Abstract
A polyclonal competitive enzyme-linked immunosorbent assay (PC-ELISA) is described for detection of antibodies to Ehrlichia (Cowdria) ruminantium by using a soluble extract of endothelial cell culture-derived E. ruminantium as the antigen and biotin-labeled polyclonal goat immunoglobulins as the competitor. For goats, the diagnostic sensitivity and specificity were both 100% with a cutoff of 80% inhibition (80 PI), with detection of antibodies for 550 days postinfection. For cattle, diagnostic sensitivity and specificity were 86 and 100%, respectively, with a cutoff of 50 PI and 79 and 100% with a cutoff of 70 PI. Cross-reactions with high-titer experimental or field antisera to other Ehrlichia and Anaplasma species were observed at up to 68 PI in cattle and up to 85 PI in sheep, and therefore to exclude these cross-reactions, cutoffs of 70 PI for bovine serology and 85 PI for small-ruminant serology were selected. Application of the PC-ELISA to bovine field sera from South Africa gave a higher proportion of positive results than application of the murine macrophage immunofluorescent antibody test or indirect ELISA, suggesting a better sensitivity for detection of recovered cattle, and results with bovine field sera from Malawi were consistent with the observed endemic state of heartwater and the level of tick control practiced at the sample sites. Reproducibility was high, with average standard deviations intraplate of 1.2 PI and interplate of 0.6 PI. The test format is simple, and the test is economical to perform and has a level of sensitivity for detection of low-titer positive bovine sera that may prove to be of value in epidemiological studies on heartwater.
Heartwater (cowdriosis) is a frequently fatal disease of susceptible domestic ruminants which has significant economic and developmental impact on livestock health and production in areas of sub-Saharan Africa where vector ticks of the genus Amblyomma are present. The disease is caused by Ehrlichia (formerly Cowdria) ruminantium, a rickettsial pathogen in the genogroup which includes the monocytotropic species Ehrlichia canis and Ehrlichia chaffeensis (3), the agents, respectively, of tropical canine pancytopenia, which has a pantropical distribution, and human monocytic ehrlichiosis, which has been reported mainly from North America in association with Amblyomma americanum ticks. The latter agent also naturally infects deer (16) and goats (2) in the United States, and differentiation of infections by E. chaffeensis and E. ruminantium would be required if the latter agent entered the United States, for example from Caribbean islands where heartwater occurs (1). Close antigenic relationships exist between these organisms, indicative of multiple shared epitopes on immunodominant antigens (12).
Serodiagnosis of E. ruminantium infection in domestic animals and wildlife has utilized a range of immunoassay methods involving reaction to E. ruminantium antigens obtained by infection of murine macrophages (5) or caprine neutrophils (17) or in vitro infection of endothelial cells (21). In vitro-infected endothelial cells have been used in immunofluorescent antibody tests (21), in Western blot tests (11, 19), in indirect enzyme-linked immunosorbent assays (ELISAs) (20, 31), and in a competitive ELISA involving a monoclonal antibody reactive with the E. ruminantium major antigenic protein MAP1 (14). A common feature of each of these tests is an unacceptable level of analytical or diagnostic specificity, the former evident at the test development stage through false-positive reactions with sera obtained after experimental infection with various Ehrlichia species and the latter evident at the test validation or application stage through high frequencies of false positives with sera from tick-exposed ruminants from heartwater-free areas (6, 12, 19). Cross-reactions with other Ehrlichia species are assumed to be the main reason for false-positive reactions, since in most cases a high specificity of these tests with sera from non-tick-exposed animals has been demonstrated. However, the inappropriate selection of cutoff values may also have contributed to reduced specificity (22). Improved specificity has been demonstrated with the use of a portion of the E. ruminantium map1 gene expressed as a recombinant peptide (MAP1-B) in an indirect ELISA format (34) and with the use of a baculovirus-expressed MAP1 antigen in combination with a monoclonal antibody in a competitive ELISA format (15).
Although improvement of specificity has been the principal concern in recent heartwater serodiagnostic test development, the lack of sensitivity is an equally if not more important concern for the use of serology in epidemiological studies in areas of endemicity (28, 29). An appropriate diagnostic sensitivity is also very important to reduce the proportion of false negatives in import-export screening. The sensitivity of tests for detection of cattle recovered from heartwater appears to be poor, with a decline of antibodies to negative or minimal levels within 3 to 7 months of infection or of removal from tick infestation, respectively, of calves exposed experimentally to or field challenged with E. ruminantium (18, 29). Down-regulation of antibody responses to E. ruminantium antigens was postulated to explain low seropositivity rates detected by MAP1-B ELISA in cattle sera from farms in Zimbabwe where heartwater is endemic (28, 29). In addition, considerable antigenic variation between E. ruminantium isolates has been detected (13, 27), resulting in serological reactions which can be stronger with homologous than with heterologous antigens, an additional factor which may decrease the sensitivity of serodiagnostic tests for heartwater.
In an attempt to overcome sensitivity problems, a simple competitive ELISA (polyclonal competitive ELISA [PC-ELISA]) was developed. The assay utilizes a relatively crude cell culture-derived antigen and competition between antibodies in test sera and in a polyclonal, biotin-labeled competitor. A heterologous competitor and antigen were used in the reactions in an attempt to improve sensitivity for detection of antibodies to diverse E. ruminantium stocks. Polyclonal competition systems have been reported previously (32) and have the potential to improve sensitivity for detection of antibodies in situations where antibodies are present at very low levels in serum and where antibodies to a range of potential antigens may be present. The methods for production of polyclonal competitor antibody, initial PC-ELISA development, and identification of cutoff levels with acceptable specificities are given below.
MATERIALS AND METHODS
Sera from animals experimentally infected with E. ruminantium.
Seven Saanen goats, 9 to 12 months of age and reared in Scotland in tick-free conditions, were infected with E. ruminantium blood stabilates by intravenous inoculation and treated with long-acting oxytetracycline on the second day of the febrile reaction (>40.5°C) to moderate the severity of the infection. Four E. ruminantium isolates were used: Ball 3 (8) and Welgevonden (4) from South Africa, Senegal (10) from Senegal in West Africa, and Gardel (33) from Guadeloupe in the Caribbean. Animals were infected once only, and sera were collected at regular intervals following infection, aliquoted, and stored at −20°C. Goats were kept tick free at the Centre for Tropical Veterinary Medicine under United Kingdom Home Office regulations for animal experimentation throughout the experiment. Sera were assayed by indirect ELISA (31), and high-titer sera were selected for production of the biotinylated competitor immunoglobulins (Ig). Bovine and ovine sera collected after experimental infections with E. ruminantium were supplied by J. Du Plessis, Onderstepoort Veterinary Institute (OVI), and had been collected from animals which had been reared under tick-free conditions in South Africa; sera from 23 calves and 5 sheep preinfection, 10 calves and 10 sheep after experimental infection with six different E. ruminantium isolates, and 19 calves after immunization with the Ball 3 vaccine stock were tested. Postinfection bovine sera were purposely selected to represent a spectrum of levels of antibody response with a bias towards sera with low titers as determined by the mouse macrophage immunofluorescent antibody test (MIFA) (5). MIFA results were supplied by J. Du Plessis, OVI, from tests performed as previously described in which a reciprocal titer of 20 or higher was considered to indicate positivity (5). Sera collected sequentially over a 26-week period from a calf (no. 456) reared under tick-free conditions and experimentally infected with the Lutale isolate of E. ruminantium (12) were supplied by F. Jongejan, University of Utrecht, Utrecht, The Netherlands.
Sera from animal populations in heartwater-free locations and areas of endemicity.
Sera from 110 cattle, 52 sheep, and 25 goats from Scotland (a heartwater-free country) were selected at random from the surplus at a local diagnostic laboratory. Samples originated from a wide range of locations across Scotland and had been collected as part of routine serosurveillance activities. Sera from adult cattle kept on a ranch in South Africa where heartwater is endemic were supplied by J. Du Plessis, OVI; the sera had been chosen for study since it was considered that the low proportion of sera from these cattle found to be positive by MIFA was not a true reflection of the exposure to E. ruminantium and the level of herd immunity on the ranch. Serum samples from 90 Malawi zebu calves were supplied by A. Soldan, Livestock Disease Evaluation Project, Lilongwe, Malawi. Calves had been born into village herds located close to six dip stations near Lilongwe (31); sera were collected when the animals were 8 to 9 months of age from calves born in May or June 1992.
Sera from animals naturally and experimentally infected with other Ehrlichia and Anaplasma species.
Antisera from animals experimentally infected with defined agents or, where these were not available, antisera from naturally infected animals were tested as indicated in Table 1. Sera collected following experimental infection of Ayrshire cattle in Kenya with the agent of bovine petechial fever (Ehrlichia ondiri) (30) were positive by indirect immunofluorescence with Anaplasma phagocytophilum (previously Ehrlichia phagocytophila) (3) antigens and by Western blotting with antigens of granulocytotropic ehrlichiae (35).
TABLE 1.
Origins of and results of PC-ELISA for sera collected following experimental or suspected natural infection with Ehrlichia or Anaplasma species other than E. ruminantium
| Species (reference) | Host species (country) | No. of sera | Sourcea (reference) | PI obtained with PC-ELISA |
|---|---|---|---|---|
| E. ovina | Sheep | 8 | F. Jongejan (12) | 0-52 |
| M. Pennisi | 0-72 | |||
| A. phagocytophilum (E. phagocytophila) | Sheep | 6 | CTVM collection (26) | 0-31 |
| A. phagocytophilum (Ehrlichia equi) (3) | Horse | 1 | ProtaTek | 0 |
| E. ondiri | Cattle | 6 | CTVM collection (30) | 0-32 |
| E. canis | Dog | 8 | J. Dawson | 52 |
| ProtaTek | 28 | |||
| F. Jongejan (12) | 0-86 | |||
| E. chaffeensis | Human | 2 | J. Dawson | 34-44 |
| Neorickettsia risticii (Ehrlichia risticii) (3) | Horse | 1 | ProtaTek | 0 |
| Suspected Ehrlichia sp. | Cattle (Namibia) | 2 | J. Du Plessis (7) | 49-68 |
| Suspected Ehrlichia sp. | Sheep (Zimbabwe) | 8 | S. Mahan | 53-85 |
F. Jongejan, Utrecht, The Netherlands; M. Pennisi, Messina, Italy; ProtaTek, St. Paul, Minn.; J. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.; J. Du Plessis, OVI, Onderstepoort, South Africa; S. Mahan, University of Florida/U. S. Agency for International Development/Southern African Development Community Heartwater Research Project, Harare, Zimbabwe. CTVM, Centre for Tropical Veterinary Medicine.
Production of biotinylated competitor Ig.
Serum Ig were purified from sera collected from goats infected 28 or 35 days previously with Senegal, Ball 3, or Gardel isolates of E. ruminantium as follows. To 2 ml of constantly stirred caprine serum, 0.54 g of ammonium sulfate (Sigma Aldrich, Poole, United Kingdom) was added slowly, and the mixture was stirred continuously at room temperature for 1 h. After centrifugation at 5,000 × g for 10 min at 4°C, the supernatant was discarded and the pellet was resuspended in 400 μl of Milli-Q purified water (Millipore system). Under constant stirring, 0.1 g of ammonium sulfate was added, and stirring continued for 60 min to reprecipitate Ig. Following further centrifugation as described before, the supernatant was discarded and the pellet was dissolved in 2 ml of Milli-Q water and dialyzed overnight against a 1,000× volume of distilled water in tubing designed to retain proteins of greater than 12 kDa (Sigma Aldrich). The dialyzed Ig were stored at −20°C until further use. Protein concentration was determined by the bicinchoninic acid protein assay method (Pierce & Warriner, Chester, United Kingdom) according to the manufacturer's protocol and Ig for labeling were diluted in 0.1 M bicarbonate buffer (pH 9.0 to 9.2; Sigma Aldrich) to 2 mg/ml. Thereafter, for biotinylation, each milliliter of diluted Ig was mixed with 30 μl of biotinamidocaproate solution (10 mg/ml in dimethyl sulfoxide; Sigma Aldrich) and incubated with constant mixing for 3 h at room temperature, followed by overnight dialysis as described before but against at least a 100× volume of phosphate-buffered saline (PBS). Sodium azide (final concentration, 0.05%) was added as a preservative, and the biotinylated Ig (BIg) was stored at −20°C.
Development of PC-ELISA.
Detergent-extracted soluble antigens of E. ruminantium (Welgevonden) elementary bodies (EB) were prepared as previously described (31) with slight modification. Briefly, supernatant from E. ruminantium-infected bovine endothelial cell cultures (24) was centrifuged for 20 min at 1,000 × g to remove cell debris and then for 20 min at 10,000 × g to pellet the EB. The EB were washed twice in PBS and then resuspended in lysis buffer (0.5% Nonidet P-40 and 0.5% sodium deoxycholate in 50 mM Tris [pH 7.4], 2 mM EDTA, and 150 mM sodium chloride) and incubated for 2 min at room temperature, followed by rapid passage through a 26-gauge needle and incubation at 37°C for 30 min. The lysate was then centrifuged at 16,000 × g for 30 min at 4°C to remove insoluble antigen. The soluble antigen extract was stored at −20°C until required. For PC-ELISA, the E. ruminantium antigen was diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6, and used to coat ELISA plates (Immulon 1; Dynatech) overnight at 4°C. Plate washing was conducted by manual addition by pipette of 300 μl of PBS containing 0.05% Tween 20 per well, with five washes between steps. An antigen concentration that was not limiting to color development in indirect ELISA (31) was chosen; this lay in the dilution range of 1/2,000 to1/8,000. The ranges were determined by titration of suitable BIg and conjugate (ExtrAvidin-peroxidase; Sigma Aldrich) concentrations that gave high levels of specific reaction to E. ruminantium antigen without significant nonspecific attachment to the plates. During optimization of the test, a panel of caprine and bovine antisera to E. ruminantium (strongly, weakly, and very weakly positive by indirect ELISA) and negative bovine and caprine sera were used to determine the effect of different test serum/BIg ratios on competitor inhibition, with each combination maintaining a final Tween 20 concentration of 0.05%. A ratio that consistently gave large differences in inhibition levels between weakly positive sera and negative control sera, and a high and reproducible level of color reaction with competitor alone, was utilized in subsequent tests. Color development was done with tetramethyl benzidine (TMB) at 0.1 mg/ml in 0.05 M phosphate citrate buffer, with addition of 1 μl of hydrogen peroxide (30% [wt/wt] solution) per ml of TMB solution immediately before use. Reactions were stopped with 1 M sulfuric acid. In routine use, these conditions resulted in almost colorless results with caprine positive sera and strong yellow reactions with negative sera.
After optimization, the test was performed essentially as described above, but well H12 of each plate was not coated with antigen to act as a no-antigen blank control. Duplicate wells received 50-μl aliquots of undiluted test or control sera; competitor control wells and the no-antigen blank wells received 50 μl of PBS instead of serum. Within 5 min of serum addition, 50 μl of BIg competitor (raised against the Senegal isolate unless otherwise specified) diluted in PBS containing 0.1% Tween 20 was added to all wells of the plate, and the plate was covered and incubated for 60 min at 37°C in a shaking incubator. After washing, 100 μl of ExtrAvidin-peroxidase conjugate (1/2,500 dilution in PBS containing 0.05% Tween 20) was added per well, and the plate was incubated for 30 min as described above. Color development and reaction termination were performed as described above, and optical densities were read at 450 nm with automatic deduction of the optical density of the no-antigen control well. Inhibition levels for test and control sera were calculated with reference to the mean of the competitor control values and expressed as percent inhibition (PI):
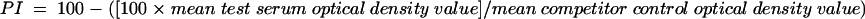 |
With occasional sera, enhanced binding of the competitor control occurred, resulting in PI values of less than zero. Reproducibility was determined by using nine sheep and goat sera from experimentally infected animals (three samples collected preinfection and two collected on each of days 21 and 62 and 18 months postinfection) and 10 assays per serum sample on four different plates.
RESULTS
(i) Identification of cutoff values in relation to reactivity of anti-Ehrlichia sera.
The cutoff value was defined by reference to inhibition levels obtained with the PC-ELISA with a panel of cattle, sheep, and goat sera from a heartwater-free country (Scotland), sera from goats and cattle experimentally infected with E. ruminantium, and sera obtained from mammals experimentally and naturally infected with various other Ehrlichia species.
Sera from 110 Scottish cattle had inhibition levels between 1 and 34 PI with the PC-ELISA, while Scottish sheep (n = 52) and goat (n = 25) sera had inhibition levels of 1 to 37 and 3 to 38 PI, respectively. In contrast, inhibition levels for sera from goats and cattle experimentally infected with E. ruminantium exceeded 40 PI in all cases. For caprine sera collected between 66 and 122 days after experimental E. ruminantium infection, inhibition levels were between 94 and 100 PI, irrespective of the E. ruminantium isolate used in the infection. Inhibition levels remained above 84 PI for four goats when tested 550 days post-experimental infection, after maintenance of the goats throughout in tick-free conditions. Inhibition levels for sera collected before and after vaccination of 19 cattle with the Ball 3 vaccine stock were below 35 PI before immunization and afterwards exceeded 40 PI in all cases; levels for 13 of the postimmunization sera exceeded 70 PI, while the remainder of the sera had values between 42 and 67 PI (Fig. 1). In the calf experimentally infected with E. ruminantium (Lutale), the inhibition level obtained with the PC-ELISA following seroconversion remained between 90 and 95 PI until week 26 postinfection.
FIG. 1.
Frequency distribution of inhibition levels obtained with PC-ELISA for sera collected from 19 cattle in South Africa pre- and postimmunization with E. ruminantium (Ball 3).
The inhibition levels for antisera raised against other Ehrlichia and Anaplasma species were then determined and are shown in Table 1. Sera collected from sheep after experimental infection with A. phagocytophilum and from cattle after E. ondiri infection gave inhibition levels within the normal range obtained for heartwater-naïve Scottish sheep and cattle (less than 40 PI). Higher inhibition levels were found with other ruminant infections; maximal values of 72 and 68 PI were obtained for ovine (Ehrlichia ovina) and bovine (presumed natural infections with other Ehrlichia species from Namibia) sera, respectively. With nonruminant infections, the highest inhibition level (86 PI) was found for a canine serum collected 9 weeks postinfection with E. canis; most sera raised against this organism gave lower inhibition levels.
Eight ovine sera from Zimbabwe from animals with presumed natural infections with other Ehrlichia species from areas where heartwater is not endemic and from a farm where Amblyomma ticks were not present on domestic livestock gave high inhibition levels (between 53 and 85 PI). These sera had been selected to represent high-titer false positives when tested by immunofluorescence and Western blotting (Table 1).
On the basis of these results, application of a cutoff value of 40 PI would result in a diagnostic sensitivity and specificity of 100% for detection of antibodies after experimental E. ruminantium infection in previously tick-free cattle and goats. However, higher cutoff values, of 70 and 85 PI for bovine and small ruminant sera, respectively, would be necessary to avoid false-positive results due to experimental or natural infections with other Ehrlichia species, with a consequent reduction in sensitivity (see Discussion and Table 2).
TABLE 2.
Suggested ranges for categorization of PC-ELISA results and sensitivity for detection of prior E. ruminantium infection
| PI | Interpretation
|
Sensitivity for detection of previous E. ruminantium infectiona (%)
|
||
|---|---|---|---|---|
| Goats | Cattle | Goats | Cattle | |
| <50 | Not previously exposed to E. ruminantium | Probably not previously exposed to E. ruminantium | NA | NA |
| 50-70 | Cross-reaction with other Ehrlichia sp. or weakly positive for E. ruminantiumb | Weakly positive for E. ruminantiumb or cross-reaction with other Ehrlichia sp. | 100 | 86 |
| 71-85 | Prior infection with or exposure to E. ruminantiumb or Ehrlichia sp. | Prior infection with or exposure to E. ruminantium | 100 | 79 |
| >85 | Prior infection with or exposure to E. ruminantium | Prior infection with or exposure to E. ruminantium | 91 | 31 |
Where the cutoff is the lowest value in the range given and sensitivity for detection of previous E. ruminantium infection is calculated from results with experimental infections. NA, not applicable.
Except in situations where suitable Amblyomma vectors are considered to be absent.
(ii) Time course of antibody detection following experimental caprine infection.
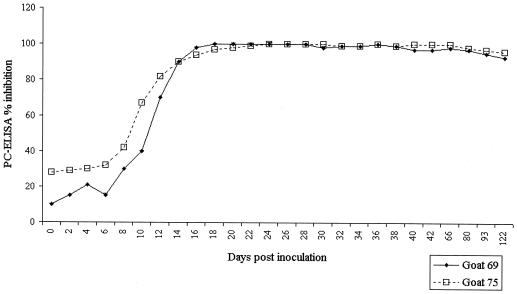
Seroconversion in the seven experimentally infected Saanen goats was detected by PC-ELISA between 8 and 16 days postinfection when a cutoff of 50 PI was used and was only slightly delayed to between 9 and 18 days for the same sera when a cutoff of 70 PI was used. In relation to the onset of the febrile response, sera were first positive at 70 PI or above on the second or third day of fever of >40.5°C. Inhibition levels exceeded 90 PI by 20 days postinfection in all animals and remained at this level throughout the period of monitoring (122 days); the inhibition levels obtained for sera collected sequentially from two of the experimentally infected goats are shown in Fig. 2. In a separate study, inhibition levels between 84 and 95 PI were obtained with sera from four goats infected 550 days previously with E. ruminantium (Welgevonden). Therefore, of 11 animals sampled for 3 months or longer after recovery, all tested positive with the 70-PI cutoff and 10 of 11 (91%) tested positive with the 85-PI cutoff.
FIG. 2.
Inhibition levels obtained with PC-ELISA for sera collected sequentially from two goats experimentally infected with the Welgevonden (goat 69) and Gardel (goat 75) isolates of E. ruminantium. Sera were collected every 2 days between days 0 and 42 and thereafter on days 66, 88, 93, and 122.
(iii) Comparison of biotinylated competitor sera raised against different E. ruminantium isolates.
Biotinylated anti-E. ruminantium Ig prepared from sera of goats infected with two geographically distinct isolates (Ball 3 from South Africa and Gardel from Guadeloupe) were investigated for the detection of infection in goats with E. ruminantium isolates of different geographical origins. Test results for the two competitor preparations were compared during the time course of infections with four E. ruminantium isolates (Ball 3, Senegal, Welgevonden, and Gardel). With a cutoff for positivity of 70 PI, there was complete agreement between tests in statuses of 176 sera.
(iv) Reproducibility of the assay.
In the PC-ELISA, the average intraplate standard deviation in PI was 1.2% (range, 0.4 to 2.6%) for nine sera. Interplate average standard deviation in PI for the same nine sera tested on four plates was 0.6% (range, 0.1 to 1.4%).
(v) Comparison of PC-ELISA and indirect ELISA for bovine and ovine sera from South Africa.
Bovine sera collected preinfection (n = 23) and postrecovery (n = 10) from animals experimentally infected with E. ruminantium or postvaccination with the Ball 3 stock (n = 19) were tested by PC-ELISA and by indirect ELISA (31). The PC-ELISA had a sensitivity and specificity of 100% with the 70-PI cutoff level for detection of infection status in cattle preinfection and after experimental infection. Of the 19 postvaccination sera, 13, 2, and 4 gave inhibition levels of >70, 50 to 70, and <50 PI, respectively. The indirect ELISA detected 50% of the postrecovery and 11% of the postvaccination bovine sera. Ovine sera collected preinfection (n = 5) and postrecovery (n = 10) from animals experimentally infected with E. ruminantium were tested by PC-ELISA and by indirect ELISA; both tests correctly identified the statuses of all 15 sera.
The two ELISAs were applied to 20 sera with low MIFA-determined titers (10, 6, and 4 sera with titers of <20, 20, and 80, respectively) from cattle kept under conditions of Amblyomma exposure on a ranch in South Africa where heartwater is endemic. Sixteen of the 20 sera (80%) tested positive by PC-ELISA when a cutoff of 70 PI was applied, and this number rose to 18 of 20 if a cutoff of 50 PI was applied, whereas only 10 of these sera were positive by MIFA (titer of 20 or higher) and 12 were positive by indirect ELISA.
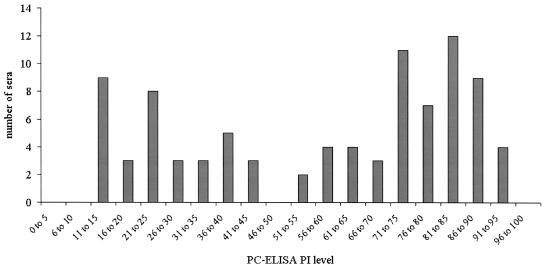
(vi) PC-ELISA results for Malawi zebu calves.
The frequency distribution of inhibition levels for 90 sera collected from calves at 8 to 9 months of age is shown in Fig. 3. A bimodal distribution of values is suggested, consistent with a mixed population of antibody-positive and -negative animals, with a division between the two groups occurring at about 50 PI.
FIG. 3.
Frequency distribution of inhibition levels obtained with PC-ELISA for sera from 90 8- to 9-month-old calves in Malawi.
DISCUSSION
The lack of sensitivity and specificity of serological tests for detection of acquired immunity to E. ruminantium has for a long period created uncertainty in the interpretation of cross-sectional studies on herd immunity. Since postexposure antibody levels in calves are observed to decline by 3 to 7 months to levels detected as negative by the MIFA (5) or between 8 and 33 weeks to levels detected as negative by the MAP1-B ELISA (29, 34), longitudinal studies on development of herd immunity and endemic stability (25) require testing of animals at intervals of no longer than 2 months in order to detect the timing of seroconversion. Longer intervals should be appropriate for sheep and goats, since their antibody levels appear to be more durable (5, 34). For cattle in particular, a sensitive and specific serological test would greatly simplify the analysis of interventions upon herd exposure to E. ruminantium by enabling cross-sectional studies on higher numbers of animals at ages at which susceptibility of naïve animals to infection is expected to be high. The exposure rate in young animals can provide an important basis for identification of appropriate tick-borne disease control measures, such as immunization or altered acaricide application, but to date has rarely been applied to heartwater control because of the unsuitability of serological tests.
The competitive ELISA described in this paper appears to offer some advantages over other tests, in particular a relatively high sensitivity for bovine infections and ease of use. Practical advantages of the format developed include the use of undiluted test and control sera, obviating a pretest dilution step, and the ability to assay sera from different animal species by using the same reagents on the same plate. The sensitivity was at least as good as that of the MIFA for experimentally infected cattle, and with field sera from South Africa, the PC-ELISA resulted in a higher proportion of seropositives than the MIFA. The PC-ELISA results for field sera were considered to be consistent with a high observed level of tick exposure and the apparent presence of endemic stability to heartwater, since the South African samples were collected from adult cattle on a ranch where heartwater was known to occur and seroconversion in Malawi was occurring in the majority of calves within the first 12 months of life (31). For determination of test sensitivity, sera from recovered long-term carrier animals are as important as those from recently recovered animals, as animals in areas of endemicity are expected to be exposed to E. ruminantium repeatedly from an early age (25). Selection of high-titer sera following experimental infection and booster challenge of goats (22) or immunization with vaccines with oil adjuvants (23) may lead to overestimation of sensitivity in natural situations. Although sera from bovine carriers were not available in the study described here, since the costs of long-term experimental containment of heartwater-infected animals are very high, difficult bovine sera were selected which had very weak positive or negative test statuses as determined by MIFA and caprine sera were collected from animals with infections which had been treated with antibiotics and without booster challenges, conditions which may favor reduced antibody responses.
Published guidelines for validation of immunoassays (9) emphasize that it is important to determine cross-reactivity with antisera to related organisms at an early stage of test development and also that sera used in test validation should closely resemble those from animals to which the test will be applied (9). In the development of the PC-ELISA, the limit of cross-reactivity with antisera raised against other Ehrlichia and Anaplasma species was determined and false-positive reactions with the PC-ELISA occurred with caprine or ovine sera at up to 85 PI and up to 68 PI with a bovine serum sample. Although these inhibition levels were high, antibody levels induced in cattle and goats by experimental infection with E. ruminantium were usually above the limit of cross-reactivity with other ehrlichiae. Although the number of sera used in sensitivity and specificity testing was small, those used were selected to represent low levels of antibody to E. ruminantium or high levels of cross-reactivity and, it is hoped, would represent the extremes to be anticipated in the application of tests. Since the application of a cutoff level of 85 PI gave a sensitivity for detection of experimental infection in goats of almost 100% and cross-reactions at above 86 PI were not detected, the performance of the PC-ELISA for this species appears to be adequate. For cattle, the 85-PI cutoff would dramatically reduce sensitivity, but since cross-reactions at above 68 PI were not detected, arguments for a cutoff of 70 PI can be advanced since the sensitivity of the test for detection of recent bovine infections was at least 79% at this cutoff level. Suggested interpretations of PC-ELISA results with caprine and bovine sera are given in Table 2. Where there is an overlap in the distribution of high numbers of false-positive Ehrlichia reactions and weak-positive E. ruminantium antibody levels, interpretation must be cautious. The absence of suitable vectors of E. ruminantium in the environment of the animal or group sampled would support a tentative classification as a cross-reaction.
Since the majority of heartwater serology concerns the detection of potentially infected animals in the field as part of studies on tick-borne disease management, it can be argued that high sensitivity is more important than high specificity so that infected or immune animals are detected. It may also be argued that, provided that specificity is not lower than 80%, the interpretation of levels of herd immunity in areas where heartwater is endemic is not unduly complicated since herd immunity levels of 20 to 40% would not be considered to indicate endemic stability whereas herd immunity rates of >80% would do so. The cutoff value applied to the test for each animal species will depend on the information required; a high cutoff would be appropriate for detection of infection in a herd or population, while a lower cutoff would allow detection of most or all infected animals within a group but would produce some false positives. Although lowering the cutoff value for bovine sera from 70 to 50 PI results in a higher sensitivity, with the resultant increase in the proportion of positive sera from field situations of endemicity (South Africa and Malawi), this can be considered to have limited effect upon the interpretation of the level of herd immunity in each of these situations. The seropositivity rates were consistent with the levels of Amblyomma challenge at each location: 80% in cattle in South Africa under heavy exposure to Amblyomma hebraeum and 77% in 8- to 9-month-old calves under Amblyomma variegatum exposure and relaxed tick control in Malawi, with the 70 PI cutoff applied with the PC-ELISA. The bimodal distribution of PC-ELISA values from the field-exposed cattle in Malawi supports the argument that the majority of immune animals have inhibition levels higher than 70 PI and that levels between 50 and 70 PI may indicate weak positives or possibly cross-reactions with other Ehrlichia species, since sera from non-tick-exposed cattle in Scotland had levels of less than 40 PI. Further studies on the sensitivity of the assay for detection of naïve and recovered cattle, sheep, and goats in areas where heartwater is endemic and comparison with other recently developed E. ruminantium diagnostic assays such as the MAP1-B ELISA (34) will be required to assess the usefulness of the PC-ELISA for field application.
Acknowledgments
This research was funded by the Department for International Development of the British government.
We gratefully acknowledge the generous assistance of all those who supplied sera and accompanying data, particularly Jan Du Plessis, Frans Jongejan, Jacqueline Dawson, Maria Pennisi, Suman Mahan, and Andrew Soldan. Alan Walker, Cornelis Bekker, and Ivan Morrison are thanked for useful comments on the manuscript.
REFERENCES
- 1.Camus, E., N. Barre, D. Martinez, and G. Uilenberg. 1996. Heartwater (cowdriosis): a review. Office International des Epizooties, Paris, France.
- 2.Dugan, V. G., S. E. Little, D. E. Stallknecht, and A. D. Beall. 2000. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 38:448-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 4.Du Plessis, J. L. 1985. A method for determining the Cowdria ruminantium infection rate of Amblyomma hebraeum: effects in mice injected with tick homogenates. Onderstepoort J. Vet. Res. 52:55-61. [PubMed] [Google Scholar]
- 5.Du Plessis, J. L., and L. Malan. 1987. The application of the indirect fluorescent antibody test in research on heartwater. Onderstepoort J. Vet. Res. 54:319-325. [PubMed] [Google Scholar]
- 6.Du Plessis, J. L., E. Camus, P. T. Oberem, and L. Malan. 1987. Heartwater serology; some problems with the interpretation of results. Onderstepoort J. Vet. Res. 54:327-329. [PubMed] [Google Scholar]
- 7.Du Plessis, J. L., J. D. Bezuidenhout, M. S. Brett, E. Camus, F. Jongejan, S. M. Mahan, and D. Martinez. 1993. The sero-diagnosis of heartwater: a comparison of five tests. Rev. Elev. Med. Vet. Pays Trop. 46:123-129. [PubMed] [Google Scholar]
- 8.Haig, D. A. 1952. Note on the use of the white mouse for the transport of strains of heartwater. J. S. Afr. Vet. Med. Assoc. 23:167-170. [Google Scholar]
- 9.Jacobson, R. H. 1998. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. Off. Int. Epizoot. 17:469-486. [DOI] [PubMed] [Google Scholar]
- 10.Jongejan, F. 1991. Protective immunity to heartwater (Cowdria ruminantium infection) is acquired after vaccination with in vitro-attenuated rickettsiae. Infect. Immun. 59:729-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jongejan, F., and M. J. C. Thielemans. 1989. Identification of an immunodominant, antigenically conserved 32-kilodalton protein from Cowdria ruminantium. Infect. Immun. 57:3243-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jongejan, F., N. de Vries, J. Nieuwenhuijs, A. H. M. van Vliet, and L. A. Wassink. 1993. The immunodominant 32-kilodalton protein of Cowdria ruminantium is conserved within the genus Ehrlichia. Rev. Elev. Med. Vet. Pays Trop. 46:145-152. [PubMed] [Google Scholar]
- 13.Jongejan, F., L. A. Wassink, M. J. C. Thielemans, N. M. Perie, and G. Uilenberg. 1989. Serotypes in Cowdria ruminantium and their relationship with Ehrlichia phagocytophila determined by immunofluorescence. Vet. Microbiol. 21:31-40. [DOI] [PubMed] [Google Scholar]
- 14.Jongejan, F., M. J. C. Thielemans, M. De Groot, P. J. S. Van Kooten, and B. A. M. Van Der Zeijst. 1991. Competitive enzyme linked immunoassay for heartwater using monoclonal antibodies to a Cowdria ruminantium-specific 32 kilodalton protein. Vet. Microbiol. 28:199-211. [DOI] [PubMed] [Google Scholar]
- 15.Katz, J. B., R. DeWald, J. E. Dawson, E. Camus, D. Martinez, and R. Mondry. 1997. Development and evaluation of a recombinant antigen, monoclonal antibody-based competitive ELISA for heartwater serodiagnosis. J. Vet. Diagn. Investig. 9:130-135. [DOI] [PubMed] [Google Scholar]
- 16.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and E. W. Howerth. 1997. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 35:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan, L. L., C. J. Holland, C. A. Mebus, and M. Ristic. 1986. Serological relationship between Cowdria ruminantium and certain Ehrlichia species. Vet. Rec. 119:458-459. [DOI] [PubMed] [Google Scholar]
- 18.Mahan, S. M., S. M. Semu, T. F. Peter, and F. Jongejan. 1998. Evaluation of the MAP-1B ELISA for cowdriosis with field sera from livestock in Zimbabwe. Ann. N. Y. Acad. Sci. 849:259-261. [DOI] [PubMed] [Google Scholar]
- 19.Mahan, S. M., N. Tebele, D. Mukwedeya, S. Semu, C. B. Nyathi, L. A. Wassink, P. J. Kelly, T. Peter, and A. F. Barbet. 1993. An immunoblotting diagnostic assay for heartwater based on the immunodominant 32-kilodalton protein of Cowdria ruminantium detects false positives in field sera. J. Clin. Microbiol. 31:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez, D., S. Coisne, C. Sheikboudou, and F. Jongejan. 1993. Detection of antibodies to Cowdria ruminantium in the serum of domestic ruminants by indirect ELISA. Rev. Elev. Med. Vet. Pays Trop. 46:115-120. [PubMed] [Google Scholar]
- 21.Martinez, D., J. Swinkels, E. Camus, and F. Jongejan. 1990. Comparaison de trois antigenes pour la serodiagnostic de la cowdriose par immunofluorescence indirecte. Rev. Elev. Med. Vet. Pays Trop. 43:159-166. [PubMed] [Google Scholar]
- 22.Mboloi, M. M., C. P. J. Bekker, C. Kruitwagen, M. Greiner, and F. Jongejan. 1999. Validation of the indirect MAP1-B enzyme-linked immunosorbent assay for diagnosis of experimental Cowdria ruminantium infection in small ruminants. Clin. Diagn. Lab. Immunol. 6:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondry, R., D. Martinez, E. Camus, A. Liebisch, J. B. Katz, R. DeWald, A. H. M. van Vliet, and F. Jongejan. 1998. Validation and comparison of three enzyme-linked immunosorbent assays for the detection of antibodies to Cowdria ruminantium infection. Ann. N. Y. Acad. Sci. 849:262-272. [DOI] [PubMed] [Google Scholar]
- 24.Mutunga, M., P. M. Preston, and K. J. Sumption. 1998. Nitric oxide is produced by Cowdria ruminantium-infected bovine pulmonary endothelial cells in vitro and is stimulated by gamma interferon. Infect. Immun. 66:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Callaghan, C. J., G. F. Medley, T. F. Peter, S. M. Mahan, and B. D Perry. 1999. Predicting the effect of vaccination on the transmission dynamics of heartwater (Cowdria ruminantium infection). Prev. Vet. Med. 42:17-38. [DOI] [PubMed] [Google Scholar]
- 26.Paxton, E. A., and G. R. Scott. 1989. Detection of antibodies to the agent of tick borne fever (Cytoecetes phagocytophila) by indirect immunofluorescence. Vet. Microbiol. 21:133-138. [DOI] [PubMed] [Google Scholar]
- 27.Perez, J. M., D. Martinez, C. Sheikboudou, F. Jongejan, and A. Bensaid. 1998. Characterisation of variable immunodominant antigens of Cowdria ruminantium by ELISA and immunoblots. Parasite Immunol. 20:613-622. [DOI] [PubMed] [Google Scholar]
- 28.Peter, T. F., C. J. O'Callaghan, G. F. Medley, B. D. Perry, S. M. Semu, and S. M. Mahan. 2002. Population-based evaluation of the Ehrlichia ruminantium MAP 1B indirect ELISA. Exp. Appl. Acarol. 25:881-897. [DOI] [PubMed] [Google Scholar]
- 29.Semu, S. M., T. F. Peter, D. Mukwedeya, A. F. Barbet, F. Jongejan, and S. M. Mahan. 2001. Antibody responses to MAP 1B and other Cowdria ruminantium antigens are down regulated in cattle challenged with tick-transmitted heartwater. Clin. Diagn. Lab. Immunol. 8:388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snodgrass, D. R. 1974. Studies on bovine petechial fever and ovine tick borne fever. Ph.D. thesis. University of Edinburgh, Edinburgh, United Kingdom.
- 31.Soldan, A. W., T. L. Norman, S. Masaka, E. A. Paxton, R. M. Edelsten, and K. J. Sumption. 1993. Seroconversion to Cowdria ruminantium of Malawi zebu calves, reared under different tick control strategies. Rev. Elev. Med. Vet. Pays Trop. 46:171-177. [PubMed] [Google Scholar]
- 32.Sorensen, K. J., R. L. Madekurozwa, and P. Dawe. 1992. Foot and mouth disease; detection of antibodies in cattle sera by blocking ELISA. Vet. Microbiol. 32:253-265. [DOI] [PubMed] [Google Scholar]
- 33.Uilenberg, G., E. Camus, and N. Barre. 1985. Quelques observations sur une souche de Cowdria ruminantium isolee en Guadeloupe (Antilles francaises). Rev. Elev. Med. Vet. Pays Trop. 38:34-42. [PubMed] [Google Scholar]
- 34.van Vliet, A. H. M., B. A. M. van der Zeijst, E. Camus, S. M. Mahan, D. Martinez, and F. Jongejan. 1995. Use of a specific immunogenic region on the Cowdria ruminantium MAP1 protein in a serological assay. J. Clin. Microbiol. 33:2405-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velez, M. P. A. 1999. Epidemiology and molecular characterisation of Ehrlichia phagocytophila in relation to emerging ehrlichiae. Ph.D. thesis. University of Edinburgh, Edinburgh, United Kingdom.