Abstract
This study aimed to clarify how concentrations of vitamin C in plasma relate to the serology of periodontitis. The random sample used comprised 431 men, 194 from Finland and 237 from Russia. The plasma vitamin C concentration was determined by o-phtaldialdehyde-fluorometry, and serum immunoglobulin G antibodies to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis were determined by a multiserotype enzyme-linked immunosorbent assay (ELISA). The mean plasma vitamin C concentration was higher (P < 0.001) in Finnish subjects (mean ± standard deviation, 4.5 ± 2.8 mg/liter) than in Russian subjects (1.4 ± 1.8 mg/liter). Mean antibody levels to both A. actinomycetemcomitans (4.7 ± 3.6 versus 5.2 ± 3.1 ELISA units [P = 0.05]) and P. gingivalis (5.7 ± 2.5 versus 7.6 ± 2.9 ELISA units [P < 0.001]) were lower in Finnish men than in Russian men. In the combined Finnish and Russian population, the antibody levels to P. gingivalis were negatively correlated with vitamin C concentrations (r = −0.22; P < 0.001); this association remained statistically significant (P = 0.010) in a linear regression model after adjustment for confounding factors. The proportion of P. gingivalis-seropositive subjects decreased with increasing vitamin C concentrations (P for trend, <0.01), but no trend was seen among A. actinomycetemcomitans-seropositive subjects. In conclusion, P. gingivalis infection is associated with low concentrations of vitamin C in plasma, which may increase colonization of P. gingivalis or disturb the healing of the infected periodontium.
Periodontitis is usually a painless, slowly progressing infectious disease in tooth-supporting tissues. Persistent bacterial colonization on the tooth surfaces leads to chronic inflammation in periodontal tissues. Periodontal inflammation results in gingival bleeding, pocket formation, destruction of alveolar bone, and eventually loss of teeth (33). Severe forms of periodontitis are relatively common, affecting up to 20% of the population worldwide (34).
Although gingival bleeding is a clinical symptom of both scurvy and periodontitis, the two conditions are distinct disease entities. Unlike for scurvy, which is caused by vitamin C deficiency, the etiological agents in periodontitis are dental plaque bacteria, especially gram-negative microorganisms, including Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. An inflammatory response to the overgrowth of periodontal bacteria in general, and to certain species in particular, leads to microulceration in the epithelium-facing tooth surface in periodontal pockets, opening a route for the bacteria to the circulation. In periodontitis, bacteria and their components are commonly spread in circulating blood (7). The continued local or systemic bacterial stimulus causes release of proinflammatory mediators, which may have a role in the pathogenesis of atherosclerosis and stroke (14, 28, 35). Accordingly, findings suggesting a role of periodontitis in cardiovascular diseases (CVD) add a new perspective to the importance of oral status for systemic health (8).
Vitamin C has long been a candidate for modulating periodontal diseases, although the exact role of vitamin C deficiency in periodontitis is not known (2, 30, 44). Even though low vitamin C intake does not cause periodontitis, it is known that additional vitamin C is required during infectious diseases and tissue regeneration (17, 37). Avitaminosis-C is associated primarily with defective collagen synthesis, causing tissue dysfunction such as impaired wound healing and ruptured capillaries because of insufficient support of the capillary walls by the connective tissues (9). Regeneration of collagen to maintain the integrity of the tooth attachment elements is especially important for periodontal health. Since vitamin C is involved in the synthesis of intercellular substances such as collagen fibers found in various forms of connective tissues and the matrix of bone and teeth (15), and since vitamin C has immunomodulating functions influencing the susceptibility of a host to infectious diseases (4, 10), it is rational to hypothesize that a low vitamin C concentration in serum is a risk factor for periodontal diseases (30).
The role of a low vitamin C concentration in plasma as a risk factor for periodontitis needs reevaluation. First, the concerted action of intensified susceptibility to infections together with quantitative and qualitative changes in dental plaque microbiota favors the growth of fastidious periodontopathogenic species. This leads to an altered oral-periodontal ecosystem and increased risk for development of periodontitis. Second, due to the irreversible nature of the destruction of tooth-supporting tissues, untreated periodontitis increases the infectious burden, even after the correction of the vitamin C deficiency. The aim of the study was to clarify how vitamin C relates to periodontitis in two culturally different populations with very different plasma vitamin C concentrations.
MATERIALS AND METHODS
Population survey.
Comparable population surveys on risk factors for chronic diseases were carried out in North Karelia, Finland, and in Pitkäranta, Russia, in the spring of 1997. The sampling and study protocols were described earlier in detail (23, 45). The survey protocol followed the World Health Organization MONICA protocol (47). During these population surveys, plasma and serum samples were collected for the analyses of vitamin C concentrations and antibody responses to periodontal pathogens. The ethics committee approved the survey plan, and the subjects signed written informed consent agreements.
Study subjects.
Vitamin C concentrations in plasma and levels of serum antibodies to periodontal pathogens were determined from a random subsample of 431 men age 25 to 64 years (194 Finnish and 237 Russian). Data on smoking and education were collected by use of a questionnaire. Trained nurses counted the number of teeth and restorations. Serum carbohydrate-deficient transferrin (CDT) concentrations were determined with a double-antibody kit (CDTect; Pharmacia & Upjohn Diagnostics, Uppsala, Sweden) to estimate alcohol consumption (24).
Plasma vitamin C determinations.
The plasma samples were taken after 4 h of fasting, and the pH of the plasma was adjusted with 5% metaphosphoric acid (1:10, vol/vol) within half an hour of collection. The samples were frozen and transported on dry ice to the laboratory of the National Public Health Institute, Helsinki, Finland. The samples were stored at −20°C, and the total vitamin C concentration was determined within 6 months by an automated method (Autoanalyzer II; Technicon, New York, N.Y.) using o-phtaldialdehyde and fluorescence detection (5). For pooled plasma pretreated and stored as were the samples, the interassay variation was 4.7% (n = 10).
Serum antibodies to periodontal pathogens.
Serum immunoglobulin G (IgG) class antibodies to the periodontal pathogens A. actinomycetemcomitans and P. gingivalis were determined by enzyme-linked immunosorbent assays (ELISAs), in which six strains of A. actinomycetemcomitans, representing serotypes a, b, c, d, and e and a nonserotypeable strain, and three strains of P. gingivalis, representing serotypes a, b, and c, were used as antigens in the form of formalin-killed whole cells (36). Two dilutions (1:1,500 and 1:3,000 for A. actinomycetemcomitans and 1:100 and 1:200 for P. gingivalis) of each serum sample (stored at −70°C) in duplicate were used for the measurements, and the results (in ELISA units [EU]), as mean absorbances, were calculated as continuous variables. The subjects were considered seropositive for A. actinomycetemcomitans or P. gingivalis when the corresponding antibody value was ≥5.0 EU, which represents the mean antibody level + 1.5 times the standard deviation (SD) for the periodontally healthy subjects in our earlier study (36). The threshold value of ≥14.0 EU for the high level of the combined antibody response (antibodies to A. actinomycetemcomitans plus antibodies to P. gingivalis), which is considered the threshold level for severe periodontitis, represents the corresponding mean value + 3 times the SD for the periodontally healthy subjects (36). The interassay variation was monitored by a “high” and a “low” serum control on each plate, and the results were corrected according to the mean value for the high control. As calculated from the results for the low control (n = 23), the interassay coefficients of variation were 6.3 and 6.1% for A. actinomycetemcomitans and P. gingivalis, respectively.
Statistical analyses.
Differences in continuous and categorical variables between Finnish and Russian subjects were examined with t tests and chi-square tests, respectively. Pearson correlation analysis was used to test the correlation between serum antibody levels and plasma vitamin C concentrations. The associations of A. actinomycetemcomitans and P. gingivalis antibody levels with vitamin C concentration and the confounders were examined with linear regression analysis for the combined Finnish and Russian study populations. The statistical analyses were carried out with SAS program version 6.0 for VAX computers.
RESULTS
The characteristics of the survey subjects, including mean ages, serum A. actinomycetemcomitans and P. gingivalis antibody levels, and plasma vitamin C concentrations as well as mean values for possible confounding factors, are presented in Table 1. Although the Finnish men were slightly older than the Russian men, the mean levels of antibodies to both A. actinomycetemcomitans (4.69 versus 5.23 EU [P = 0.05]) and P. gingivalis (5.68 versus 7.61 EU [P < 0.001]) were significantly lower in Finland than in Russia, respectively. In contrast to the case for the antibody levels, the mean plasma vitamin C concentration was significantly (P < 0.001) higher in Finland (4.46 mg/liter) than in Russia (1.41 mg/liter). The mean number of teeth per subject was similar in the two populations, but the Russians had fewer (P < 0.001) dental fillings than the Finns. The Russian men were also more frequently (P < 0.001) current smokers (62.9 versus 29.8%), and, based on serum CDT values, they consumed more (P < 0.001) alcohol than the Finnish men (Table 1).
TABLE 1.
Characteristics of Finnish and Russian subjects
| Characteristic | Mean (SD) for group
|
P value | |
|---|---|---|---|
| Finnish | Russian | ||
| n | 194 | 237 | |
| Age (yr) | 48.2 (13.6) | 44.8 (11.4) | 0.005a |
| Serum antibodies (EU) to: | |||
| A. actinomycetemcomitans | 4.69 (2.63) | 5.23 (3.09) | 0.05a |
| P. gingivalis | 5.68 (2.52) | 7.61 (2.92) | <0.001a |
| % of subjects with a high combined antibody response | 19.1 | 32.5 | <0.001b |
| Plasma vitamin C (mg/liter)c | 4.46 (2.76) | 1.41 (1.84) | <0.001a |
| No. of teeth | 20.6 (12.6) | 21.1 (9.4) | nsa,d |
| No. of fillings | 6.4 (5.7) | 2.1 (2.5) | <0.001a |
| CDT (U/liter) | 13.7 (7.7) | 20.9 (14.9) | <0.001a |
| Smokers (%) | 29.8 | 62.9 | <0.001b |
| Yr of education | 10.0 (3.7) | 11.0 (3.1) | 0.003a |
t test.
Chi-square test.
1 mg/liter = 5.68 μmol/liter.
NS, not significant.
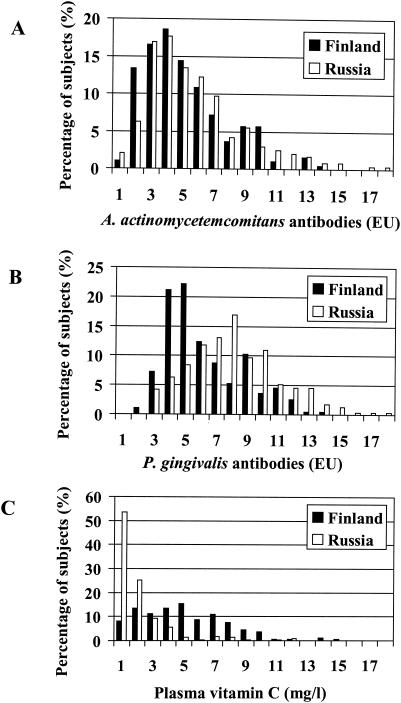
The distributions of A. actinomycetemcomitans and P. gingivalis antibody levels, as well as the distribution of plasma vitamin C concentrations, are shown in Fig. 1, separately for Finland and Russia. The proportions of subjects with a high combined antibody response (antibody to A. actinomycetemcomitans plus antibody to P. gingivalis of ≥14.0 EU), representing severe periodontitis, were 19.1 and 32.5% in Finland and in Russia (P < 0.001), respectively. The proportions of seronegative subjects (antibody level of <5.0 EU) for A. actinomycetemcomitans and P. gingivalis were 63.9 and 56.5% (P = 0.120) in Finland and 51.5 and 18.9% (P < 0.001) in Russia. A very low plasma vitamin C concentration (≤2.0 mg/liter) was found in 80.2% of Russian men and in 22.2% of Finnish men (P < 0.001).
FIG. 1.
Distributions of levels of antibodies to periodontal pathogens and plasma vitamin C concentrations. Serum IgG class antibodies to A. actinomycetemcomitans (A) and P. gingivalis (B) were determined by multiserotype ELISA, and plasma vitamin C concentrations (C) were determined by o-phtaldialdehyde-fluorometry, The sample comprised 194 Finnish men and 237 Russian men.
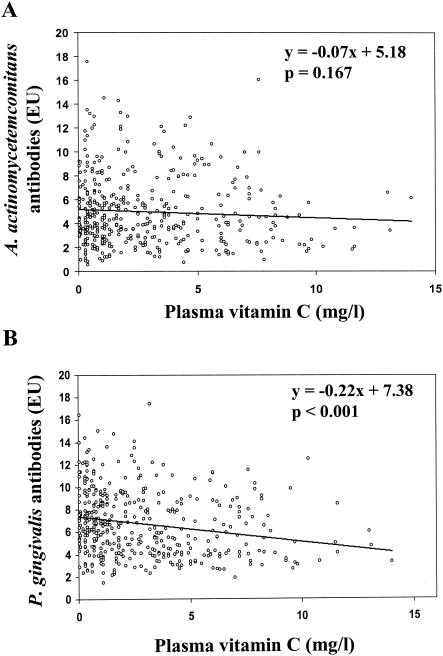
In the combined study population, levels of antibodies to A. actinomycetemcomitans and P. gingivalis correlated positively with each other (r = 0.23; P < 0.001). Most importantly, levels of antibodies to both pathogens were negatively correlated with plasma vitamin C concentrations (Fig. 2); the r values for A. actinomycetemcomitans and P. gingivalis were −0.07 (P = 0.167) and −0.22 (P < 0.001), respectively. In a linear regression model after adjustment for age, both antibodies were associated with plasma vitamin C concentrations, but this association was not significant for A. actinomycetemcomitans (P = 0.220). The inverse association (β = −0.20) between vitamin C concentrations and P. gingivalis antibody levels again was highly significant (P < 0.001). The association remained significant (β = −0.14; P = 0.010) after adjustment for age, number of teeth and fillings, CDT, and number of cigarettes smoked per day (Table 2). When adjusted for vitamin C, both antibody levels were positively associated with age: the β value for A. actinomycetemcomitans was 0.02 (P = 0.035), and that for P. gingivalis was 0.07 (P < 0.001). In addition, A. actinomycetemcomitans antibody levels were associated with smoking, and P. gingivalis antibody levels were associated with the number of fillings and teeth as well as CDT in the full model. The number of years of education, which was used to describe socioeconomic status, did not explain the differences in the antibody levels.
FIG. 2.
Correlations between plasma vitamin C concentrations and serum antibodies to the periodontal pathogens A. actinomycetemcomitans (A) and P. gingivalis (B). The formulas and P values for the regression lines are shown (n = 431).
TABLE 2.
Linear regression model for A. actinomycetemcomitans and P. gingivalis antibody levels with response variables added stepwise
| Parameter and response variable |
β (P)
|
||||
|---|---|---|---|---|---|
| Model I | Model II | Model III | Model IV | Model V | |
| A. actinomycetemcomitans antibodies (EU)a | |||||
| Vitamin C (mg/liter)b | −0.06 (0.220) | −0.07 (0.188) | −0.04 (0.400) | −0.05 (0.427) | −0.08 (0.179) |
| Age (yr) | 0.02 (0.035) | 0.04 (0.004) | 0.04 (0.007) | 0.04 (0.007) | 0.03 (0.043) |
| + No. of teeth | 0.03 (0.045) | 0.04 (0.023) | 0.04 (0.024) | 0.04 (0.061) | |
| + No. of fillings | −0.04 (0.250) | −0.04 (0.279) | −0.06 (0.132) | ||
| + CDT (U/liter) | 0.00 (0.942) | 0.02 (0.235) | |||
| + Smoking (cigarettes/day) | −0.07 (0.000) | ||||
| P. gingivalis antibodies (EU) | |||||
| Vitamin C (mg/liter) | −0.20 (0.000) | −0.21 (0.000) | −0.13 (0.011) | −0.12 (0.015) | −0.14 (0.010) |
| Age (yr) | 0.07 (0.000) | 0.07 (0.000) | 0.07 (0.000) | 0.07 (0.000) | 0.07 (0.000) |
| + No. of teeth | 0.01 (0.423) | 0.04 (0.010) | 0.04 (0.011) | 0.04 (0.018) | |
| + No. of fillings | −0.17 (0.000) | −0.17 (0.000) | −0.17 (0.000) | ||
| + CDT (U/liter) | 0.02 (0.046) | 0.03 (0.023) | |||
| + Smoking (cigarettes/day) | −0.02 (0.345) | ||||
Change in other variables/1-EU change in the antibody level.
1.0 mg/liter = 5.68 μmol/liter.
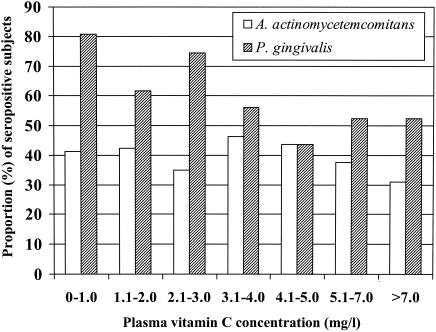
In the combined study population, mean antibody levels for both pathogens were higher among subjects with low vitamin C concentrations than among subjects with higher vitamin C concentrations. For subjects with plasma vitamin C concentrations of ≤4.0 mg/liter versus >4.0 mg/liter, the mean (± SD) levels of antibodies to A. actinomycetemcomitans and P. gingivalis were 5.06 ± 2.90 EU versus 4.78 ± 2.91 EU (P = 0.376) and 7.09 ± 2.95 versus 5.79 ± 2.55 EU (P < 0.001), respectively. The proportion of A. actinomycetemcomitans-seropositive (antibody level of ≥5.0 EU) subjects ranged between 24 and 46% in the vitamin C categories, and no clear trend between the categories could be observed (Fig. 3). On the other hand, the proportion of P. gingivalis-seropositive subjects decreased almost linearly (P for trend, <0.01) from 81 to 52% with increasing plasma vitamin C concentrations.
FIG. 3.
Proportions of seropositive subjects for different categories of plasma vitamin C concentrations. The Finnish (n = 194) and Russian (n = 237) subjects with levels of serum IgG antibodies to A. actinomycetemcomitans and P. gingivalis of ≥5.0 EU as determined by a multiserotype ELISA were classified as seropositive for the pathogen. The proportion of seropositive subjects was calculated separately for each vitamin C category.
DISCUSSION
In the present study we found a weak association between plasma vitamin C concentration and levels of antibody to A. actinomycetemcomitans in serum (r = −0.07) but a significant association between the vitamin C concentration and levels of antibody to P. gingivalis (r = −0.22). Although a low plasma vitamin C concentration or vitamin C intake has long been known to have an effect on periodontal diseases, the majority of epidemiological and biochemical studies have failed to show an association between vitamin C deficiency and the prevalence or severity of periodontitis (18, 38), nor have they demonstrated that patients suffering from periodontal disease benefit from vitamin C supplementation (16, 39). In the Third National Health and Nutrition Examination Survey, comprising 12,419 adult subjects, only a weak association was found between periodontal disease as diagnosed by clinical examinations and a low vitamin C intake as assessed by dietary information (30).
A hypothetical association between periodontitis and vitamin C is supported by the observations that additional vitamin C is required during infectious diseases, due to increased oxidative stress (17, 43). Nowadays it is well established that periodontitis is a chronic infection caused predominantly by gram-negative bacteria, especially A. actinomycetemcomitans and P. gingivalis (48). Vitamin C is highly concentrated in leukocytes and is used rapidly during infection (e.g., to prevent oxidative damage). In humans, the essentiality of vitamin C to the immune system is most clearly illustrated during scurvy, where infections occur and where poor or immeasurable responses are measured throughout the whole immune system (3).
In this study we exploited a new approach using serology to diagnose periodontitis. Since several genetically and serologically heterogeneous bacterial species are involved in the pathogenesis of periodontitis, creating a reliable serological method for aid in diagnosis has been problematic, and the antibody results have been controversial. However, our recently developed and validated multiserotype ELISA has a sensitivity of 71% and a specificity of 90% for identifying periodontitis (36). Since the antigen mixtures in the ELISA consist of reference strains representing all known serotypes of the pathogens, geographic differences in the serotype distribution (1) cannot bias the results. This is especially important for the determination of the levels of antibody to A. actinomycetemcomitans, since most patients with an oral A. actinomycetemcomitans infection harbor only one serotype of the pathogen (49). This leads to an elevated serum antibody level against only this particular serotype (46). By the multiserotype ELISA, the subjects who were PCR positive for subgingival A. actinomycetemcomitans or P. gingivalis could also be reliably distinguished from those who were PCR negative.
The fact that the antibodies of both pathogens were not significantly associated with vitamin C concentration is interesting and may be explained by the different characteristics of these pathogens (41). A. actinomycetemcomitans is particularly associated with aggressive periodontitis in young individuals or with refractory periodontitis in adults. P. gingivalis again occurs specifically in severe periodontitis at adult age. Unlike A. actinomycetemcomitans, P. gingivalis cultures will survive for only a limited number of generations in the absence of a source of heme (32). In a periodontal pocket, blood is a likely source of heme, since gingival bleeding increases significantly after a 1-month period of ascorbic acid depletion (25). As the most effective physiological antioxidant (13), vitamin C may also generate a disadvantageous environment for the optimal growth and survival of P. gingivalis (12). Accordingly, it is possible that an extremely low vitamin C concentration may increase colonization of P. gingivalis, but it is also conceivable that it disturbs the healing of the periodontal tissues. Since the major function of ascorbic acid is its involvement in the synthesis of collagen fibers (20), a very low vitamin C status may prevent the regeneration of periodontal tissues. However, the attachment ligaments or alveolar bone lost due to the inflammation response will not be revived.
The sample chosen for this study is especially good for finding the association between vitamin C and periodontitis, since the combined population includes a large number of subjects with uncommonly low plasma vitamin C concentrations as well as a high proportion of subjects with exceptionally high levels of antibodies to periodontal pathogens. In general, vitamin C deficiency is rarely seen in contemporary western societies, although it is found among elderly people, alcoholics, drug addicts, and those who survive prolonged starvation (42). Therefore, the significance of the severity and duration of vitamin C deficiency on chronic infections, such as periodontitis, is not well known. In our study, which was conducted in the spring (samples were taken in April), the prevalence of severe vitamin C deficiency among Russian men (79.8%) was comparable with earlier results from 1992 (29) and in accordance to their relatively low average vitamin C intake of 60 mg/day (40). The proportion of Finnish men with a very low plasma vitamin C concentration (≤2.0 mg/liter) was larger in the present study than in the previous survey (29) (22.2 versus 2.2%, respectively). This difference is supported by a 17% lower intake of vitamin C in 1997 (11) than in 1992 (21). Therefore, in the present study, the plasma vitamin C concentrations describe the dietary intake well. However, it is likely that the low intake of vitamin C is temporary and will increase during the harvest period (26). Also, the common smoking and high consumption of alcohol observed among the Russian men are known factors associated with a low vitamin C status and intake, respectively (27). Overall, the mean plasma vitamin C concentrations in this study were low compared to, for example, those in the EPIC-Norfolk prospective study (19). We are confident, however, that our plasma vitamin C results are accurate, since the collection and storage of the samples followed a strict protocol.
Like in most epidemiological studies, in our study we used education as a measure of socioeconomic status. However, this did not explain the differences in antibody or plasma vitamin C levels, although dental care and diet are connected to socioeconomic status. It has been claimed that if only one parameter is used to describe socioeconomic status, education is the best predictor for health (22). On the other hand, years of education do not necessarily give comparable estimates of socioeconomic status when different populations are compared. It is possible that in some populations, such as in the Republic of Karelia, Russia, education does not necessarily lead to a high income or occupational status. Nonetheless, the high levels of periodontal antibodies and the low number of dental fillings in the Russian population suggest that dental care services are not used as frequently in Russia as in Finland.
In this study, we found that periodontitis may be associated with vitamin C deficiency. As assessed by plasma vitamin C concentrations, vitamin C deficiency is also an independent risk factor for myocardial infarction (31). Furthermore, among a variety of chronic infectious diseases, periodontitis is implicated in CVD pathogenesis (6). In order to determine the possible combined role of periodontitis and extreme vitamin C deficiency in the development of CVD, prospective studies should be conducted.
Acknowledgments
We thank Tiina Karvonen and Pirjo Laakso for excellent technical assistance.
This study was financed by the Academy of Finland (grants 77613 and 75953).
REFERENCES
- 1.Asikainen, S., C. H. Lai, S. Alaluusua, and J. Slots. 1991. Distribution of Actinobacillus actinomycetemcomitans serotypes in periodontal health and disease. Oral Microbiol. Immunol. 6:115-118. [DOI] [PubMed] [Google Scholar]
- 2.Aurer-Kozelj, J., N. Kralj-Klobucar, R. Buzina, and M. Bacic. 1982. The effect of ascorbic acid supplementation on periodontal tissue ultrastructure in subjects with progressive periodontitis. Int. J. Vit. Nutr. Res. 52:333-341. [PubMed] [Google Scholar]
- 3.Basu, T., and J. W. T. Dickerson. 1996. Vitamins in human health and disease. CAB International, Wallingford, Conn.
- 4.Bhaskaram, P. 2002. Micronutrient malnutrition, infection, and immunity: an overview. Nutr. Rev. 60:S40-S45. [DOI] [PubMed]
- 5.Brubacher, G., and J. P. Vuilleumier. 1978. Vitamin C, p. 989-997. In H. C. Curtius and M. Roth (ed.), Clinical biochemistry. Principles and methods, vol 2. Walter de Gruyter, Berlin, Germany.
- 6.DeStefano, F., R. F. Anda, H. S. Kahn, D. F. Williamson, and C. M. Russell. 1993. Dental disease and risk of coronary heart disease and mortality. Br. Med. J. 306:688-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebersole, J. L. 1990. Systemic humoral immune responses in periodontal disease. Crit. Rev. Oral Biol. Med. 1:283-331. [DOI] [PubMed] [Google Scholar]
- 8.Epstein, S. E., Y. F. Zhou, and J. Zhu. 1999. Infection and atherosclerosis. Emerging mechanistic paradigms. Circulation 100:20-28. [DOI] [PubMed] [Google Scholar]
- 9.Exton-Smith, A. N. 1979. The clinical diagnosis of vitamin C deficiencies in everyday medical practice, p. 127-138. In T. G. Taylor (ed.), The importance of vitamins to human health. MTP Press, Lancaster, United Kingdom.
- 10.Field, C. J., I. R. Johnson, and P. D. Schley. 2002. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 71:16-32. [PubMed] [Google Scholar]
- 11.Findiet Study Group. 1998. The 1997 dietary survey of Finnish adults. Publications of the National Public Health Institute, B8. National Public Health Institute, Helsinki, Finland.
- 12.Forng, R. Y., C. Champagne, W. Simpson, and C. A. Genco. 2000. Environmental cues and gene expression in Porphyromonas gingivalis and Actinobacillus actinomycetem-comitans. Oral Dis. 6:351-365. [DOI] [PubMed] [Google Scholar]
- 13.Frei, B., L. England, and B. N. Ames. 1989. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 86:6377-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk, J. L., K. R. Feingold, A. H. Moser, and C. Grunfeld. 1993. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 98:67-82. [DOI] [PubMed] [Google Scholar]
- 15.Geesin, J. C., D. Darr, R. Kaufman, S. Murad, and S. R. Pinnell. 1988. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 90:420-424. [DOI] [PubMed] [Google Scholar]
- 16.Glickman, I. 1972. Clinical periodontology, 4th ed., p. 372-374. W. B. Saunders, Philadelphia, Pa.
- 17.Irvin, T. T., D. K. Chattopadhyay, and A. Amythe. 1978. Ascorbic acid requirements in post-operative patients. Surg. Gynecol. Obstet. 149:49-55. [PubMed] [Google Scholar]
- 18.Ismail, A. I., B. A. Burt, and S. A. Eklund. 1983. Relationship between ascorbic acid intake and periodontal disease in the United States. J. Am. Dent. Assoc. 107:927-931. [DOI] [PubMed] [Google Scholar]
- 19.Khaw, K. T., S. Bingham, A. Welch, R. Luben, N. Wareham, S. Oakes, and N. Day. 2001. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. Lancet 357:657-663. [DOI] [PubMed] [Google Scholar]
- 20.Kivirikko, K. I., and D. J. Prokop. 1967. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc. Natl. Acad. Sci. USA 57:782-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleemola, P., M. Virtanen, and P. Pietinen. 1994. The 1992 dietary survey of Finnish adults. Publications of the National Public Health Institute, B2. National Public Health Institute, Helsinki, Finland.
- 22.Laatikainen, T. 2000. Cardiovascular risk in the Republic of Karelia, Russia: comparison of major risk factors with North Karelia, Finland. Publications of the National Public Health Institute, A2. National Public Health Institute, Helsinki, Finland.
- 23.Laatikainen, T., L. Delong, S. Pokusajeva, M. Uhanov, E. Vartiainen, and P. Puska. 2002. Changes in cardiovascular risk factors and health behaviours from 1992 to 1997 in the Republic of Karelia, Russia. Eur. J. Public Health 12:37-43. [DOI] [PubMed] [Google Scholar]
- 24.Laatikainen, T., H. Alho, E. Vartiainen, P. Jousilahti, P. Sillanaukee, and P. Puska. 2002. Self-reported alcohol consumption and association to carbohydrate-deficient transferrin and gamma-glutamyltransferase in a random sample of the general population in the Republic of Karelia, Russia and in North Karelia, Finland. Alcohol Alcohol. 37:282-288. [DOI] [PubMed] [Google Scholar]
- 25.Leggott, P. J., P. B. Robertson, R. A. Jacob, J. J. Zambon, M. Walsh, and G. C. Armitage. 1991. Effects of ascorbic acid depletion and supplementation on periodontal health and subgingival microflora in humans. J. Dent. Res. 70:1531-1536. [DOI] [PubMed] [Google Scholar]
- 26.Lenton, K. J., H. Therriault, A. M. Cantin, T. Fulop, H. Payette, and J. R. Wagner. 2000. Direct correlation of glutathione and ascorbate and their dependence on age and season in human lymphocytes. Am. J. Clin. Nutr. 71:1194-1200. [DOI] [PubMed] [Google Scholar]
- 27.Loria, C. M., M. J. Klag, L. E. Caulfield, and P. K. Whelton. 2000. Vitamin C status and mortality in US adults. Am. J. Clin. Nutr. 72:139-145. [DOI] [PubMed] [Google Scholar]
- 28.Marcus, A. J., and D. P. Hajjar. 1993. Vascular transcellular signaling. J. Lipid Res. 34:2017-2031. [PubMed] [Google Scholar]
- 29.Matilainen, T., E. Vartiainen, P. Puska, G. Alfthan, S. Pokusajeva, N. Moisejeva, and M. Uhanov. 1996. Plasma ascorbic acid concentrations in the Republic of Karelia, Russia and in North Karelia, Finland. Eur. J. Clin. Nutr. 50:115-120. [PubMed] [Google Scholar]
- 30.Nishida, M., S. G. Grossi, R. G. Dunford, A. W. Ho, M. Trevisan, and R. J. Genco. 2000. Dietary vitamin C and the risk for periodontal disease. J. Periodontol. 71:1215-1223. [DOI] [PubMed] [Google Scholar]
- 31.Nyyssönen, K., M. T. Parviainen, R. Salonen, J. Tuomilehto, and J. T. Salonen. 1997. Vitamin C deficiency and risk of myocardial infarction: prospective population study of men from eastern Finland. Br. Med. J. 314:634-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen, I., H. N. Shah, and S. E. Gharbia. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: taxonomy and biochemical characteristics of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontology 2000 20:14-52. [DOI] [PubMed] [Google Scholar]
- 33.Page, R. C. 1999. Milestones in periodontal research and the remaining critical issues. J. Periodont. Res. 34:331-339. [DOI] [PubMed] [Google Scholar]
- 34.Papapanou, P. N., S. P. Engebretson, and I. B. Lamster. 1999. Current and future approaches for diagnosis of periodontal diseases. N.Y. State Dent. J. 65:32-37. [PubMed] [Google Scholar]
- 35.Pearce, W. H., I. Sweis, J. S. Yao, W. J. McCarthy, and A. E. Koch. 1992. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J. Vasc. Surg. 16:784-789. [PubMed] [Google Scholar]
- 36.Pussinen, P. J., T. Vilkuna-Rautiainen, G. Alfthan, K. Mattila, and S. Asikainen. 2002. Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J. Clin. Microbiol. 40:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin, M. B. 1984. Vitamin and wound healing. Plast. Surg. Nurs. 4:16-19. [DOI] [PubMed] [Google Scholar]
- 38.Russel, A. L. 1963. International nutrition surveys: a preliminary summary of dental findings. J. Dent. Res. 42:223-225. [DOI] [PubMed] [Google Scholar]
- 39.Shannon, I., and W. A. Gibson. 1965. Intravenous ascorbic acid loading in subjects classified as to periodontal status. J. Dent. Res. 44:355-361. [DOI] [PubMed] [Google Scholar]
- 40.Shaternikov, V. A., E. N. Stepanova, and G. M. Geller. 1981. The level of the water-soluble vitamin intake with foods in the USSR, p. 159-163. In Nutrition in health and disease and international development. Symposia from the XII International Congress of Nutrition. Alan R. Liss, New York, N.Y. [PubMed]
- 41.Slots, J. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease: introduction. Periodontology 2000 20:7-13. [DOI] [PubMed] [Google Scholar]
- 42.Touyz, L. Z. G. 1997. Oral scurvy and periodontal disease. J. Can. Dent. Assoc. 63:837-845. [PubMed] [Google Scholar]
- 43.Tsai, K., T. Hsu, C. Kong, and F. Lu. 2000. Is the endogeneous peroxyl-radical scavenging capacity of plasma protective in systemic inflammatory disorders in humans? Free Radic. Biol. Med. 28:926-933. [DOI] [PubMed] [Google Scholar]
- 44.Väänänen, M. K., H. A. Markkanen, V. J. Tuovinen, A. M. Kullaa, A. M. Karinpää, and E. A. Kumpusalo. 1993. Periodontal health related to plasma ascorbic acid. Proc. Finn. Dent. Soc. 89:51-59. [PubMed] [Google Scholar]
- 45.Vartiainen, E., P. Jousilahti, G. Alfthan, J. Sundvall, P. Pietinen, and P. Puska. 2000. Cardiovascular risk factor changes in Finland, 1972-1997. Int. J. Epidemiol. 29:49-56. [DOI] [PubMed] [Google Scholar]
- 46.Vilkuna-Rautiainen, T., P. J. Pussinen, K. Mattila, M. Vesanen, H. Åhman, B. Dogan, and S. Asikainen. 2002. Antigenically diverse reference strains and autologous strains of Actinobacillus actinomycetemcomitans are equally efficient antigens in enzyme-linked immunosorbent assay analysis. J. Clin. Microbiol. 40:4640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 1988. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): major international collaboration. J. Clin. Epidemiol. 41:105-114. [DOI] [PubMed] [Google Scholar]
- 48.World Workshop in Periodontics. 1996. Periodontal diseases: pathogenesis and microbial factors. Consensus report. Ann. Periodontol. 1:926-932. [DOI] [PubMed] [Google Scholar]
- 49.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]