutional Support (SMI 821-109).
ABBREVIATIONS
- ZDF
Zucker diabetic fatty
- FFA
free fatty acid
- NIDDM
non-insulin-dependent diabetes mellitus
- iNOS
inducible nitric oxide synthase
References
- 1.National Center for Health Statistics. Newsweek. 1994;127:62. [Google Scholar]
- 2.Rahier J, Goebbles R M, Henquin J C. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 3.Peterson R G, Shaw W N, Neel M, Little L A, Eichberg J. ILAR News. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Hirose H, Zhou Y-T, Esser V, McGarry J D, Unger R H. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 7.Milburn J L, Hirose H, Lee Y, Nagasawa Y, Ogawa A, Ohneda M, Beltrandel Rio H, Newgard C B, Johnson J H, Unger R H. J Biol Chem. 1994;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 8.Hirose H, Lee Y, Inman L R, Nagasawa Y, Johnson J H, Unger R H. J Biol Chem. 1996;271:5633–5637. doi: 10.1074/jbc.271.10.5633. [DOI] [PubMed] [Google Scholar]
- 9.Shimabukuro M, Ohneda M, Lee Y, Unger R H. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 11.Corbett J A, Wang J L, Sweetland M A, Lancaster J R, Jr, McDaniel M L. J Clin Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneto H, Fujii J, Seo H G, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- 13.Ohneda M, Inman L, Unger R H. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- 14.Yang S Q, Lin H Z, Lane M D, Clemens M, Diehl A M. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel S, Merrill A H., Jr FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro M, Koyama K, Lee Y, Unger R H. J Clin Invest. 1997;100:1750–1754. doi: 10.1172/JCI119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimabukuro, M., Zhou, Y.-T., Lee, Y. & Unger, R. H. (1998) J. Biol. Chem. 273, in press. [DOI] [PubMed]
- 19.Naber S P, McDonald J M, Jarett L, McDaniel M L, Ludvigsen C W, Lacy P E. Diabetologia. 1980;19:439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- 20.Duke R C, Sellins C B. In: Cellular Basis of Immune Modulation. Kaplan J G, editor. New York: Liss; 1989. pp. 311–314. [Google Scholar]
- 21.Hopcroft D W, Mason D R, Scott R S. Horm Metab Res. 1985;17:559–561. doi: 10.1055/s-2007-1013606. [DOI] [PubMed] [Google Scholar]
- 22.Preiss J, Loomis C R, Bishop W R, Stein R, Niedel J E, Bell R M. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 23.Okazaki T, Bielawska A, Bell R M, Hannun Y A. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 24.Bligh E A, Dyer W J. Can J Biochem Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Merrill A H, Jr, Jones D D. Biochem Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 27.Watts J D, Gu M, Polverino A J, Patterson S D, Aebersold R. Proc Natl Acad Sci USA. 1997;94:7292–7296. doi: 10.1073/pnas.94.14.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paumen M B, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 29.Corbett J A, McDaniel M L. Methods Enzymol. 1996;268:398–408. doi: 10.1016/s0076-6879(96)68042-2. [DOI] [PubMed] [Google Scholar]
- 30.Noel R J, Antinozzi P A, McGarry J D, Newgard C B. J Biol Chem. 1997;272:18621–18627. doi: 10.1074/jbc.272.30.18621. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka S, Nishino H, Shiraki T, Ikeda K, Koike H, Okuno A, Wada M, Fujiwara T, Horikoshi H. Metabolism. 1993;42:75–80. doi: 10.1016/0026-0495(93)90175-n. [DOI] [PubMed] [Google Scholar]
- 33.Sreenan S, Sturis J, Pugh W, Burant C F, Polonsky K S. Am J Physiol. 1996;271:E742–E747. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]