The major complication in the therapeutic or prophylactic use of heparin in medical treatment is type II heparin-induced thrombocytopenia (HIT II), a unique form of drug-induced immune-mediated thrombocytopenia. Due to the extensive use of heparin, this side effect is widespread; up to 3% of patients treated with unfractionated heparin (UFH) develop HIT II (69). Clinically, HIT II is characterized by thrombocytopenia which paradoxically is associated with thrombosis (HIT II-induced thrombosis [HITTS]) (i.e., deep vein thrombosis, pulmonary embolism, and venous gangrene) in about 50% of the cases.
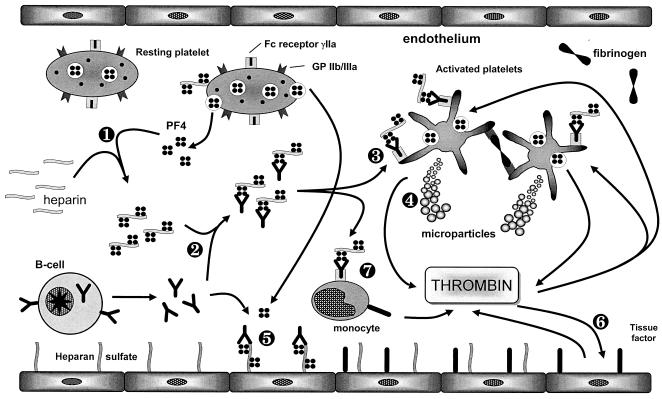
In the pathogenesis of HIT II, the formation of platelet factor 4 (PF4)/heparin complexes seems to be the major determinant (2, 3, 20). The binding of PF4 to heparin induces antibody production directed against a neoepitope created by the three-dimensional assembly of PF4 and heparin. Subsequently, PF4/heparin-antibody complexes induce platelet activation, followed by the shedding of platelet microparticles, thrombin generation, and the involvement of endothelial cells (Fig. 1).
FIG. 1.
Current model for the pathophysiological mechanism of HIT II. Step 1, binding of i.v. applied heparin to PF4 results in the formation of a neoepitope. Step 2, induction of an immune response against the PF4/heparin complex with antibody formation. Step 3, the complex of PF4/heparin and specific antibody associates with platelets via binding of the antibody Fc part to the platelet immunoglobulin receptor FcγRIIa, representing the major step in platelet activation in HIT II. Step 4, activated platelets shed procoagulant microparticles which enhance thrombin generation. Step 5, association of PF4 with endothelial cell-bound heparan sulfate and subsequent antibody binding result in direct activation of endothelial cells, accelerating the procoagulant capacity. Step 6, activation of endothelial cells leads to expression of tissue factor, further increasing thrombin generation. Step 7, monocytes activated by PF4/heparin antibody binding tissue factor. (Reproduced from reference 71 with permission of the publisher.)
Remarkably, the incidence of HIT II, HIT II-associated thrombotic complications, and PF4/heparin-antibodies (HIT II antibodies) in patients receiving therapy with UFH is clearly dependent on the clinical setting of the patient. Cardiosurgical patients are at a high risk to develop HIT II antibodies, while orthopedic patients undergoing hip replacement have a high risk for developing thromboembolic complications (Iceberg model [66]). In medical patients the risk for developing HIT II is substantially lower. Comparing low-molecular-weight heparins (LMWH) with UFH, the use of LMWH coincides with a substantially lower risk of HIT II, but this effect holds true only within the same group of patients (77, 78).
It is generally accepted that the clinical diagnosis of HIT II should be followed by immediate cessation of heparin therapy and initiation of therapy with alternative anticoagulants. Negative results in the concomitant laboratory testing should not serve as the sole criterion for restarting heparin therapy.
In almost every case of HIT II, administration of alternative anticoagulants is essential either to continue prophylactic anticoagulation or to treat and prevent thrombotic complications due to HIT II. Three substances (danaparoid, hirudin, and argatroban) are available. Since these substances have not been compared directly with each other in the treatment of HIT II, criteria such as half-life and/or route of excretion should be considered for selection.
This review focuses on (i) the present laboratory methods for diagnosing HIT II, including their principles, time consumption, and significance, and (ii) the alternative anticoagulants, including their characteristics and indications in certain clinical settings.
LABORATORY ASSAYS FOR HIT II DIAGNOSIS
Laboratory testing in the diagnosis of HIT II is based on either the immunological detection of antibodies directed against the PF4/heparin complex or the functional platelet-activating potential of the emerging immuncomplexes.
In immunological assays, the antibodies are detected by reaction with surface-bound PF4/heparin or polyvinylsulfate/PF4 complexes (2, 3, 21, 57) or with PF4/heparin complexes in a fluid phase (40). Two ready-to-use enzyme-linked immunosorbent assay (ELISA)-based assays using these principles are commercially available (Asserachrom HPIA [Diagnostica Stago, Asnières, France] and GTI-PF4 [GTI Corp., Brookfield, Wis.]). Recently, two other types of immunological assays have been introduced, the immunofiltration assay (either fluorescence linked [FLIFA] [64] or peroxidase linked [ELIFA] [59]) and the gel agglutination technique assay (ID-PF4/heparin antibody test; Diamed AG, Cressier, Switzerland) (39). In the FLIFA, antibodies in the patient's serum are immobilized on a nitrocellulose membrane and detected with fluorescein isothiocyanate-conjugated PF4/heparin complexes, while in the ELIFA, nitrocellulose-bound PF4/heparin complexes are used to detect HIT antibodies via an enzymatic reaction. The ID-PF4/heparin antibody test utilizes polystyrene beads coated with PF4/heparin complexes which are adapted to the ID microtyping system, which is widely used in immunohematology. An immunochromatographic test strip device has been introduced in a prototype release (Serbio, Gennevielliers, France) (based on a Pall Corporation strip device).
In the second group of assays, the functional or activation assays, the platelet-activating capacity of PF4/heparin-antibody complexes is measured. Serum from the patient is incubated with fresh donor platelets in the presence of heparin. The readout of these assays rests on the measurement of different platelet activation parameters or the macroscopic detection of platelet aggregation. The first functional assay, the platelet aggregation test (PAT), was introduced in 1984 (29). The “gold standard” for the detection of PF4/heparin-antibodies is the [14C]serotonin release assay (14C-SRA) (50). Widely used, at least in Europe, is the heparin-induced platelet activation assay (HIPA) (19). Platelet aggregometry with determination of ADP release is utilized in lumiaggregometry (51). Flow cytometry is applied in the detection of platelet microparticles (33), the expression of the platelet activation marker P-selectin (CD62p) (28), or annexin V binding to phosphatidylserine (43, 54).
Technical performance.
The immunological assays are easy to perform, acceptable in time consumption (with results within 0.5 to 4 h), and standardized in interpretation. For the ID-PF4/heparin antibody test and the prototype immunochromatographic test strip (Pall), technical handling is very easy and the time required is very short (0.5 h and 10 min, respectively).
These advantages do not hold true for the functional or activation assays. Depending on the particular test, a high level of expertise is necessary, readouts are delicate, and the time consumption is sometimes high. Even in the 14C-SRA, radioactive tracers may be necessary (methods for measuring the serotonin release by ELISA or high-pressure liquid chromatography assays have been published recently [24a, 29a]).
Sensitivity and specificity.
As for every laboratory assay, the clinician should be aware of the specificity and sensitivity of the assay used. A large number of studies comparing the sensitivities and specificities of immunological and functional assays in detecting heparin-induced drug-dependent antibodies have been published (6, 9, 12, 14, 15, 22, 26, 27, 37, 39, 43, 47, 49, 51, 54, 58, 59, 62, 78). In general it should be pointed out that defining specificity and sensitivity in diagnostic assays depends on the definition of reference groups expressing or not expressing the value to be calculated. In the laboratory diagnosis of HIT II, there are three different possibilities for reference groups: (i) HIT II patients defined by clinical aspects, (ii) a gold standard in testing for HIT II antibodies, or (iii) a combination of both. Since these studies did not all use the same reference group, clear-cut definitions of the specificities and sensitivities of the assays available are not given. Nevertheless, the different kinds of assays exhibit obvious differences in specificity and sensitivity.
In the majority of these studies, three different groups of subjects have been investigated: (i) patients with clinical symptoms of HIT II and proven HIT II antibodies, (ii) patients with clinical symptoms of HIT II but without occurrence of HIT II antibodies, and (iii) healthy subjects.
Regarding the specificity and sensitivity calculated from these studies, the following conclusions can be drawn. The traditional immunological assays (i.e., ELISAs) exhibit a high sensitivity for HIT II antibodies in patients with HIT II or HITTS, ranging from 80 to 100%, but have a lower specificity (3, 6, 7, 58, 62). Specificity also clearly depends on the type of patient population (78). In a significant number of patients undergoing heparin therapy but without clinical symptoms of HIT II, or even in healthy blood donors, ELISAs detect HIT II antibodies (6, 14, 15, 62). Two explanations for this phenomenon have been suggested: (i) in heparin-treated patients HIT II antibodies may be formed without inducing HIT II, and (ii) in vitro fixation of PF4 on a solid phase may alter the three-dimensional structure of the protein in such a way that subsequent formation of neoantigens allows binding of antibodies which are not specific for the PF4/heparin complex in a native state (2, 59). Two recently developed immunological assays, the FLIFA and the ELIFA, circumvent the latter problem by avoiding fixation of the PF4/heparin complexes on a solid surface. Indeed, the specificity of these assays seems to be enhanced (23, 59, 64, 65).
Immunological assays detect HIT II antibodies for a significantly longer period than functional assays. While functional assays turn negative within 50 days (confidence interval, 32 to 64 days), immunological assays do not turn negative until after 85 days (confidence interval, 64 to 124 days) (76). This observation reflects the higher sensitivity of immunological assays. Another significant problem leading to false-negative results in immunological assays is that antigens other than PF4/heparin complexes seem to be involved in the pathogenesis of HIT II (4, 15). In a few cases antibodies directed against the chemokine interleukin 8 or neutrophil-activating peptide 2 are able to induce HIT II, but the presence of heparin is not necessary for antibody binding. While the overwhelming majority of HIT II (and HITTS) cases are mediated by PF4/heparin complex antibodies of the immunoglobulin G (IgG) class, antibodies of the IgM or IgA class have also been described (5). This is remarkable, since it is generally accepted that the pathogenesis in HIT II is based on platelet activation via the binding of PF4/heparin-IgG antibody complexes to the FcγRIIa receptor.
Functional or activation assays have a lower sensitivity for the detection of PF4/heparin complex antibodies, but they have a higher probability of identifying cases which are also clinically diagnosed as HIT II (33, 67). Within the group of functional assays, substantial differences in performance have been observed. One reason is the use of platelet-rich plasma versus washed donor platelets. Washed donor platelets result in enhanced sensitivity and specificity (9, 15, 19, 33, 50, 54, 74). By washing the platelets, the role of interfering substances such as IgG, acute-phase proteins (e.g., fibrinogen), or variable calcium concentrations is excluded, thereby minimizing nonspecific platelet activation.
Comparing the different types of assays, the PAT, which has the advantage of easy handling, lacks adequate sensitivity, presumably due to the use of platelet-rich plasma (see above). The second assay, introduced in 1986, is the SRA. In terms of specificity and sensitivity, this assay represents the gold standard (both sensitivity and specificity reach levels of 65% to >90%). Consequently, numerous studies using the SRA as a standard for the calculation of the sensitivity and specificity of newly introduced assays have been published (9, 62, 74, 77).
The HIPA, introduced in 1991, offers similar sensitivity and specificity. More recent functional tests, based on luminometry, flow cytometry, or calculation of serotonin release by nonradioactive methods, have also been proven to exhibit similar sensitivity and specificity.
In functional assays, the selection of donor platelets seems to represent the major factor influencing test sensitivity, due to significant interindividual variability in platelet activation responsiveness.
However, despite variable sensitivity and specificity of functional and immunological assays, the overall performance is superior to those of other tests in platelet serology. For example, analysis of the sensitivity and specificity of laboratory testing in HIT II diagnosis by means of a receiver operating characteristic (simultaneous statistical comparison of sensitivity and specificity) revealed significantly enhanced performance compared to assays such as the monoclonal antibody immobilization of platelet antigens (78).
The main characteristics of functional and immunological assays are given in Table 1.
TABLE 1.
Characteristic features of functional and immunological assays in HIT II diagnosis
| Type of assay | Test principle or measurement | Technical performance | Sensitivity and specificity | Costs/technical equipment |
|---|---|---|---|---|
| Functional | ||||
| General aspects | Platelet activation | Large interassay variation | Depends on selection and responsiveness of donor platelets | Variable, in general moderate |
| SRA | Platelet serotonin release | Time-consuming, high level of expertise required, radioisotopes neededa | High sensitivity, high specificity (gold standard) | Moderate |
| PAT | Platelet aggregation | Easy to perform | Low sensitivity, moderate specificity | Low |
| HIPA | Platelet aggregation | Moderate level of expertise required, moderate time consumption | High sensitivity, high specificity | Moderate |
| Luminometry | Platelet ATP release | Easy to perform, rapid | Similar to SRA and HIPA | Low/luminometer |
| Flow cytometry | Platelet activation parameters (CD62, annexin V binding) | Moderate level of expertise required, rapid | Variable, depends on platelet activation parameter | Low/flow cytometer |
| Immunological or antigen | ||||
| General aspects | Binding of IgG (or IgA or IgM) antibodies to PF4/heparin complexes | Easy, rapid | High sensitivity, moderate specificity | Variable |
| ELISA | Surface-bound PF4/heparin or PF4/polyvinylsulfate complexes as target antigens | Easy to perform | High sensitivity, moderate specificity | High/ELISA reader |
| Solid-phase detection | PF4/heparin complexes captured on nitrocellulose | Moderate | Similar to ELISA | Low |
| Fluid-phase detection | PF4/heparin complex binding antibodies in fluid phase | Moderate | Similar to ELISA | Moderate/ELISA reader |
| ID system | Agglutination of latex particles coated with PF4/heparin complexes | Easy, very rapid | First reports indicate high sensitivity and good specificity, awaits further validation | Low/gel card centrifuge |
| Pall test strip device | Immunochromatography | Simple and rapid, point-of-care testing | Initial results promising, awaits further validation | Unknown/none |
Alternative methods for measuring serotonin release have been proposed.
Cross-reactivity of UFH to LMWH and danaparoid.
Functional tests such as the HIPA allow testing of possible cross-reactivity of UFH to LMWH or danaparoid, which can be demonstrated in about 10 to 50% of cases with PF4/UFH complex antibodies. While LMWH is generally contraindicated in cases of HIT II, cross-reactivity with Orgaran does not seem to influence the clinical outcome under Orgaran therapy. Therefore, testing for cross-reactivity should be limited to cases with continued thrombosis after a change to Orgaran therapy (40, 68).
Time point of blood sampling.
Regarding the effect of the time point of blood sampling on the likelihood of demonstrating HIT II antibodies, conflicting results have been published. According to Harenberg et al., the sensitivity in antibody detection during therapy with heparin is considerably lower than when PF4/heparin complexes are removed from circulation (24) (this study used a small number of patients). In contrast, a second survey could not detect any differences in assay sensitivity depending on the time point of blood sampling within the acute phase of the disease (61).
Antibody titer.
The antibody titer in patients with HIT II or HITTS should not be used as a predictor of the severity of the disease, the development of thrombosis, or the clinical outcome, since there is no correlation with these parameters (60-62).
OPTIMAL DIAGNOSTIC STRATEGY
Summarizing the aforementioned characteristics of the different types of assays, the following general statements can be made. (i) With regard to the two groups of assays (functional and immunological), a high proportion of patients with clinically defined HIT II may be recognized by one or both types of assays, but there is a small but significant number of patients whom are not recognized by at least one type of assay. (ii) On the other hand, detection of HIT II antibodies is not definite proof of HIT II. (iii) The combined use of a functional assay and an immunological assay with repetitive blood sampling enhances the probability of correctly diagnosing HIT II. (iv) In the application of functional tests, the expertise of the laboratory personnel and the careful selection of donor platelets have a significant influence on the sensitivity and the specificity of the assay. (v) In the selection of assays for routine testing, other factors, such as time consumption and costs, should be included. (vi) To evaluate the diagnosis of HIT II, the results of laboratory testing have to be assessed in direct combination with the clinical probability of HIT II.
Regarding these factors, an algorithm has been developed to calculate the probability of HIT II (70, 73). Using such an algorithm, one should be aware that difficulties in distinguishing moderate from high clinical probability of HIT II or inter- and intra-assay variations may direct the decision the wrong way. Additionally, while for obvious reasons a high clinical probability and a positive test result or, alternatively, a moderate probability and a negative test result indicate the presence or absence of HIT II, respectively, with a high likelihood, in cases with a questionable probability of HIT II from the clinical point of view, indeterminate or conflicting laboratory results need further assessment.
The request of the clinician for laboratory testing always follows a moderate or high probability of clinical diagnosis of HIT II. Heparin therapy has to be discontinued, since the period of time until return of the results of laboratory testing does not allow acceptance of a possible progression of HIT II. Generally, an antigen test is used as a first-level screening test, since it combines high sensitivity with rapid and simple performance (with the disadvantage of high costs, at least for the enzyme immunoassays). In labs with a high level of expertise, functional and immunological tests are performed simultaneously, ensuring a maximum level of information in the laboratory diagnosis of HIT II.
The combination of clinical assessment and laboratory testing may not lead to a final appraisal, even with the use of both a functional and an immunological assay. Clinical reassessment and repeated testing may be helpful in this situation, but it has to be accepted that a number of cases cannot be assessed exhaustively.
In the first line of testing, the recently introduced ID-PF4/heparin antibody test and the immunochromatography test strip device may accelerate diagnosis due to rapid and easy performance, but both assays await further evaluation.
ALTERNATIVE ANTICOAGULANTS IN HIT II THERAPY
Immediate cessation of heparin is mandatory when HIT II becomes clinically manifest. Even if the clinical probability of HIT II is moderate or low, results from the laboratory should not be awaited, because there is a high risk for thrombotic events. After discontinuing heparin therapy, 40 to 50% of patients will suffer from thromboembolic events in the following weeks (75). Additionally, heparin cessation alone is not an appropriate therapeutic strategy in HIT II, as many patients require further parenteral anticoagulation because of the underlying disease or HIT II-associated vessel occlusions. LMWH is contraindicated in the treatment of acute HIT II. Although it is less likely to cause the formation of HIT II antibodies, it cross-reacts with the antibodies in patients with acute HIT II and causes recurrent thrombocytopenia or thrombosis (20, 77).
The transfusion of platelet concentrates should be avoided, since this may induce or aggravate thromboembolic complications. This is underlined by the fact that bleeding complications are a rare event in most cases of acute HIT II despite low platelet counts.
The therapeutic strategy depends on the clinical situation. Detection of HIT II antibodies alone without clinical signs of HIT II (a drop in platelet count and/or new thromboembolic events) does not justify the change to an alternative anticoagulant substance. Clinical diagnosis of HIT II without thrombosis should be followed by immediate cessation of heparin therapy and replacement with an alternative anticoagulant in a therapeutic dose until normalization of platelet counts. Inapparent thrombosis in the patient should be ruled out by diagnostic means. If acute HIT II is aggravated by thromboembolic events (HITTS), the immediate cessation of heparin therapy should be followed by parenteral anticoagulation in a therapeutic dose with one of the alternative anticoagulants during the acute episode. Subsequently, an oral anticoagulant should be administered for several months (75). The duration of treatment is not yet well defined. The risk for thrombosis in patients with HIT II can persist for up to at least 6 weeks, and therefore anticoagulation is recommended for a period of at least 2 to 3 months.
In the meantime, several anticoagulants are available as alternatives to heparin in patients with suspected or proven HIT II. These are the thrombin-specific inhibitors hirudin (lepirudin, Refludan) and argatroban and the heparinoid danaparoid-natrium (Orgaran). Their compositions and pharmacological properties are summarized in Table 2. All of them require careful dosing and monitoring, since there is no antidote available.
TABLE 2.
Alternative anticoagulants for patients with HIT II
| Anti- coagulant | Structure | Approx molecular mass (Da) | Mode of action | Half-life | Excretion | Half-life in:
|
Monitoring | Immunological side effects | |
|---|---|---|---|---|---|---|---|---|---|
| Hepatic disease | Renal disease | ||||||||
| Hirudin (lepirudin) | Recombinant protein | 7,000 | Inhibition of thrombin | 90 min | 90% renal | Normal | Prolonged (up to 52 h [55]) | aPTT, ECT | Drug-induced antibodies enhance anticoagulant effect |
| Argatroban | Synthetic peptide | 500 | Direct inhibition of thrombin | 40-50 min | Mainly through liver | Prolonged | Normal | aPTT | None reported so far |
| Danaparoid | Heparinoid (84% heparan sulfate, 12% dermatan sulfate, 4% chondroitin sulfate) | 6,000 | Mainly inhibition of factor Xa, also antithrombin activity | Approx 24 h for factor Xa inhibition and 3 h for antithrombin activity | Mainly renal | Normal | Prolonged | Anti-factor Xa activity | Rarely antibodies to PF4 (without clinical significance) |
As studies which directly compare the clinical benefits of these substances are lacking, other aspects, such as the physician's prior experience or the pharmacokinetic profile of the substance, might be considered in the therapeutic decision. The long half-life of danaparoid (25 h), the hepatic excretion of argatroban (for patients with renal failure), and the short half-lives of both argatroban (40 to 50 min) and lepirudin (1.5 h) may influence the decision-making process (76).
Lepirudin.
Lepirudin is the recombinant form of hirudin, which originates from the saliva of the medicinal leech and is the most potent natural thrombin inhibitor. Hirudin, a single-polypeptide-chain protein of 7,000 Da, specifically inhibits thrombin by blocking its active site (63). The interaction with thrombin is more specific and faster than that between thrombin and its natural substrate fibrinogen. In two prospective studies the initiation of hirudin therapy has been shown to be effective by decreasing the risk of death, new thromboembolic complications, and limb amputation from 6.1% per day to 1.3% per day (16).
Hirudin is not inactivated by PF4, is also capable of inhibiting platelet-bound thrombin, and does not cross-react with HIT II antibodies. It has a short half-life of about 1.5 h after parenteral application and is excreted renally to over 90%. Thus, the half-life of the drug is dramatically prolonged in patients with impaired renal function (e.g., in the case of nephrectomy, up to 150 h) (55).
Patients with HIT II should receive lepirudin for 2 to 10 days according to the clinical course. The dose should be adjusted as follows (17): (i) for treatment of patients with proven thromboembolic complications (TECs), 0.4-mg/kg bolus intravenously (i.v.), followed by 0.15 mg/kg/h; (ii) for treatment of patients with proven TECs under thrombolytic therapy, 0.2-mg/kg bolus i.v., followed by 0.1 mg/kg/h; and (iii) for prophylaxis in patients without (proven) TECs, 0.1 mg/kg/h. For patients with impaired renal function, the dose should be reduced as follows (17) for the indicated serum creatinine level (in milligrams per deciliter): up to 1.5, bolus reduced to 0.2 mg/kg; 1.6 to 2.0, infusion rate reduced by 50%; 2.1 to 2.5, infusion rate reduced by 75%; 2.6 to 6.0, infusion rate reduced by 90%. Patients with a serum creatinine level of >6 mg/dl should receive only boli of 0.1 mg/kg every second day if the activated partial thromboplastin time (aPTT) ratio is <1.5 (17).
There are two major problems concerning therapy with lepirudin. One is the tendency to develop antihirudin antibodies, which enhance the anticoagulant activity of lepirudin due to prolonged elimination of the drug-antibody complexes. In a prospective study of patients receiving long-term lepirudin i.v., 84% of patients developed antilepirudin antibodies between days 10 and 30 (25). The formation of antibodies also leads to a moderate increase in aPTT. Second, the lack of an antidote necessitates careful monitoring, since overdosing can cause excessive bleeding.
Laboratory monitoring.
The aPTT is the preferred test for monitoring the anticoagulant activity of hirudin in most clinical settings but not in aortocoronary venous bypass. An aPTT ratio of 1.5 to 3.0 (for Actin FS or Neothromtin) or of 1.5 to 2.5 (for other reagents) should be achieved (17). At least daily monitoring of the aPTT is mandatory in order to assess the need for dose adjustment (1, 13). If normal, the patient's own baseline level before anticoagulation may serve as the aPTT reference. If not, the median of the hospital's normal range should be used. A therapeutically induced prolongation of the aPTT can be achieved in around 70% of patients, but the interindividual variability is high (17). In a study with healthy volunteers, the aPTT was doubled by 0.4 μg of recombinant hirudin (r-hirudin) per ml of plasma, and in a case report on a patient awaiting surgery for aortic valve replacement, a prolonged aPTT of between 60 and 80 s was associated with a plasma r-hirudin concentration of 1 to 1.5 μg/ml (8, 48). Potzsch et al. found a correlation coefficient of 0.61 between prolongation of the aPTT and the plasma r-hirudin concentration in r-hirudin-treated patients (44). An aPTT exceeding 70 s is increasingly insensitive to increasing concentrations of r-hirudin and therefore is not suitable for procedures and clinical settings with the need for high concentrations of hirudin (e.g., cardiopulmonary bypass surgery [CPB]) (18).
As an alternative, the ecarin clotting time (ECT) method, as a rapid, sensitive, and reproducible assay, may serve to calculate hirudin concentrations in whole blood or plasma. It is based on the measurement of coagulation activity. In this test the snake venom ecarin activates prothrombin in an alternative way to its intermediate meizothrombin independently of the presence of phospholipids and calcium. Hirudin inhibits meizothrombin. The degree of prolongation of the resulting clotting time correlates in a linear way with the plasma hirudin level (r = 0.94) (44, 46). This linear correlation can be found in a wide range of concentrations (0.05 to 5 μg/ml), and thus no dilution procedures are necessary for measurement of subtherapeutic, therapeutic, or toxic concentrations. Heparin or oral anticoagulants in the sample do not influence the performance of the ECT method, just as little as fibrinogen (from 60 to 100%) and prothrombin (from 20 to 100%). The test is applicable to different types of coagulation analyzers. A device to monitor the ECT during CPB has been approved by the Food and Drug Administration (30). Moreover, if whole blood is used, the ECT takes on the character of a bedside test because the time-consuming centrifugation step does not need to be performed. The disadvantages of the whole-blood ECT method are poor reproducibility compared to plasma ECT and variations due to fluctuation in hematocrit levels.
There are also assays available for directly measuring the hirudin concentration (using a chromogenic substrate or ELISA). These are designed primarily for scientific purposes or the assessment of activity of hirudin preparations, because they are expensive and the time required makes them unsuitable for routine testing or bedside monitoring (35).
Hirudin levels in plasma for use in various clinical situations are listed in Table 3. If hirudin levels are measured in whole blood, the resulting concentrations have to be converted according to the hematocrit value.
TABLE 3.
Hirudin plasma concentrations for various settings
| Setting | Hirudin level (μg/ml) in plasma |
|---|---|
| Therapeutic | 0.5-1.5 |
| Prophylactic | 0.25-0.75 |
| Extracorporal circulation in: | |
| CPB | 3-5 |
| Hemofiltration | 0.8-1.5 |
| Hemodialysis | 0.6-1 |
In October 2002 the European Agency for the Evaluation of Medical Products (EMEA) published a statement on seven recent reports of severe anaphylactic reactions in patients receiving Refludan. In six of these cases the patients had been reexposed to hirudin; five cases were fatal. Physicians should be aware of this fact and therefore should consider treatment only in the case of approved indications (adult patients with HIT II requiring parenteral anticoagulation), especially if the patient recently had been treated with hirudin. There should be access to treatment for anaphylactic shock reactions. The revised product information is published on the EMEA website (http://www.emea.eu.int).
Argatroban.
Argatroban, a synthetic peptide, directly inhibits thrombin through reversible and specific binding to the catalytic domain of thrombin. It has been evaluated in patients with probable HIT II (34). It has a half-life of about 40 min and is eliminated predominantly via hepatic excretion. The usual treatment dose is 2 μg/kg/min; with this dose the therapeutic range of the aPTT (1.5 to 3 times the baseline level) as the routine monitoring parameter will be achieved within 4 to 5 h of initiation of therapy (34). Other coagulation parameters (i.e., activated clotting time, INR, and ECT) are also prolonged, a fact that should be taken into account if a switch to oral coagulation is planned.
As argatroban is not excreted renally, a dose reduction is not required in patients with renal insufficiency. When administered to patients with compromised liver function, a fourfold decrease in the starting dose is recommended.
Danaparoid-natrium.
Danaparoid is a heparin-like substance with a low antithrombin activity (half-life 2 to 4 h) and major and long-lasting activity against clotting factor Xa (half-life of 24 h). The ratio of anti-factor Xa activity to anti-factor IIa activity is 28:1 (for heparin it is 1:1). A steady state is reached after 4 to 5 days of administration, and 40 to 50% of danaparoid is excreted renally. Danaparoid is primarily indicated as an immediate substitute for heparin in HIT II patients requiring postoperative prophylaxis against venous thromboembolism. The recommended dosage is two doses of 750 anti-factor Xa units daily subcutaneously for prophylaxis and a bolus of 1,500 U i.v., followed by 1,500 U subcutaneously twice a day for therapeutic reasons and 2,500 U as a bolus i.v., followed by 400 U/h for 4 h, then 300 U for 4 h, and finally 150 to 200 U/h for parenteral anticoagulation to maintain anti-factor Xa activity at 0.5 to 0.8 U/ml. Therapy can be monitored by quantifying anti-factor Xa activity (in an assay with danaparoid as a standard) (31). Monitoring is mandatory in patients with severe renal impairment, extremely low or high body weight, or thromboembolic or bleeding complications during therapy. There is no specific antidote available. Cross-reacting PF4/heparin complex antibodies can be detected in 7 to 50% of patients, but two retrospective studies showed no difference between patients treated with danaparoid independently of the presence of cross-reacting antibodies (53).
Duration of therapy and switch to oral anticoagulants.
Duration of treatment is not yet well defined. The risk for thrombosis in patients with HIT II can persist for up to at least 6 weeks after cessation of heparin therapy. Therefore, anticoagulation should be continued for at least 2 to 3 months. Warfarin should be initiated in low doses while the patient is still receiving danaparoid or a thrombin-specific inhibitor at a time when the platelet count is near normal, because the risk of thrombosis persists and cryptic thrombosis may occur (53). Once the platelet count has recovered to at least 100 to 150/nl, warfarin should be introduced cautiously, with a minimum of a 5-day overlap with the parenterally applied anticoagulant (20).
Discontinuation of heparin and initiation of warfarin therapy without intermittent application of one of the alternative substances mentioned has been associated with venous limb gangrene. This is due to a transient decrease in the protein C concentration caused by warfarin and the ongoing thromboembolic process (72).
In patients receiving lepirudin, the drug infusion rate should be lowered to an aPTT ratio of 1.5 before the administration of warfarin starts. Lepirudin should be discontinued when the international normalized ratio reaches levels of >2 (17).
Anticoagulation in patients with a history of HIT II.
Patients often require further anticoagulation months or years following their episode of HIT II. In principle, all of the anticoagulants mentioned above are suitable, but therapy with danaparoid and hirudin carries the risk of life-threatening hemorrhage due to the need for sophisticated monitoring and the lack of a neutralizing agent. Therefore, in certain clinical situations (e.g., cardiac or vascular surgery), heparin is the drug of choice because of the extensive experience with this substance and the possibility of rapid neutralization by protamine. Generally, HIT II antibodies are transient and detectable for only 2 to 3 months following an episode of acute HIT II, and they do not recur regularly if the patient is reexposed to heparin. In the case of recurrence they appear at the earliest after 3 days. This aspect creates the option for short-term heparin reexposure in the absence of HIT II antibodies (exclusion by a sensitive assay) in certain clinical situations such as CPB (45, 76). Physicians should be aware that factor concentrates and antithrombin III preparations may contain heparin.
MANAGEMENT OF ACUTE HIT II IN PARTICULAR CLINICAL SETTINGS
HIT II in pregnancy and childhood.
Danaparoid has been successfully used during pregnancy, and there is no evidence for crossing of the placental barrier or appearance in breast milk (10). Hirudin should be administered cautiously in pregnancy, because embryotoxic effects have been demonstrated in rabbits after administration of high concentrations of hirudin (38). Hirudin is not secreted into the breast milk (36). For the application of argatroban in pregnancy, no data are available.
HIT II in the intensive care unit.
Thrombin-specific inhibitors are perfectly suited for patients in the intensive care unit due to their short half-lives. Since antidotes are lacking, in cases of severe bleeding under therapy with these inhibitors the infusion of recombinant factor VIIa should be considered. If danaparoid is administered in doses greater than those used for prophylactic purposes, monitoring assays should be readily available.
HIT II in dialysis patients.
For dialysis patients argatroban is the ideal alternative to heparin, since it is not excreted renally and therefore does not require dose adjustment (52). The half-life of lepirudin in patients undergoing dialysis is 52 h. It has been successfully used to maintain anticoagulation during dialysis when given as a single bolus prior to dialysis at a dose of 0.08 mg/kg (56). Danaparoid was administered to patients undergoing hemofiltration with an initial bolus of 2,500 U followed by an infusion of 200 to 600 U/h (11).
HIT II during CPB.
The application of heparin should be considered first for the above-mentioned reasons (short half-life and availability of the antidote protamine). If surgery can be delayed until the platelet count has normalized, then short-term heparin therapy could be used perioperatively. In the pre- and postoperative periods, an alternative anticoagulant should be given. If the need for surgery emerges in a patient with HIT II, danaparoid or lepirudin must be administered with frequent perioperative monitoring of the anticoagulant activity. Such a proceeding should be carefully coordinated among the specialties involved (32).
In the last decade, lepirudin has been used in selected patients with HIT II during CPB, with a whole-blood lepirudin level of 3 to 4 μg/ml throughout the procedure (32, 46). These are much higher levels than those that are effective in patients with deep venous thrombosis (0.6 μg/ml) (41). Such high levels cannot be quantified accurately by activated clotting time or aPTT, as they would exceed 120 s and the correlation to the hirudin level would be poor. The use of hirudin during CPB demands close monitoring of the anticoagulation activity by ECT, which shows a linear correlation to hirudin concentration up to 4 μg/ml. Another advantage is that measuring the ECT in whole blood gives rapid results for close monitoring. A scheme for dosing of hirudin during CPB is proposed in Table 4 (46). For the management of patients with a history of HIT II, see above.
TABLE 4.
Anticoagulation with r-hirudin during CPBa
| CPB stage | Action and r-hirudin concn |
|---|---|
| Preoperative | Determine an individual standard curve for the ECT with the patient's own blood |
| Before starting CPB (target concn of hirudin, 3.0-5.0 μg/ml) | Priming of the machine, 0.2 mg/kg; IV bolus 10 min before CPB start, 0.25 mg/kg; infusion of hirudin (50 mg/50 ml), 0.5 ml/min |
| ECT after one circulation step | For r-hirudin concn of >3 μg/ml, CPB start possible; for r-hirudin concn of <3 μg/ml, CPB start not possible; hirudin infusion at 60 ml/h, control of ECT after 5 min |
| During CPB (target concn of hirudin, 3.5-5.0 μg/ml) | Control of ECT every 15 min; for r-hirudin concn of >4.5 μg/ml, reduce infusion vol by 10 ml/h; for r-hirudin concn of <3.5 μg/ml, increase infusion vol by 10 ml/h; for r-hirudin concn of <3 μg/ml, 5 mg of hirudin as a single bolus |
| 30 min before finishing CPB (target concn of hirudin, 2.5-3.5 μg/ml) | Control of ECT every 5 min; gradually reduce infusion by 5 ml/h after finishing CPB; put 5 mg of r-hirudin in recirculating machine |
Adapted from reference 42 with permission.
Some investigators have adapted the ECT to mechanical systems with ready-to-use cartridges to be able to measure the ECT in the operating room as a point-of-care test (30).
If danaparoid should be used in the CPB setting, the anti-factor Xa activity should be adjusted to 0.7 to 1.5 U/ml to avoid excessive bleeding (1).
CONCLUSIONS
To diagnose and to treat the most threatening complication in heparin therapy, immune-mediated HIT II, the correct interpretation of laboratory results and knowledge about the characteristics of alternative substances are absolute essentials to supplement clinical decision making.
In cases where a strong clinical probability is accompanied by positive results in both functional and immunological assays, the diagnosis of HIT II can be made without doubt. Otherwise, variable sensitivity and specificity of laboratory assays in combination with a questionable probability of the clinical diagnosis of HIT II may generate situations in which a final statement about the presence or absence of HIT II is not possible.
Diagnosis of HIT II, even in cases with moderate probability, results in the mandatory replacement of heparin with alternative substances. Results of laboratory testing should not be awaited before their use, since in the acute phase of HIT II, cessation of heparin alone does not prevent thromboembolic complications. Studies comparing the three available alternative substances are still lacking. Therefore, the selection of a certain substance should be based on the experience of the physician, the pharmacokinetic characteristics of the substance, the mode of excretion, and the underlying clinical situation which created the initial application of heparin.
Despite the improvement in laboratory testing and the introduction of new anticoagulants, the diagnosis and treatment of HIT II still remain a challenge for the treating physician, and this review may aid in the diagnostic process.
REFERENCES
- 1.Alving, B. M. 2003. How I treat heparin-induced thrombocytopenia and thrombosis. Blood 101:31-37. [DOI] [PubMed] [Google Scholar]
- 2.Amiral, J., F. Bridey, M. Dreyfus, A. M. Vissoc, E. Fressinaud, M. Wolf, and D. Meyer. 1992. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb. Haemost. 68:95-96. [PubMed] [Google Scholar]
- 3.Amiral, J., F. Bridey, M. Wolf, C. Boyer-Neumann, E. Fressinaud, A. M. Vissac, E. Peynaud-Debayle, M. Dreyfus, and D. Meyer. 1995. Antibodies to macromolecular platelet factor 4-heparin complexes in heparin-induced thrombocytopenia: a study of 44 cases. Thromb. Haemost. 73:21-28. [PubMed] [Google Scholar]
- 4.Amiral, J., A. Marfaing-Koka, M. Wolf, M. C. Alessi, B. Tardy, C. Boyer-Neumann, A. M. Vissac, E. Fressinaud, M. Poncz, and D. Meyer. 1996. Presence of autoantibodies to interleukin-8 or neutrophil-activating peptide-2 in patients with heparin-associated thrombocytopenia. Blood 88:410-416. [PubMed] [Google Scholar]
- 5.Amiral, J., M. Wolf, A. Fischer, C. Boyer-Neumann, A. Vissac, and D. Meyer. 1996. Pathogenicity of IgA and/or IgM antibodies to heparin-PF4 complexes in patients with heparin-induced thrombocytopenia. Br. J. Haematol 92:954-959. [DOI] [PubMed] [Google Scholar]
- 6.Arepally, G., C. Reynolds, A. Tomaski, J. Amiral, A. Jawad, M. Poncz, and D. B. Cines. 1995. Comparison of PF4/heparin ELISA assay with the 14C-serotonin release assay in the diagnosis of heparin-induced thrombocytopenia. Am. J. Clin. Pathol. 104:648-654. [DOI] [PubMed] [Google Scholar]
- 7.Bauer, T. L., G. Arepally, B. A. Konkle, B. Mestichelli, S. S. Shapiro, D. B. Cines, M. Poncz, S. McNulty, J. Amiral, W. W. Hauck, R. N. Edie, and J. D. Mannion. 1997. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation 95:1242-1246. [DOI] [PubMed] [Google Scholar]
- 8.Bichler, J., and H. Fritz. 1991. Hirudin, a new therapeutic tool? Ann. Hematol. 63:67-76. [DOI] [PubMed] [Google Scholar]
- 9.Chong, B. H., J. Burgess, and F. Ismail. 1993. The clinical usefulness of the platelet aggregation test for the diagnosis of heparin-induced thrombocytopenia. Thromb. Haemost. 69:344-350. [PubMed] [Google Scholar]
- 10.Chong, B. H., and H. N. Magnani. 2001. Danaparoid for the treatment of heparin-induced thrombocytopenia, p. 323. In T. E. Warkentin and A. Greinacher (ed.), Heparin-induced thrombocytopenia, 2nd ed. Marcel Dekker, New York, N.Y.
- 11.Chong, B. H., and H. N. Magnani. 1992. Orgaran in heparin-induced thrombocytopenia. Haemostasis 22:85-91. [DOI] [PubMed] [Google Scholar]
- 12.Eichler, P., U. Budde, S. Haas, H. Kroll, R. M. Loreth, O. Meyer, U. Pachmann, B. Potzsch, A. Schabel, D. Albrecht, and A. Greinacher. 1999. First workshop for detection of heparin-induced antibodies: validation of the heparin-induced platelet-activation test (HIPA) in comparison with a PF4/heparin ELISA. Thromb. Haemost. 81:625-629. [PubMed] [Google Scholar]
- 13.Eichler, P., H. J. Friesen, N. Lubenow, B. Jaeger, and A. Greinacher. 2000. Antihirudin antibodies in patients with heparin-induced thrombocytopenia treated with lepirudin: incidence, effects on aPTT, and clinical relevance. Blood 96:2373-2378. [PubMed] [Google Scholar]
- 14.Eichler, P., R. Raschke, N. Lubenow, O. Meyer, P. Schwind, and A. Greinacher. 2002. The new ID-heparin/PF4 antibody test for rapid detection of heparin-induced antibodies in comparison with functional and antigenic assays. Br. J. Haematol. 116:887-891. [DOI] [PubMed] [Google Scholar]
- 15.Greinacher, A., J. Amiral, V. Dummel, A. Vissac, V. Kiefel, and C. Mueller-Eckhardt. 1994. Laboratory diagnosis of heparin-associated thrombocytopenia and comparison of platelet aggregation test, heparin-induced platelet activation test, and platelet factor 4/heparin enzyme-linked immunosorbent assay. Transfusion 34:381-385. [DOI] [PubMed] [Google Scholar]
- 16.Greinacher, A., P. Eichler, N. Lubenow, H. Kwasny, and M. Luz. 2000. Heparin-induced thrombocytopenia with thromboembolic complications: meta-analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood 96:846-851. [PubMed] [Google Scholar]
- 17.Greinacher, A., U. Janssens, G. Berg, M. Bock, H. Kwasny, B. Kemkes-Matthes, P. Eichler, H. Volpel, B. Potzsch, M. Luz, et al. 1999. Lepirudin (recombinant hirudin) for parenteral anticoagulation in patients with heparin-induced thrombocytopenia. Circulation 100:587-593. [DOI] [PubMed] [Google Scholar]
- 18.Greinacher, A., and N. Lubenow. 2002. Heparininduzierte Thrombozytopenie. Die gelben Hefte 42:61-70. [Google Scholar]
- 19.Greinacher, A., I. Michels, V. Kiefel, and C. Mueller-Eckhardt. 1991. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb. Haemost. 66:734-736. [PubMed] [Google Scholar]
- 20.Greinacher, A., and T. E. Warkentin. 2001. Treatment of heparin-induced thrombocytopenia: an overview, p. 291-322. In T. E. Warkentin and A. Greinacher (ed.), Heparin-induced thrombocytopenia., 2nd ed. Marcel Dekker, New York, N.Y.
- 21.Griffiths, E., and W. H. Dzik. 1997. Assays for heparin-induced thrombocytopenia. Transfus. Med. 7:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Harenberg, J., G. Huhle, C. Giese, L. C. Wang, M. Feuring, X. H. Song, and U. Hoffmann. 2000. Determination of serotonin release from platelets by enzyme immunoassay in the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 109:182-186. [DOI] [PubMed] [Google Scholar]
- 23.Harenberg, J., L. Wang, U. Hoffmann, G. Huhle, and M. Feuring. 2001. Improved laboratory confirmation of heparin-induced thrombocytopenia type II. Time course of antibodies and combination of antigen and biologic assays. Am. J. Clin. Pathol. 115:432-438. [PubMed] [Google Scholar]
- 24.Harenberg, J., L. C. Wang, U. Hoffmann, G. Huhle, and M. Feuring. 2001. Laboratory diagnosis of heparin-induced thrombocytopenia type II after clearance of platelet factor 4/heparin complex. J. Lab. Clin. Med. 137:408-413. [DOI] [PubMed] [Google Scholar]
- 24a.Harenberg, J., G. Huhle, C. Giese, L. C. Wang, M. Feuring, X. H. Song, and U. Hoffmann. 2000. Determination of serotonin release from platelets by enzyme immunoassay in the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 109:182-186. [DOI] [PubMed]
- 25.Huhle, G., U. Hoffmann, X. Song, L. C. Wang, D. L. Heene, and J. Harenberg. 1999. Immunologic response to recombinant hirudin in HIT type II patients during long-term treatment. Br. J. Haematol. 106:195-201. [DOI] [PubMed] [Google Scholar]
- 26.Izban, K. F., H. W. Lietz, D. A. Hoppensteadt, W. P. Jeske, J. Fareed, M. Bakhos, and J. M. Walenga. 1999. Comparison of two PF4/heparin ELISA assays for the laboratory diagnosis of heparin-induced thrombocytopenia. Semin. Thromb. Hemost. 25:51-56. [PubMed] [Google Scholar]
- 27.Jackson, M. R., C. Krishnamurti, C. A. Aylesworth, and B. M. Alving. 1997. Diagnosis of heparin-induced thrombocytopenia in the vascular surgery patient. Surgery 121:419-424. [DOI] [PubMed] [Google Scholar]
- 28.Jy, W., W. W. Mao, L. L. Horstman, P. A. Valant, and Y. S. Ahn. 1999. A flow cytometric assay of platelet activation marker P-selectin (CD62P) distinguishes heparin-induced thrombocytopenia (HIT) from HIT with thrombosis (HITT). Thromb. Haemost. 82:1255-1259. [PubMed] [Google Scholar]
- 29.Kelton, J. G., D. Sheridan, H. Brain, P. J. Powers, A. G. Turpie, and C. J. Carter. 1984. Clinical usefulness of testing for a heparin-dependent platelet-aggregating factor in patients with suspected heparin-associated thrombocytopenia. J. Lab. Clin. Med. 103:606-612. [PubMed] [Google Scholar]
- 29a.Koch, S., J. Harenberg, M. Odel, H. Schmidt-Gayk, S. Walch, and U. Budde. 2002. Development of a high-pressure liquid chromatography method for diagnosis of heparin-induced thrombocytopenia. Am. J. Clin. Pathol. 117:900-904. [DOI] [PubMed]
- 30.Koster, A., M. Loebe, R. Hansen, M. Bauer, F. Mertzlufft, H. Kuppe, and R. Hetzer. 2000. A quick assay for monitoring recombinant hirudin during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II: adaptation of the ecarin clotting time to the act II device. J. Thorac. Cardiovasc. Surg. 119:1278-1283. [DOI] [PubMed] [Google Scholar]
- 31.Laposata, M., D. Green, E. M. Van Cott, T. W. Barrowcliffe, S. H. Goodnight, and R. C. Sosolik. 1998. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: the clinical use and laboratory monitoring of low-molecular-weight heparin, danaparoid, hirudin and related compounds, and argatroban. Arch. Pathol. Lab. Med. 122:799-807. [PubMed] [Google Scholar]
- 32.Latham, P., A. F. Revelis, G. P. Joshi, J. M. DiMaio, and M. E. Jessen. 2000. Use of recombinant hirudin in patients with heparin-induced thrombocytopenia with thrombosis requiring cardiopulmonary bypass. Anesthesiology 92:263-266. [DOI] [PubMed] [Google Scholar]
- 33.Lee, D. H., T. E. Warkentin, G. A. Denomme, C. P. Hayward, and J. G. Kelton. 1996. A diagnostic test for heparin-induced thrombocytopenia: detection of platelet microparticles using flow cytometry. Br. J. Haematol. 95:724-731. [DOI] [PubMed] [Google Scholar]
- 34.Lewis, B. E., D. E. Wallis, S. D. Berkowitz, W. H. Matthai, J. Fareed, J. M. Walenga, J. Bartholomew, R. Sham, R. G. Lerner, Z. R. Zeigler, P. K. Rustagi, I. K. Jang, S. D. Rifkin, J. Moran, M. J. Hursting, and J. G. Kelton. 2001. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation 103:1838-1843. [DOI] [PubMed] [Google Scholar]
- 35.Lindhoff-Last, E., G. P. Piechottka, F. Rabe, and R. Bauersachs. 2000. Hirudin determination in plasma can be strongly influenced by the prothrombin level. Thromb. Res. 100:55-60. [DOI] [PubMed]
- 36.Lindhoff-Last, E., A. Willeke, C. Thalhammer, G. Nowak, and R. Bauersachs. 2000. Hirudin treatment in a breastfeeding woman. Lancet 355:467-468. [DOI] [PubMed] [Google Scholar]
- 37.Look, K. A., M. Sahud, S. Flaherty, and J. L. Zehnder. 1997. Heparin-induced platelet aggregation vs platelet factor 4 enzyme-linked immunosorbent assay in the diagnosis of heparin-induced thrombocytopenia-thrombosis. Am. J. Clin. Pathol. 108:78-82. [PubMed] [Google Scholar]
- 38.Lubenow, N., and A. Greinacher. 2000. Management of patients with heparin-induced thrombocytopenia: focus on recombinant hirudin. J. Thromb. Thrombolysis 10:47-57. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, O., A. Salama, N. Pittet, and P. Schwind. 1999. Rapid detection of heparin-induced platelet antibodies with particle gel immunoassay (ID-HPF4). Lancet 354:1525-1526. [DOI] [PubMed] [Google Scholar]
- 40.Newman, P. M., R. L. Swanson, and B. H. Chong. 1998. Heparin-induced thrombocytopenia: IgG binding to PF4-heparin complexes in the fluid phase and cross-reactivity with low molecular weight heparin and heparinoid. Thromb. Haemost. 80:292-297. [PubMed] [Google Scholar]
- 41.Parent, F., F. Bridey, M. Dreyfus, D. Musset, G. Grimon, P. Duroux, D. Meyer, and G. Simonneau. 1993. Treatment of severe venous thrombo-embolism with intravenous Hirudin (HBW 023): an open pilot study. Thromb. Haemost. 70:386-388. [PubMed] [Google Scholar]
- 42.Poetzsch, B., and K. Madlener. 1997. Antikoagulationsregime mit Hirudin während des kardiopulmonalen Bypass, p. 17. In Workshop “Heparin-induzierte Thrombozytopenie in der Herzchirurgie.” Kerckhoff Clinics, Bad Nauheim, Germany.
- 43.Poley, S., and W. Mempel. 2001. Laboratory diagnosis of heparin-induced thrombocytopenia: advantages of a functional flow cytometric test in comparison to the heparin-induced platelet-activation test. Eur. J. Haematol. 66:253-262. [DOI] [PubMed] [Google Scholar]
- 44.Potzsch, B., S. Hund, K. Madlener, C. Unkrig, and G. Muller-Berghaus. 1997. Monitoring of recombinant hirudin: assessment of a plasma-based ecarin clotting time assay. Thromb. Res. 86:373-383. [DOI] [PubMed] [Google Scholar]
- 45.Potzsch, B., W. P. Klovekorn, and K. Madlener. 2000. Use of heparin during cardiopulmonary bypass in patients with a history of heparin-induced thrombocytopenia. N. Engl. J. Med. 343:515. [DOI] [PubMed] [Google Scholar]
- 46.Potzsch, B., K. Madlener, C. Seelig, C. F. Riess, A. Greinacher, and G. Muller-Berghaus. 1997. Monitoring of r-hirudin anticoagulation during cardiopulmonary bypass—assessment of the whole blood ecarin clotting time. Thromb. Haemost. 77:920-925. [PubMed] [Google Scholar]
- 47.Pouplard, C., J. Amiral, J. Y. Borg, A. M. Vissac, B. Delahousse, and Y. Gruel. 1997. Differences in specificity of heparin-dependent antibodies developed in heparin-induced thrombocytopenia and consequences on cross-reactivity with danaparoid sodium. Br. J. Haematol. 99:273-280. [DOI] [PubMed] [Google Scholar]
- 48.Riess, F. C., C. Lower, C. Seelig, N. Bleese, J. Kormann, G. Muller-Berghaus, and B. Potzsch. 1995. Recombinant hirudin as a new anticoagulant during cardiac operations instead of heparin: successful for aortic valve replacement in man. J. Thorac. Cardiovasc. Surg. 110:265-267. [DOI] [PubMed] [Google Scholar]
- 49.Rugeri, L., A. Bauters, N. Trillot, S. Susen, C. Decoen, A. Watel, and B. Jude. 1999. Hemostasis and thrombosis: clinical usefulness of combined use of platelet aggregation test and anti PF4-H antibodies Elisa test for the diagnosis of heparin induced thrombocytopenia. Hematology 4:367-372. [DOI] [PubMed] [Google Scholar]
- 50.Sheridan, D., C. Carter, and J. G. Kelton. 1986. A diagnostic test for heparin-induced thrombocytopenia. Blood 67:27-30. [PubMed] [Google Scholar]
- 51.Stewart, M. W., W. S. Etches, L. K. Boshkov, and P. A. Gordon. 1995. Heparin-induced thrombocytopenia: an improved method of detection based on lumi-aggregometry. Br. J. Haematol. 91:173-177. [DOI] [PubMed] [Google Scholar]
- 52.Swan, S. K., and M. J. Hursting. 2000. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy 20:318-329. [DOI] [PubMed] [Google Scholar]
- 53.Tardy, B., B. Tardy-Poncet, P. Fournel, C. Venet, R. Jospe, and A. Dacosta. 1999. Lower limb veins should be systematically explored in patients with isolated heparin-induced thrombocytopenia. Thromb. Haemost. 82:1199-1200. [PubMed] [Google Scholar]
- 54.Tomer, A. 1997. A sensitive and specific functional flow cytometric assay for the diagnosis of heparin-induced thrombocytopenia. Br. J. Haematol. 98:648-656. [DOI] [PubMed] [Google Scholar]
- 55.Vanholder, R., A. Camez, N. Veys, A. Van Loo, A. M. Dhondt, and S. Ringoir. 1997. Pharmacokinetics of recombinant hirudin in hemodialyzed end-stage renal failure patients. Thromb. Haemost. 77:650-655. [PubMed] [Google Scholar]
- 56.Vanholder, R. C., A. A. Camez, N. M. Veys, J. Soria, M. Mirshahi, C. Soria, and S. Ringoir. 1994. Recombinant hirudin: a specific thrombin inhibiting anticoagulant for hemodialysis. Kidney Int. 45:1754-1759. [DOI] [PubMed] [Google Scholar]
- 57.Visentin, G. P., S. E. Ford, J. P. Scott, and R. H. Aster. 1994. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J. Clin. Investig. 93:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitale, M., P. Tazzari, F. Ricci, M. A. Mazza, G. Zauli, G. Martini, L. Caimi, F. A. Manzoli, and R. Conte. 2001. Comparison between different laboratory tests for the detection and prevention of heparin-induced thrombocytopenia. Cytometry 46:290-295. [DOI] [PubMed] [Google Scholar]
- 59.Vun, C. M., S. Evans, and C. N. Chesterman. 2001. Anti-PF4-heparin immunoglobulin G is the major class of heparin-induced thrombocytopenia antibody: findings of an enzyme-linked immunofiltration assay using membrane-bound hPF4-heparin. Br. J. Haematol. 112:69-75. [DOI] [PubMed] [Google Scholar]
- 60.Walenga, J. M., W. P. Jeske, A. R. Fasanella, J. J. Wood, S. Ahmad, and M. Bakhos. 1999. Laboratory diagnosis of heparin-induced thrombocytopenia. Clin. Appl. Thromb. Hemost. 5:S21-S27. [DOI] [PubMed]
- 61.Walenga, J. M., W. P. Jeske, A. R. Fasanella, J. J. Wood, and M. Bakhos. 1999. Laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Semin. Thromb. Hemost. 25:43-49. [PubMed] [Google Scholar]
- 62.Walenga, J. M., W. P. Jeske, J. J. Wood, S. Ahmad, B. E. Lewis, and M. Bakhos. 1999. Laboratory tests for heparin-induced thrombocytopenia: a multicenter study. Semin. Hematol. 36:22-28. [PubMed] [Google Scholar]
- 63.Wallis, R. B. 1996. Hirudins: from leeches to man. Semin. Thromb. Hemost. 22:185-196. [DOI] [PubMed] [Google Scholar]
- 64.Wang, L., G. Huhle, R. Malsch, U. Hoffmann, X. Song, and J. Harenberg. 1999. Determination of heparin-induced IgG antibody by fluorescence-linked immunofiltration assay (FLIFA). J. Immunol. Methods 222:93-99. [DOI] [PubMed] [Google Scholar]
- 65.Wang, L. C., G. Huhle, R. Malsch, V. Hoffmann, X. L. Song, and J. Harenberg. 1999. Determination of heparin-induced IgG antibody in heparin-induced thrombocytopenia type II. Eur. J. Clin. Investig. 29:232-237. [DOI] [PubMed] [Google Scholar]
- 66.Warkentin, T. E. 2001. Clinical picture of heparin-induced thrombocytopenia, p. 43-86. In T. E. Warkentin and A. Greinacher (ed.), Heparin-induced thrombocytopenia., 2nd ed. Marcel Dekker, New York, N.Y.
- 67.Warkentin, T. E. 1999. Heparin-induced thrombocytopenia: a ten-year retrospective. Annu. Rev. Med. 50:129-147. [DOI] [PubMed] [Google Scholar]
- 68.Warkentin, T. E. 1996. Heparin-induced thrombocytopenia: IgG-mediated platelet activation, platelet microparticle generation, and altered procoagulant/anticoagulant balance in the pathogenesis of thrombosis and venous limb gangrene complicating heparin-induced thrombocytopenia. Transfus. Med. Rev. 10:249-258. [DOI] [PubMed] [Google Scholar]
- 69.Warkentin, T. E. 1997. Heparin-induced thrombocytopenia. Pathogenesis, frequency, avoidance and management. Drug Safety 17:325-341. [DOI] [PubMed] [Google Scholar]
- 70.Warkentin, T. E. 2000. Laboratory testing for heparin-induced thrombocytopenia. J. Thromb. Thrombolysis 10:35-45. [DOI] [PubMed] [Google Scholar]
- 71.Warkentin, T. E., B. H. Chong, and A. Greinacher. 1998. Heparin-induced thrombocytopenia: towards consensus. Thromb. Haemost. 79:1-7. [PubMed] [Google Scholar]
- 72.Warkentin, T. E., L. J. Elavathil, C. P. Hayward, M. A. Johnston, J. I. Russett, and J. G. Kelton. 1997. The pathogenesis of venous limb gangrene associated with heparin-induced thrombocytopenia. Ann. Intern. Med. 127:804-812. [DOI] [PubMed] [Google Scholar]
- 73.Warkentin, T. E., and A. Greinacher. 2000. Laboratory testing for heparin-induced thrombocytopenia, p. 211-244. In T. E. Warkentin and A. Greinacher (ed.), Heparin-induced thrombocytopenia. Marcel Dekker, New York, N.Y.
- 74.Warkentin, T. E., C. P. Hayward, C. A. Smith, P. M. Kelly, and J. G. Kelton. 1992. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J. Lab. Clin. Med. 120:371-379. [PubMed] [Google Scholar]
- 75.Warkentin, T. E., and J. G. Kelton. 1996. A 14-year study of heparin-induced thrombocytopenia. Am. J. Med. 101:502-507. [DOI] [PubMed] [Google Scholar]
- 76.Warkentin, T. E., and J. G. Kelton. 2001. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 344:1286-1292. [DOI] [PubMed] [Google Scholar]
- 77.Warkentin, T. E., M. N. Levine, J. Hirsh, P. Horsewood, R. S. Roberts, M. Gent, and J. G. Kelton. 1995. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N. Engl. J. Med. 332:1330-1335. [DOI] [PubMed] [Google Scholar]
- 78.Warkentin, T. E., J.-A. I. Sheppard, P. Horsewood, P. J. Simpson, J. C. Moore, and J. G. Kelton. 2000. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood 96:1703-1708. [PubMed] [Google Scholar]