Abstract
Two serological tests for detection of antibodies to Ehrlichia (previously Cowdria) ruminantium, the causative agent of heartwater, were compared by using field sera collected from sheep and cattle as part of serosurveys in Ghana. Sera selected as either negative or positive by a new polyclonal competitive enzyme-linked immunosorbent assay (PC-ELISA) were tested by the indirect MAP1-B ELISA. Cutoff values of 14 percent positivity (14 PP) for both ruminant species were obtained for the MAP1-B ELISA by using preseroconversion Ghanaian sera and were compared with previously recommended cutoff values of 29 PP for sheep and 38 PP for cattle. With the 14-PP cutoff, of 151 sheep sera which tested negative by PC-ELISA, 89% were also negative by MAP1-B ELISA, while of 419 sheep sera positive by PC-ELISA, 98% were also positive by MAP1-B ELISA. Of 261 bovine sera negative by PC-ELISA, 82% were also negative by MAP1-B ELISA. Of 511 bovine sera positive by PC-ELISA, only 47% were positive by MAP1-B ELISA; these included 168 sera collected from cattle following first seroconversion as detected by both tests, with 125 of these sera positive by PC-ELISA but only 59 and 5 positive by MAP1-B ELISA with the 14- and 38-PP cutoff levels, respectively. These results indicate that both assays are highly sensitive and specific for detection of E. ruminantium exposure in sheep but that the MAP1-B ELISA lacks sensitivity for postseroconversion bovine sera in comparison to the PC-ELISA. Both tests confirm E. ruminantium seroprevalence of at least 70% in Ghanaian sheep; levels of exposure among Amblyomma variegatum-infested Ghanaian cattle are likely to be higher than the seroprevalence value of 66% obtained with the PC-ELISA.
Heartwater, or cowdriosis, caused by the rickettsia Ehrlichia (previously Cowdria) ruminantium (10) and transmitted by ticks of the genus Amblyomma, is an often fatal disease of susceptible domestic ruminants in sub-Saharan Africa and some Caribbean islands (7). Epidemiological surveys of E. ruminantium prevalence in areas of endemicity have been limited by the lack of suitably sensitive and specific serodiagnostic tests (15). Fluorescent antibody tests based on E. ruminantium-infected mouse macrophages (11), neutrophils and bovine endothelial cell cultures (27), and immunoblotting assays (20, 25) lacked specificity (12, 16, 25) and were too labor-intensive for mass screening. Several enzyme-linked immunosorbent assay (ELISA) systems have been developed over the last decade, with progressive improvement in performance. Indirect ELISA protocols using crude elementary body-derived antigen (26, 34) and a monoclonal antibody-based competitive ELISA (17) resulted in cross-reactions with other ovine and bovine Ehrlichia species (12, 16). Recently, a new competitive ELISA, which uses crude, detergent-solubilized elementary body antigen and polyclonal biotinylated competitor antibody (polyclonal competitive ELISA [PC-ELISA]), has been reported to have reduced cross-reactivity with other ruminant Ehrlichia species and improved sensitivity compared to crude antigen-based indirect ELISAs (35). Serological tests utilizing recombinant antigens include an indirect ELISA based on a specific fragment of the E. ruminantium major antigenic protein (MAP1), the MAP1-B ELISA (38), and a competitive ELISA using recombinant MAP1 antigen and anti-MAP1 monoclonal antibody competitor (18). These have been shown to have increased specificity, though both antigens cross-react with antibodies to Ehrlichia chaffeensis, which infects deer and domestic goats in the southern United States (9), and sensitivity for bovine sera appears to be poor (24, 33).
As part of an epidemiological survey of the incidence and prevalence of heartwater in domestic ruminants in the West African country of Ghana, serum samples were collected monthly or on single occasions from sheep and cattle and tested for antibodies to E. ruminantium by using the PC-ELISA (35). Based on postmortem diagnosis, heartwater was reported to occur throughout Ghana (2). Preliminary investigations using the PC-ELISA indicated the presence of E. ruminantium antibodies in sera from traditionally managed lambs, kids, and calves (4) and in naïve sheep experimentally infected with field isolates of E. ruminantium (5). In the present study, the relative sensitivity and specificity of the PC-ELISA were assessed by comparison of the assay with the MAP1-B ELISA (38) by using panels of Ghanaian ovine and bovine sera of known PC-ELISA reactivities.
MATERIALS AND METHODS
Serum samples.
Blood was collected by jugular puncture from sheep and cattle in the field into 10-ml uncoated Vacutainer tubes (Becton-Dickinson), returned to the laboratory, incubated at 37°C for 1 h, and held at 4°C overnight; the sera were separated by centrifugation at 2,000 × g for 30 min at 4°C and stored at −20°C. The sheep were the local West African dwarf, long-legged Sahel type or crosses with this type; the cattle were Ghana Sanga, N′Dama, West African shorthorn, Friesian, or Friesian crossed with Sanga or N′Dama. Two panels of test sera were selected on the basis of their E. ruminantium PC-ELISA reactivities from samples collected during longitudinal and point prevalence serosurveys carried out in Ghana between March 1994 and December 1996. The panel from the longitudinal survey, in which sera were collected monthly from tagged animals of known histories at six field sites in the Greater Accra Region, comprised 328 sera positive by PC-ELISA and 109 sera negative by PC-ELISA from 60 sheep and 173 sera positive by PC-ELISA and 89 sera negative by PC-ELISA (of which 43 samples were from animals which had previously seroconverted as determined by PC-ELISA) from 50 cattle. The panel from the point prevalence survey, in which sera were collected on a single occasion from animals of unknown histories at sites in all 10 regions of Ghana, comprised 91 sera positive by PC-ELISA and 42 sera negative by PC-ELISA from adult sheep and 338 sera positive by PC-ELISA and 172 sera negative by PC-ELISA from calves (subdivided into age groups of 4 to 6 and 7 to 12 months) and cattle >12 months old.
PC-ELISA.
The E. ruminantium PC-ELISA (35) was carried out as follows: Immulon 1 ELISA plates (Dynatech) were coated (100 μl/well) with E. ruminantium (Welgevonden) antigen diluted in carbonate-bicarbonate buffer (Sigma), and the plates were held at 4°C overnight. One well was left empty as a blank control. The plates were then washed manually five times with phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBST). Fifty microliters of test sera, control positive (obtained postrecovery from a goat experimentally infected with the Senegal isolate of E. ruminantium) or negative (from a pool of Scottish cattle) sera, or PBS was added to each well, followed by 50 μl of biotinylated competitor antibody (raised against the Senegal isolate) diluted in PBS with 0.1% Tween 20, and the plates were incubated on a plate shaker at 37°C for 1 h. The plates were then washed as before, 100 μl of ExtrAvidin-peroxidase conjugate (Sigma) diluted 1 in 2,500 in PBST was added to each well, and the plates were incubated as before at 37°C for 30 min and washed as before. Every well then received 100 μl of tetramethyl benzidine substrate (Sigma) dissolved in phosphate-citrate buffer (Sigma) with 0.1% hydrogen peroxide (30% solution; Sigma) added just before use. The plates were incubated at room temperature (22 to 32°C) for 20 min, and the reaction was stopped by adding 100 μl of 1 M sulfuric acid to every well. The plates were read on a Titertek Multiscan plate reader at 450 nm, and the optical density (OD) values obtained after automatic deduction of the OD value of the no-antigen control well were used to calculate the percent inhibition (PI) for each serum sample as follows:
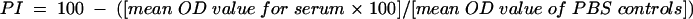 |
As they are percentage calculations that adjust the data to inbuilt controls, PI values can fall outside the 0-to-100% scale. PI levels of >85 for sheep sera and >70 for cattle sera were considered to indicate positivity for E. ruminantium (35).
MAP1-B ELISA.
The MAP1-B indirect ELISA was supplied in kit form by Utrecht University and was carried out as previously described (38) except that Immulon 1 ELISA plates were used and the initial blocking step was carried out by using 100 μl of PBST-1% bovine milk (Marvel; 99% fat free) per well, with the plates stationary during incubation at 37°C. A preliminary titration of the anti-species conjugates provided with the kit was carried out, and dilutions of 1 in 2,000 for sheep and 1 in 1,000 for cattle were subsequently used. Results were presented as percent positivity (PP) calculated as a percentage of the OD value of the reference species positive control sera provided with the kit. As they are percentage calculations that adjust the data to inbuilt controls, PP values can fall outside the 0-to-100% scale. Cutoff points of 14 PP for both species, calculated as the mean + 2 standard deviations of PP (29) of sera from Ghanaian sheep (n = 45) and calves (n = 48) tested prior to seroconversion by the PC-ELISA (Table 1), were compared with previously recommended cutoff points of 29 PP for sheep and 38 PP for cattle (38).
TABLE 1.
Calculation of MAP1-B ELISA cutoff values based on results for Ghanaian sheep and calves determined by PC-ELISA to be seronegative
| Species | No. tested | Mean PI ± SD (range) obtained with PC-ELISA | Mean PP ± SD (range) obtained with MAP1-B ELISA | Cutoff point (mean PP + [2 × SD]) |
|---|---|---|---|---|
| Sheep | 45 | 21.1 ± 18.26 (−3 to 71) | 4.13 ± 4.73 (0.3 to 30.9) | 13.59, or 14 PP |
| Calves | 48 | 17.7 ± 14.3 (−5 to 66) | 3.66 ± 5.13 (−2.9 to 26.9) | 13.92, or 14 PP |
Correlation analysis.
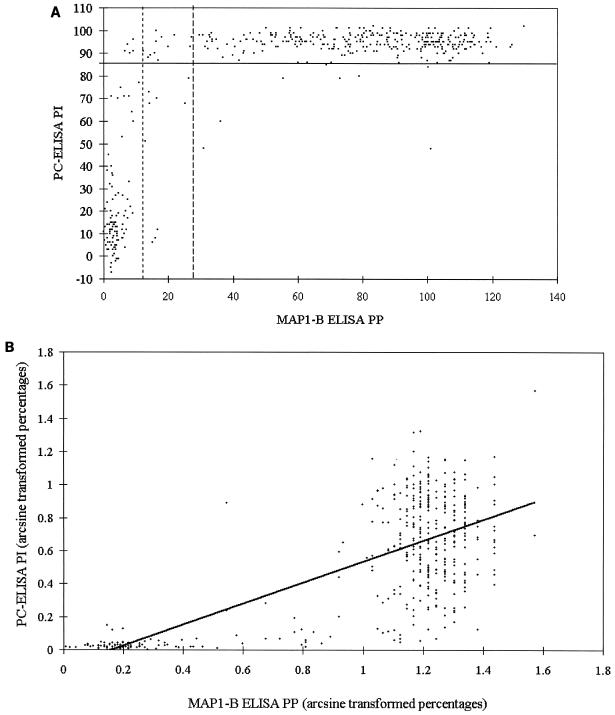
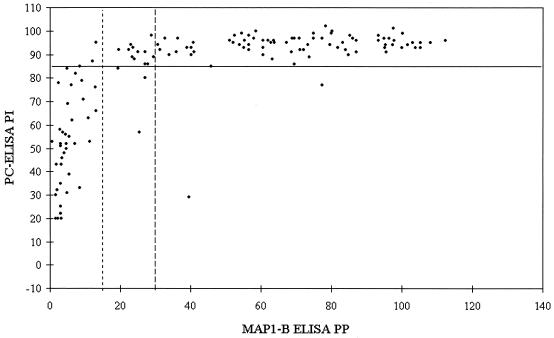
To examine the level of correlation between the two ELISAs for longitudinal and point prevalence survey sheep and cattle sera, the test results were transformed as follows. First, each set of data was converted to a decimal percentage scale by addition of the maximum negative value to the whole data series followed by division by the maximum positive value for the whole series. These decimal percentages were converted by arcsine to normalize them. The paired data were then correlated by using Pearson's product-moment correlation test. Figure 1 shows a representative set of data presented as scatter plots of percentage values (PI and PP) obtained with the two tests (Fig. 1A) and of values after transformation of the percentages by arcsine and fitting of a linear regression line (Fig. 1B). The remaining data sets are presented only as scatter plots of PI and PP (see Fig. 2 to 5) to demonstrate the appropriate positioning of the cutoff levels and the clustering of the data in distinct compartments.
FIG. 1.
(A) Scatter plot of paired PI values (y axis; solid line indicates cutoff of 85 PI) obtained with PC-ELISA and PP values (x axis; dotted line on left indicates cutoff of 14 PP, and broken line on right indicates cutoff of 29 PP) obtained with MAP1-B ELISA for sera (n = 437) from longitudinal survey sheep. (B) Scatter plot of the same paired data shown in panel A but after transformation of the percentages by arcsine and fitting of a linear regression trend line.
FIG. 2.
Scatter plot of paired PI values (y axis; solid line indicates cutoff of 85 PI) obtained with PC-ELISA and PP values (x axis; dotted line on left indicates cutoff of 14 PP, and broken line on right indicates cutoff of 29 PP) obtained with MAP1-B ELISA for sera (n = 133) from point prevalence survey adult sheep.
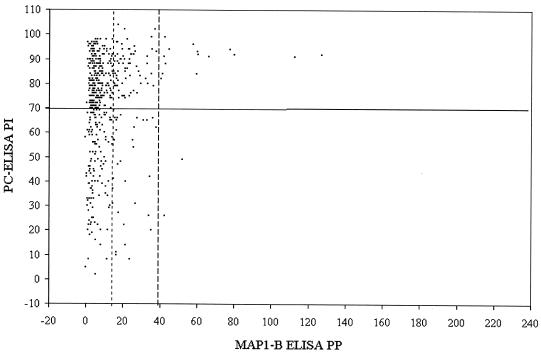
FIG. 5.
Scatter plot of paired PI values (y axis; solid line indicates cutoff of 70 PI) obtained with PC-ELISA and PP values (x axis; dotted line on left indicates cutoff of 14 PP, and broken line on right indicates cutoff of 38 PP) obtained with MAP1-B ELISA for sera (n = 510) from point prevalence calves and adult cattle.
RESULTS
Sheep sera. (i) Longitudinal survey.
Sixty sheep were monitored for up to 34 months; of these, 16 were adult when first sampled and the remainder were monitored from <1 month of age. Forty-six animals either were seropositive throughout or seroconverted at some point as determined by both tests; four lambs did not seroconvert until they were >12 months old. After seroconversion, PI values for 4% of serum samples (n = 661) taken from the 46 animals fell below 85 in the PC-ELISA (data not shown). Figure 1A shows a scatter plot of the results for the 437 individual sheep sera tested by the two assays, with results summarized in Table 2. There was overall agreement between the two tests of 97%, with the MAP1-B ELISA detecting slightly more seropositives than the PC-ELISA with the 14-PP cutoff and slightly fewer with the 29-PP cutoff. Of the 106 preseroconversion sera which tested negative in the PC-ELISA, 10 and 2 tested positive in the MAP1-B ELISA with the lower and higher cutoff levels, respectively, indicating relative specificity for the former test of >90% if the latter test is assumed to be 100% specific. Lowering the cutoff in the MAP1-B ELISA from 29 to 14 PP increased sensitivity by around 3% but reduced specificity by a greater degree.
TABLE 2.
Results for longitudinal survey sheep sera grouped according to PC-ELISA reactivities and tested by MAP1-B ELISA
| PC-ELISA reactivity | No. of sera tested | No. of sera positive by MAP1-B ELISA (% agreement) with cutoff of:
|
|
|---|---|---|---|
| 14 PP | 29 PP | ||
| Positive | 328 | 324 (99) | 314 (96) |
| Negative preseroconversion | 106 | 10 (91) | 2 (98) |
| Negative postseroconversion | 3 | 3 (0) | 3 (0) |
(ii) Point prevalence survey.
Results for the 133 sera from adult sheep of unknown histories are shown in Fig. 2 and Table 3. Of 42 sera which tested negative by the PC-ELISA, 4 and 2 were positive by MAP1-B ELISA with the 14- and 29-PP cutoff levels, respectively, resulting in >90% agreement between the two tests. Of the 91 adult sheep sera which tested positive by the PC-ELISA, 88 and 78 were positive by MAP1-B ELISA with the lower and higher cutoff levels, respectively. In this case, lowering the MAP1-B ELISA cutoff point increased the agreement between the two tests from 86 to 97%.
TABLE 3.
Results from point prevalence survey sheep and cattle sera grouped according to PC-ELISA reactivities and tested by MAP1-B ELISA with low (14 PP for both species) and high (29 PP for sheep and 38 PP for cattle) cutoff levels
| Species (age in mos) | PC-ELISA reactivity | No. of sera tested | No. of sera positive by MAP1-B ELISA (% agreement) with:
|
|
|---|---|---|---|---|
| Low cutoff | High cutoff | |||
| Adult sheep | Positive | 91 | 88 (97) | 78 (86) |
| Negative | 42 | 4 (92) | 2 (95) | |
| Calves (4-6) | Positive | 77 | 25 (33) | 8 (10) |
| Negative | 43 | 8 (81) | 0 (100) | |
| Calves (7-12) | Positive | 114 | 32 (28) | 3 (3) |
| Negative | 42 | 9 (79) | 1 (98) | |
| Adult cattle | Positive | 147 | 31 (21) | 4 (4) |
| Negative | 87 | 16 (82) | 2 (98) | |
| All cattle | Positive | 338 | 88 (26) | 15 (4) |
| Negative | 172 | 33 (81) | 3 (98) | |
Cattle sera. (i) Longitudinal survey.
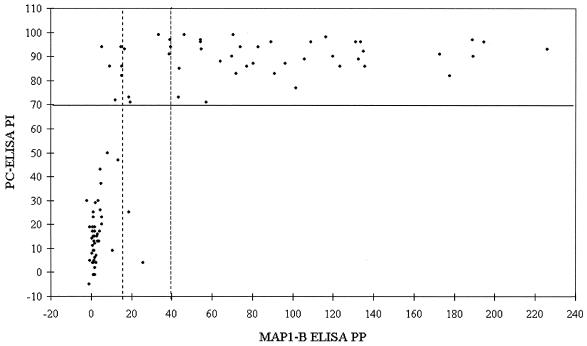
Fifty animals were monitored from ages of 0 to 3 months for up to 34 months. Of these, 49 calves seroconverted, as determined by PC-ELISA, within 12 months and one seroconverted at age 20 months. After seroconversion, PI values for 24% of serum samples (n = 1,063) taken from these animals fell below 70 in the PC-ELISA (data not shown). Figures 3 and 4 show scatter plots of the results from the two assays for, respectively, sera up to and including first seroconversion as detected by PC-ELISA and sera collected after first seroconversion as detected by PC-ELISA. Table 4 summarizes the results from the 262 sera tested in both assays. Of the 46 preseroconversion sera which tested negative by PC-ELISA, none were positive by MAP1-B ELISA with the 38-PP cutoff, while results for 2 sera exceeded the 14-PP cutoff level, indicating a high level of agreement between the tests. In addition, the majority of the postseroconversion samples which tested negative by PC-ELISA were also negative by MAP1-B ELISA. Of the 48 sera considered to represent first-seroconversion samples, 45 and 38 were positive by MAP1-B ELISA with the lower and higher cutoff levels, respectively, giving at least 79% agreement between the two tests. However, of 125 sera which tested positive by PC-ELISA, taken from the same animals following first seroconversion, only 5 were positive by MAP1-B ELISA with the 38-PP cutoff level. With the 14-PP cutoff, 59 postseroconversion sera tested positive by MAP1-B ELISA, improving agreement between the tests to close to 50%.
FIG. 3.
Scatter plot of paired PI values (y axis; solid line indicates cutoff of 70 PI) obtained with PC-ELISA and PP values (x axis; dotted line on left indicates cutoff of 14 PP, and broken line on right indicates cutoff of 38 PP) obtained with MAP1-B ELISA for sera (n = 94) from longitudinal survey calves up to and including first seroconversion.
FIG. 4.
Scatter plot of paired PI values (y axis; solid line indicates cutoff of 70 PI) obtained with PC-ELISA and PP values (x axis; dotted line on left indicates cutoff of 14 PP, and broken line on right indicates cutoff of 38 PP) obtained with MAP1-B ELISA for sera (n = 168) from longitudinal survey cattle after first seroconversion.
TABLE 4.
Results from longitudinal survey cattle sera grouped according to PC-ELISA reactivities and tested by MAP1-B ELISA
| PC-ELISA reactivity | No. of sera tested | No. of sera positive by MAP1-B ELISA (% agreement) with cutoff of:
|
|
|---|---|---|---|
| 14 PP | 38 PP | ||
| Positive at first seroconversion | 48 | 45 (94) | 38 (79) |
| Positive postseroconversion | 125 | 59 (47) | 5 (4) |
| Negative preseroconversion | 46 | 2 (96) | 0 (100) |
| Negative postseroconversion | 43 | 12 (72) | 2 (95) |
| Total positive | 173 | 104 (60) | 43 (22) |
| Total negative | 89 | 14 (84) | 2 (98) |
(ii) Point prevalence survey.
The results of the point prevalence survey are shown in Table 3. Overall, of 172 bovine sera from animals of unknown histories which tested negative by the PC-ELISA, 33 and 3 tested positive by MAP1-B ELISA with 14- and 38-PP cutoff levels, giving agreement of >98 and >80%, respectively. Of the 338 sera which tested positive by PC-ELISA, only 88 and 15 were positive by MAP1-B ELISA with the lower and higher cutoff levels, respectively, giving at best an agreement of 26% between the tests. Figure 5 shows a scatter plot of results from the two assays for all point prevalence bovine sera. Results for the longitudinal survey sera indicated that the MAP1-B ELISA was more effective at detecting first seroconversion, which in 98% of the calves occurred within the first 12 months of life, than subsequent exposure to E. ruminantium. When the point prevalence sera were grouped according to ages of animals (Table 3), the numbers of sera found positive by MAP1-B ELISA were highest among calves aged 4 to 6 months with both cutoff levels, giving seroprevalences for the group of 28 and 7% with the lower and higher cutoff levels, compared with 64% with the PC-ELISA. Overall MAP1-B ELISA-determined seroprevalences were 24 and 4% with the lower and higher cutoff levels, respectively, compared with 66% with the PC-ELISA.
Correlation analysis.
The correlation coefficients (r) and probability values (P) for the paired data sets presented in Fig. 1A and 2 to 5 are shown in Table 5. They were all statistically significant; a high level of correlation (r ≥ 0.66) was found with the data for longitudinal survey and point prevalence sheep (Fig. 1A and 2) and longitudinal survey calves up to and including first seroconversion (Fig. 3). The correlation level was much lower (r ≤ 0.18) for the longitudinal survey cattle after first seroconversion (Fig. 4) and point prevalence cattle (Fig. 5).
TABLE 5.
Correlation coefficients and probability values calculated by using Pearson's product-moment correlation test from paired PC-ELISA and MAP1-B ELISA values for longitudinal and point prevalence survey sheep and cattle sera
| Sample group | r | P |
|---|---|---|
| Longitudinal survey sheep (Fig. 1) | 0.73 | >0.001 |
| Point prevalence sheep (Fig. 2) | 0.69 | >0.001 |
| Longitudinal survey calves up to and including first seroconversion (Fig. 3) | 0.66 | >0.001 |
| Longitudinal survey cattle after first seroconversion (Fig. 4) | 0.18 | >0.01 |
| Point prevalence cattle (Fig. 5) | 0.13 | >0.01 |
Overall seroprevalence.
When the results for all sera tested were combined (Table 6), both tests indicated a high level, at least 70%, of E. ruminantium seropositivity among Ghanaian sheep. The PC-ELISA also indicated a high level (66%) of seropositivity among Ghanaian cattle, although the test failed to detect as positive nearly a quarter of known postseroconversion bovine sera. Overall seroprevalence in cattle measured by the MAP1-B ELISA was much lower—less than 10% with the high cutoff level—and was improved only to 31% by lowering of the cutoff level.
TABLE 6.
Overall E. ruminantium seroprevalences in Ghanaian sheep and cattle as measured by PC-ELISA and MAP1-B ELISA at low (14 PP for both species) and high (29 PP for sheep and 38 PP for cattle) cutoff levels
| Sample group | Seroprevalence (%) as measured by:
|
||
|---|---|---|---|
| PC-ELISA | MAP1-B ELISA (low cutoff) | MAP1-B ELISA (high cutoff) | |
| Point prevalence sheep sera (n = 133) | 68.4 | 69.2 | 60.2 |
| All sheep sera (n = 570) | 73.5 | 83.3 | 70.0 |
| Point prevalence cattle sera (n = 510) | 66.3 | 23.7 | 3.5 |
| All cattle sera (n = 772) | 66.2 | 30.7 | 8.2 |
DISCUSSION
The PC-ELISA has several practical advantages over other E. ruminantium ELISA protocols: the crude antigen and polyclonal competitor antibody are relatively simple to produce, sera are used undiluted, and the same test can be used for any host species, including wildlife for which no specific conjugate would be available. For sheep, sensitivity was very high, with less than 5% of serum samples from known positive animals testing negative over a period of up to 32 months after first seroconversion. For cattle, sensitivity was lower but still useful, with 76% of postseroconversion sera testing positive over the same period.
Comparison of the assay with the MAP1-B ELISA (29, 38) allowed assessment of the relative specificity of the PC-ELISA for Ghanaian animal sera, which was otherwise difficult to evaluate with field samples. Results from the MAP1-B ELISA, which is reported to be highly specific for E. ruminantium, with no cross-reactions with other ruminant Ehrlichia species with cutoff levels of 29 and 38 PP for ovine and bovine sera, respectively (38), strongly supported the view that the organism causing PC-ELISA-detected seroconversion in Ghanaian sheep and cattle was E. ruminantium. The high level of agreement between the two tests with sheep sera indicated that either the level of cross-reactivity in the PC-ELISA with other ovine Ehrlichia species was negligible or that prevalence of such infections in Ghanaian sheep was very low. Ovine ehrlichiosis caused by Ehrlichia ovina has not been reported to occur in Ghana, though its presence in other West African countries (13, 23, 37) and the presence of known or suspected vector tick species in Ghana (22, 39) suggest that the disease is also likely to be present. A rickettsial disease with similarities to monocytic ehrlichiosis in imported exotic goats in the Accra area has been described previously (14). Due to the apparent inability of the MAP1-B ELISA to detect known E. ruminantium-exposed cattle after first seroconversion, it was not possible to draw conclusions on the possible occurrence of other bovine Ehrlichia species in Ghanaian cattle, though the same predisposing factors mentioned above for ovine ehrlichiosis are present in the country. Thus, the PC-ELISA can be considered to be highly specific for sheep sera, while the results presented here do not permit assessment of its specificity for cattle.
The importance of establishing an appropriate cutoff point for the MAP1-B ELISA has been stressed (29); cutoff points calculated by using the preseroconversion Ghanaian sera (14 PP for both species) were much lower than the values of 29 PP for sheep and 38 PP for cattle initially recommended for the test (38). Overall consideration of the results from comparison of the two tests suggests that the ideal cutoff point for both ruminant species would lie somewhere between the value calculated for Ghanaian sera and initially recommended values, with the aim of improving sensitivity without loss of specificity. MAP1-B ELISA cutoff points for bovine sera between 15.6 and 50 PP, calculated in a variety of ways, have been used in field surveys and experimental studies in areas of Africa and the Caribbean where heartwater is endemic (8, 19, 28, 30, 33).
There was good agreement between the two tests with sheep sera, with the PC-ELISA exhibiting levels of sensitivity and specificity comparable to those of the MAP1-B ELISA. Once seroconverted, most longitudinal survey sheep continued to exhibit high antibody levels, testing positive in both assays, though 5 out of the 46 sheep in this category tested negative by one or both tests on some occasions after first seroconversion. Persistently high E. ruminantium antibody levels have been reported in sheep following immunization (11, 30) or recovery from experimental heartwater (38).
Both tests performed worse with bovine sera than with sheep sera; the PC-ELISA gave false-negative results (PI values for 24% of samples taken from the longitudinal survey cattle, after seroconversion detected by both tests, fell below the 70-PI cutoff point, and those for 6% of samples fell below 50 PI) (data not shown), but not nearly as many as the MAP1-B ELISA (PP values for 96 and 57% of postseroconversion samples tested fell below the 38- and 14-PP cutoff points, respectively). This may be due to lack of sensitivity of the latter test for bovine field sera in general compared to that for experimental sera, though its high level of agreement with the PC-ELISA at first seroconversion does not support this view. Values for sera from experimentally infected cattle fell below 38 PP in the MAP1-B ELISA between 50 and 100 days postinoculation (38). A seroprevalence of 33% among nearly 400 bovine field sera tested by MAP1-B ELISA was reported from areas of Zimbabwe where heartwater is endemic, places where seroprevalence in goats exceeded 90% (24), and these values are quite similar to the overall seroprevalences of 31 and 83% in Ghanaian cattle and sheep found with the low MAP1-B ELISA cutoff. A similar seroprevalence of E. ruminantium of 31% was found with the MAP1-B ELISA in traditionally managed N′Dama cattle exposed to year-round Amblyomma variegatum challenge in Côte d'Ivoire (19). A larger field study in Zimbabwe confirmed the disparity between MAP1-B ELISA-determined seroprevalences in cattle and goats in areas where heartwater is endemic (32).
It has been suggested that polymorphism among MAP1 antigens of different E. ruminantium isolates may affect the ability of the MAP1-B ELISA to detect heterologous antibodies (24); however, since the test successfully detected numerous seropositive adult Ghanaian sheep and first seroconversion in most of the Ghanaian calves, MAP1 polymorphism would not explain the negative results with subsequently collected sera from the same cattle. It has also been postulated that the MAP1-B-specific antibody response may be down-regulated in cattle following exposure to E. ruminantium (24). Variable and generally lower antibody levels detected in immune cattle by the PC-ELISA in the present study and by other serological tests (11, 25) indicate that there may be a reduced host antibody response to E. ruminantium in general following first exposure. Indeed, a recent study in Zimbabwe of field-exposed and experimentally infected cattle confirmed that MAP1-B-specific immunoglobulin G and other antibody responses declined despite repeated tick and E. ruminantium challenge (33). With the use of the MAP1-B ELISA on groups of field-exposed N′Dama and Gobra zebu cattle in The Gambia, variation in monthly seroprevalences between 7.5 and 98.1% was found, depending on season, tick control regime, and consequent level of A. variegatum challenge (28).
Tick control for the longitudinal survey cattle was irregular, and Amblyomma ticks were present on at least some animals at each site on sampling days throughout the year, presumably resulting in repeated E. ruminantium challenge. A. variegatum ticks were also found on cattle in all the herds sampled for the point prevalence survey (39). Immunosuppression in cattle due to A. variegatum infestation has been reported (21); it is not known what effect this might have on the humoral immune response to E. ruminantium in immune, tick-infested animals. Since protective immunity to heartwater is considered to be primarily cell mediated (36) and since the seronegative adult cattle (at least one-third of the point prevalence survey animals) were surviving in an area of endemicity under regular A. variegatum, and presumably E. ruminantium, challenge, it can be concluded that the absence of detectable levels of antibodies in sera of cattle does not correlate with susceptibility to heartwater. The true proportion of heartwater-immune adult cattle in Ghana is likely to be much higher than the levels indicated by either of the serological assays used in the present study. In neighboring Côte d'Ivoire, the true prevalence of E. ruminantium infection in cattle was suspected to be higher than the 31% found by MAP1-B ELISA (19), while the same test was suggested to be an unreliable indicator of past exposure to heartwater in field-infected cattle in Zimbabwe (32).
Heartwater was first recognized as a disease problem in Ghana 70 years ago (3). Subsequent studies have confirmed its importance as a cause of mortality, particularly in small ruminants (2, 6, 31). However, until the present study, estimates of disease incidence were gathered from postmortem diagnosis data, with diagnoses in some cases based on macroscopic lesions alone, without microscopic confirmation of the presence of rickettsiae in brain smears (2, 6). The classic postmortem signs, hydrothorax and hydropericardium, do not always occur in heartwater cases, and conversely their presence may be due to other causes such as haemonchosis and plant poisoning, both also significant causes of mortality in Ghanaian sheep and goats (1, 6, 31). The results obtained from testing ovine and bovine field sera from locations throughout the country with the PC-ELISA and MAP1-B ELISA confirm the widespread exposure of ruminants in Ghana to E. ruminantium. However, among adult sheep there exists a significant proportion of animals that are seronegative by both tests, and these animals can be assumed to be naïve (true seronegatives) and at risk from heartwater. The levels of false negatives in both tests do not allow conclusions to be drawn on the occurrence of true seronegatives among adult cattle. In the absence of a serodiagnostic assay combining high levels of sensitivity and specificity for field sera from all domestic ruminant species, the present study confirms the usefulness of the PC-ELISA in carrying out field surveys of E. ruminantium seroprevalence in West Africa, either alone or in conjunction with the MAP1-B ELISA.
Acknowledgments
The research was carried out in Ghana by kind permission of the Director of Veterinary Services and was supported by the United Kingdom Government ODA (now DFID) Animal Health Programme project R5971CB.
The cooperation and assistance of staff of the Ghana Government Veterinary Services Department and the Animal Research Institute, Achimota, and livestock owners and herdsmen in the Greater Accra Region and at sampling sites throughout Ghana are gratefully appreciated. Raffaele Mattioli, Cornelis Bekker, and Ivan Morrison are thanked for critical comments on the manuscript.
REFERENCES
- 1.Aklaku, I. K. 1980. Principal causes of mortality in small ruminants in Ghana. Bull. Off. Int. Epizoot. 92:1227-1231. [Google Scholar]
- 2.Aning, K. G. 1982. Heartwater disease in sheep and goats in Ghana: its distribution, incidence and control, p. 23-28. In Proceedings of the 13th Ghana Animal Science Symposium. University of Science and Technology, Kumasi, Ghana.
- 3.Anonymous. 1933. Annual report of the Veterinary Department, Gold Coast Colony. The Government Printer, Accra, Ghana.
- 4.Bell-Sakyi, L., E. B. M. Koney, O. Dogbey, and K. J. Sumption. 1996. Heartwater in Ghana: implications for control of ticks. Trop. Anim. Health Prod. 28:59S-64S. [DOI] [PubMed]
- 5.Bell-Sakyi, L., E. B. M. Koney, O. Dogbey, J. A. Abbam, and K. G. Aning. 1997. Isolation and in vitro cultivation in Ghana of Cowdria ruminantium, the causative agent of heartwater, p. 46-51. In E. B. M. Koney and K. G. Aning (ed.), Proceedings of the 22nd Annual Conference of the Ghana Veterinary Medical Association. Ministry of Food and Agriculture, Accra, Ghana.
- 6.Bonniwell, M. A. 1978. Veterinary problems associated with sheep rearing in the Ashanti Region of Ghana, p. 116-127. In J. K. Obinim, K. G. Aning, and I. K. Aklaku (ed.), Proceedings of the First African Veterinary Conference, Accra. TAD Pharmazeutischers Werk GMBH, Cuxhaven, Germany.
- 7.Camus, E., N. Barre, D. Martinez, and G. Uilenberg. 1996. Heartwater (cowdriosis), a review, 2nd ed. Office International des Epizooties, Paris, France.
- 8.De Waal, D. T., O. Matthee, and F. Jongejan. 2000. Evaluation of the MAP1b ELISA for the diagnosis of heartwater in South Africa. Ann. N. Y. Acad. Sci. 916:622-627. [DOI] [PubMed] [Google Scholar]
- 9.Dugan, V. G., S. E. Little, D. E. Stallknecht, and A. D. Beall. 2000. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 38:448-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 11.Du Plessis, J. L. 1981. The application of the indirect fluorescent antibody test to the serology of heartwater, p. 47-52. In G. B. Whitehead and J. D. Gibson (ed.), Tick biology and control. Tick Research Unit, Grahamstown, South Africa.
- 12.Du Plessis, J. L., J. D. Bezuidenhout, M. S. Brett, E. Camus, F. Jongejan, S. M. Mahan, and D. Martinez. 1993. The sero-diagnosis of heartwater: a comparison of five tests. Rev. Elev. Med. Vet. Pays Trop. 46:123-129. [PubMed] [Google Scholar]
- 13.Gueye, A., M. Mbengue, and A. Diouf. 1989. Tiques et hemoparasitoses du betail au Senegal. IV. La zone sud-soudanienne. Rev. Elev. Med. Vet. Pays Trop. 42:517-528. [PubMed] [Google Scholar]
- 14.Hughes, M. H. 1953. A rickettsial disease of goats in the Gold Coast. Ann. Trop. Med. Parasitol. 47:299-303. [DOI] [PubMed] [Google Scholar]
- 15.Jongejan, F., and C. P. J. Bekker. 1999. Cowdria ruminantium: recent developments in diagnostic methods, molecular characterization and vaccines, p. 373-386. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millennium. Elsevier, Paris, France.
- 16.Jongejan, F., N. de Vries, J. Nieuwenhuijs, A. H. M. van Vliet, and L. A. Wassink. 1993. The immunodominant 32-kilodalton protein of Cowdria ruminantium is conserved within the genus Ehrlichia. Rev. Elev. Med. Vet. Pays Trop. 46:145-152. [PubMed] [Google Scholar]
- 17.Jongejan, F., M. J. C. Thielemans, M. De Groot, P. J. S. Van Kooten, and B. A. M. Van Der Zeijst. 1991. Competitive enzyme linked immunoassay for heartwater using monoclonal antibodies to a Cowdria ruminantium-specific 32 kilodalton protein. Vet. Microbiol. 28:199-211. [DOI] [PubMed] [Google Scholar]
- 18.Katz, J. B., R. DeWald, J. E. Dawson, E. Camus, D. Martinez, and R. Mondry. 1997. Development and evaluation of a recombinant antigen, monoclonal antibody-based competitive ELISA for heartwater serodiagnosis. J. Vet. Diagn. Investig. 9:130-135. [DOI] [PubMed] [Google Scholar]
- 19.Knopf, L., C. Komoin-Oka, B. Betschart, F. Jongejan, B. Gottstein, and J. Zinsstag. 2002. Seasonal epidemiology of ticks and aspects of cowdriosis in N′Dama village cattle in the Central Guinea savannah of Cote d'Ivoire. Prev. Vet. Med. 53:21-30. [DOI] [PubMed] [Google Scholar]
- 20.Kobold, A. M., D. Martinez, E. Camus, and F. Jongejan. 1992. Distribution of heartwater in the Caribbean determined on the basis of detection of antibodies to the conserved 32-kilodalton protein of Cowdria ruminantium. J. Clin. Microbiol. 30:1870-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koney, E. B. M., A. N. Morrow, I. Heron, N. C. Ambrose, and G. R. Scott. 1994. Lymphocyte proliferative responses and the occurrence of dermatophilosis in cattle naturally infested with Amblyomma variegatum. Vet. Parasitol. 55:245-256. [DOI] [PubMed] [Google Scholar]
- 22.Koney, E. B. M., A. R. Walker, I. D. Heron, A. N. Morrow, and N. C. Ambrose. 1994. Seasonal prevalence of ticks and their association with dermatophilosis in cattle on the Accra plains of Ghana. Rev. Elev. Med. Vet. Pays Trop. 47:163-167. [PubMed] [Google Scholar]
- 23.Leeflang, P., and A. A. Ilemobade. 1977. Tick-borne diseases of domestic animals in northern Nigeria. II. Research summary, 1966 to 1976. Trop. Anim. Health Prod. 9:211-218. [DOI] [PubMed] [Google Scholar]
- 24.Mahan, S. M., S. M. Semu, T. F. Peter, and F. Jongejan. 1998. Evaluation of the MAP-1B ELISA for cowdriosis with field sera from livestock in Zimbabwe. Ann. N. Y. Acad. Sci. 849:259-261. [DOI] [PubMed] [Google Scholar]
- 25.Mahan, S. M., N. Tebele, D. Mukwedeya, S. Semu, C. B. Nyathi, L. A. Wassink, P. J. Kelly, T. Peter, and A. F. Barbet. 1993. An immunoblotting diagnostic assay for heartwater based on the immunodominant 32-kilodalton protein of Cowdria ruminantium detects false positives in field sera. J. Clin. Microbiol. 31:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez, D., S. Coisne, C. Sheikboudou, and F. Jongejan. 1993. Detection of antibodies to Cowdria ruminantium in the serum of domestic ruminants by indirect ELISA. Rev. Elev. Med. Vet. Pays Trop. 46:115-120. [PubMed] [Google Scholar]
- 27.Martinez, D., J. Swinkels, E. Camus, and F. Jongejan. 1990. Comparaison de trois antigenes pour la serodiagnostic de la cowdriose par immunofluorescence indirecte. Rev. Elev. Med. Vet. Pays Trop. 43:159-166. [PubMed] [Google Scholar]
- 28.Mattioli, R. C., M. Bah, R. Reibel, and F. Jongejan. 2000. Cowdria ruminantium antibodies in acaricide-treated and untreated cattle exposed to Amblyomma variegatum ticks in The Gambia. Exp. Appl. Acarol. 24:957-969. [DOI] [PubMed] [Google Scholar]
- 29.Mboloi, M. M., C. P. J. Bekker, C. Kruitwagen, M. Greiner, and F. Jongejan. 1999. Validation of the indirect MAP1-B enzyme-linked immunosorbent assay for diagnosis of experimental Cowdria ruminantium infection in small ruminants. Clin. Diagn. Lab. Immunol. 6:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondry, R., D. Martinez, E. Camus, A. Liebisch, J. B. Katz, R. DeWald, A. H. M. van Vliet, and F. Jongejan. 1998. Validation and comparison of three enzyme-linked immunosorbent assays for the detection of antibodies to Cowdria ruminantium infection. Ann. N. Y. Acad. Sci. 849:262-272. [DOI] [PubMed] [Google Scholar]
- 31.Oppong, E. N. W. 1973. Diseases of sheep in Ghana. Ghana J. Agric. Sci. 6:3-7. [Google Scholar]
- 32.Peter, T. F., C. J. O'Callaghan, G. F. Medley, B. D. Perry, S. M. Semu, and S. M. Mahan. 2002. Population-based evaluation of the Ehrlichia ruminantium MAP 1B indirect ELISA. Exp. Appl. Acarol. 25:881-897. [DOI] [PubMed] [Google Scholar]
- 33.Semu, S. M., T. F. Peter, D. Mukwedeya, A. F. Barbet, F. Jongejan, and S. M. Mahan. 2001. Antibody responses to MAP 1B and other Cowdria ruminantium antigens are down regulated in cattle challenged with tick-transmitted heartwater. Clin. Diagn. Lab. Immunol. 8:388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soldan, A. W., T. L. Norman, S. Masaka, E. A. Paxton, M. Edelsten, and K. J. Sumption. 1993. Seroconversion to Cowdria ruminantium of Malawi zebu calves, reared under different tick control strategies. Rev. Elev. Med. Vet. Pays Trop. 46:171-177. [PubMed] [Google Scholar]
- 35.Sumption, K. J., E. A. Paxton, and L. Bell-Sakyi. 2003. Development of a polyclonal competitive enzyme-linked immunosorbent assay for detection of antibodies to Ehrlichia ruminantium. Clin. Diagn. Lab. Immunol. 10:910-916. [DOI] [PMC free article] [PubMed]
- 36.Totte, P., A. Bensaid, S. M. Mahan, D. Martinez, and D. J. McKeever. 1999. Immune responses to Cowdria ruminantium infections. Parasitol. Today 15:286-290. [DOI] [PubMed] [Google Scholar]
- 37.Uilenberg, G. 1993. Other ehrlichioses of ruminants, p. 269-279. In Z. Woldehiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Oxford, United Kingdom.
- 38.van Vliet, A. H. M., B. A. M. Van Der Zeijst, E. Camus, S. M. Mahan, D. Martinez, and F. Jongejan. 1995. Use of a specific immunogenic region on the Cowdria ruminantium MAP1 protein in a serological assay. J. Clin. Microbiol. 33:2405-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, A. R., and E. B. M. Koney. 1999. Distribution of ticks (Acari: Ixodida) infesting domestic ruminants in Ghana. Bull. Entomol. Res. 89:473-479. [Google Scholar]