Abstract
Serum immunoglobulin transudation into the murine gut after intragastric immunization with the model antigen ovalbumin and cholera toxin adjuvant was investigated with regard to the mucosal sampling technique applied. The levels of serum-derived immunoglobulin A (IgA) turned out to be lowest in feces, intermediate in gut lavage fluid specimens, and highest in filter wick-collected samples. However, these levels did not exceed 2% of total and specific IgA in any mucosal sample type, except after the administration of very high antigen doses (≥1 mg of antigen per g of body weight), when transudation rates of up to 31% could be measured in filter wick-collected samples from individual animals. Luminal IgG was plasma transudate and/or bile borne and appeared to be reabsorbed at the mucosa to some extent.
In order to exert its full protective power, mucosal immunoglobulin A (IgA) must be dimeric (13) and carry the secretory component (12), which is provided by the epithelia from which it is released. When specific IgA levels at mucosal surfaces are measured, it is therefore important to discriminate between transudated monomeric and locally produced, actively secreted dimeric IgA, as only the latter is able to carry the secretory component with which the immune complex is immobilized in mucus (12). Since the way in which mucosal secretions are collected may have a considerable influence on the extent of contamination with monomeric serum IgA, we investigated the contributions of serum-borne IgA and IgG in feces, intestinal lavage fluid, and filter wick-collected local intestinal secretions, the three most commonly used samples obtained after experimental mucosal immunization (5).
MATERIALS AND METHODS
Immunization and sample production.
For immunization, groups of six female BALB/c mice (age, 8 weeks; Charles River Wiga, Sulzfeld, Germany) were gavaged four times on days 0, 21, 35, and 49 with 0.2, 2, or 20 mg ovalbumin (Calbiochem, Bad Soden, Germany) plus 10 μg of cholera toxin (List Biological Laboratories, Campbell, Calif.) in 300 μl of 3% (wt/vol) sodium bicarbonate or with buffer alone (controls). Ten to 11 days after the last immunization, feces, blood, intestinal lavage fluid, and local intestinal secretions were collected and processed as described previously (2, 6).
Determination of transudation marker and immunoglobulin concentrations.
Murine serum albumin (MSA) was chosen as the transudation marker and was assayed by capture enzyme-linked immunosorbent assay. Each well of high-binding enzyme immunoassay plates (Corning Costar, Bodenheim, Germany) were coated with 75 μl of 40 ng of goat anti-mouse albumin (Bethyl, Montgomery, Tenn.) per ml in 10 mM sodium phosphate (pH 7.0)-10 mM NaCl overnight at 4°C, washed three times with Dulbecco's phosphate-buffered saline (D-PBS) containing 0.05% (vol/vol) Tween 20, and blocked with D-PBS containing 5% (wt/vol) nonfat dry milk (PBS-Blotto) for 5 h at room temperature. After four washes, 75 μl of serially diluted samples and immunoglobulin-free MSA standard (ICN, Eschwege, Germany) in PBS-Blotto were applied to each well and the plates were incubated overnight at 4°C. After another four washes, 75 μl of horseradish peroxidase-labeled goat anti-mouse albumin (Bethyl) diluted 1:1,000 in PBS-Blotto was applied to each well, the plates were incubated for 90 min at room temperature and again washed six times, and the color was developed by using a highly sensitive tetramethylbenzidine substrate reagent (3).
Quantitation of total and specific immunoglobulins was carried out as described previously (2). Antibody cross-reactivities were determined under conditions analogous to those of the quantitation of total immunoglobulins as the ratios of the detection limits for the potentially cross-reacting analyte to those for the original target analyte.
Total MSA and immunoglobulin concentrations were determined on the basis of four-parameter curve fit approximations of standard titration curves by using the readouts for the unknown samples at the steepest slopes of their titration curves (SOFTmax Pro, version 1.0; Molecular Devices, Sunnyvale, Calif.). Specific antibody responses were expressed as endpoint titers, being the reciprocal for the highest dilution that gave a reading above the cutoff, with the cutoff being the upper limit of a 99.5% confidence interval above the mean control level (4).
For determination of the relative amount of serum antibody transudate in a mucosal sample, unhindered plasma flow was assumed for the site of leakage (i.e., leakage of immunoglobulin analyte and serum marker transudate equally well). With this, the ratio of the concentration of transudating immunoglobulin analyte to the concentration of the transudating marker is identical for the serum and the mucosal sample, as expressed by equation 1:
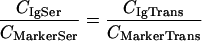 |
(1) |
where CIgSer and CMarkerSer are the measured total concentrations of immunoglobulin and transudation marker in serum, respectively, while CIgTrans and CMarkerTrans are the concentrations of transudated immunoglobulin and transudated marker in the mucosal sample, respectively. The relative amounts of serum antibody (percent TransudateIgMuc) and marker (percent TransudateMarkerMuc) in mucosal specimens can be described by equations 2 and 3:
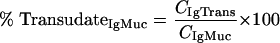 |
(2) |
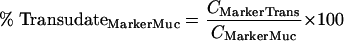 |
(3) |
where CIgMuc and CMarkerMuc are the measured total immunoglobulin analyte and marker concentrations in the mucosal sample, respectively. These equations can be resolved into a term (equation 4) that describes the relative amount of serum antibody transudate in a mucosal sample (percent TransudateIgMuc):
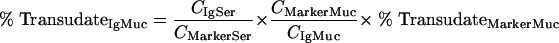 |
(4) |
In this equation, all variables except the relative amount of marker in the mucosal sample are experimentally accessible, but the relative amount of marker in the mucosal sample can safely be set to 100% in the case of MSA since serum albumin is neither produced nor actively transported by the mucosal epithelium.
On the basis of equation 4, mucosal immunoglobulins that are entirely plasma derived must display transudation rates close to 100%, whereas active translocation results in rates below this level and active absorption results in rates above this level.
Data analysis.
Statistical analyses were carried out with the Statview program (version 4.5; Abacus Concepts, Berkeley, Calif.). Between-group comparisons were performed by one-way analysis of variance (one-way ANOVA) with Fisher's protected least significant difference test. Paired t tests were used for paired samples. The results of the statistical analyses were considered significant only if P was <0.05.
RESULTS AND DISCUSSION
Analyte specificities of detection systems.
Massive cross-reactivities of capture and detection antibodies with other than their original target analyte result in erroneously higher target analyte concentrations. For that reason the mutual cross-reactivities of all capture enzyme-linked immunosorbent assays with all analytes were determined. When the measured titers and protein concentrations were corrected for cross-reactivities, the deviations between corrected and uncorrected values were below 0.034% ± 0.002% (mean ± standard error of the mean [SEM]) and were considered negligible for all titers and protein concentrations measured except serum IgA concentrations. The differences between corrected and uncorrected values for serum IgA were 2.71% ± 0.21% (mean ± SEM). For that reason, cross-reactivity-corrected concentrations were used only for serum IgA concentrations.
Total transudation marker and immunoglobulin contents in sampled specimens.
For the MSA contents of the specimens sampled by invasive means, i.e., serum, intestinal lavage fluid, and filter wick-collected samples, no persistent statistically significant differences between the different immunization groups were observed (one-way ANOVA, P ≥ 0.056), which indicates that all groups were comparable with respect to their serum marker concentration and were treated alike during sample collection. The total MSA and immunoglobulin concentrations of all samples along with the relative amounts of serum IgA and IgG in mucosal samples are given in Table 1.
TABLE 1.
Total immunoglobulin and albumin contents of collected samples and percent transudated immunoglobulina
| Sample type | Antigen dose (mg) | Analyte concn (mean ± SEM)
|
% Total immunoglobulin in transudate (mean ± SEM)
|
|||
|---|---|---|---|---|---|---|
| Albumin (μg/ml or μg/g)b | IgG (μg/ml or μg/g) | IgA (μg/ml or μg/g) | IgG | IgA | ||
| Serum | 0c | 25,700 ± 2,780 | 3,830 ± 449 | 250 ± 23 | NAd | NA |
| 0.2 | 31,300 ± 1,330 | 3,720 ± 472 | 145 ± 15 | NA | NA | |
| 2 | 33,000 ± 1,590 | 3,680 ± 388 | 253 ± 25 | NA | NA | |
| 20 | 29,100 ± 1,260 | 4,940 ± 1,250 | 200 ± 25 | NA | NA | |
| Alle | 29,800 ± 1,030 | 4,040 ± 358 | 212 ± 14 | NA | NA | |
| Feces | 0 | 118 ± 18 | 17.3 ± 2.7 | 4,900 ± 801 | 115 ± 25 | 0.030 ± 0.008 |
| 0.2 | 110 ± 23 | 25.5 ± 3.3 | 4,520 ± 579 | 47.0 ± 6.8 | 0.011 ± 0.002 | |
| 2 | 704 ± 251 | 66.4 ± 12.1 | 5,670 ± 608 | 109 ± 22 | 0.102 ± 0.042 | |
| 20 | 176 ± 50 | 44.1 ± 15.2 | 4,480 ± 1,000 | 60.9 ± 13.4 | 0.027 ± 0.007 | |
| All | 277 ± 79 | 38.3 ± 6.1 | 4,890 ± 371 | 83.1 ± 10.5 | 0.043 ± 0.013 | |
| Small intestinal lavage fluidf | 0 | 5.20 ± 1.42 | 1.08 ± 0.20 | 29.1 ± 5.5 | 66.1 ± 7.9 | 0.199 ± 0.047 |
| 0.2 | 8.56 ± 2.97 | 1.53 ± 0.20 | 24.3 ± 7.7 | 55.9 ± 10.5 | 0.286 ± 0.110 | |
| 2 | 0.728 ± 0.155 | 1.00 ± 0.27 | 38.8 ± 7.6 | 9.54 ± 2.40 | 0.021 ± 0.007 | |
| 20 | 4.82 ± 1.83 | 3.42 ± 1.31 | 40.9 ± 9.5 | 30.0 ± 12.8 | 0.100 ± 0.034 | |
| All | 4.83 ± 1.05 | 1.76 ± 0.38 | 33.3 ± 3.9 | 40.4 ± 6.3 | 0.152 ± 0.036 | |
| Filter wick-collected secretions from proximal small intestine | 0 | 405 ± 114 | 34.3 ± 7.1 | 214 ± 26 | 175 ± 43 | 1.98 ± 0.54 |
| 0.2 | 765 ± 147 | 66.6 ± 8.5 | 239 ± 22 | 126 ± 18 | 1.46 ± 0.29 | |
| 2 | 395 ± 77 | 38.5 ± 6.5 | 137 ± 22 | 117 ± 19 | 2.45 ± 0.56 | |
| 20 | 817 ± 162 | 97.4 ± 13.5 | 384 ± 9 | 126 ± 13 | 1.46 ± 0.33 | |
| All | 604 ± 74 | 60.3 ± 7.0 | 245 ± 22 | 134 ± 12 | 1.83 ± 0.22 | |
| Filter wick-collected secretions from distal small intestine | 0 | 578 ± 137 | 52.1 ± 8.4 | 257 ± 38 | 155 ± 11 | 2.31 ± 0.45 |
| 0.2 | 611 ± 75 | 55.9 ± 7.0 | 271 ± 25 | 129 ± 14 | 1.05 ± 0.13 | |
| 2 | 501 ± 137 | 43.6 ± 8.2 | 196 ± 33 | 127 ± 16 | 1.95 ± 0.37 | |
| 20 | 735 ± 66 | 90.2 ± 17.9 | 377 ± 45 | 136 ± 14 | 1.40 ± 0.17 | |
| All | 606 ± 54 | 60.4 ± 6.4 | 275 ± 22 | 136 ± 7 | 1.68 ± 0.18 | |
Some of the IgA concentrations have been reported earlier in another context (2).
Analyte contents in feces are given as micrograms per gram of dry fecal matter; all other contents are given as micrograms per milliliter of the undiluted sample.
Control animals which received buffer only.
NA, not applicable.
Mean for all groups.
Concentrations depend on the amount of lavage fluid used. Percent transudate values are absolute measures; i.e., they are independent of the lavage fluid volume.
Transudation of serum IgA into feces, intestinal lavage fluid, and filter wick-collected samples.
Throughout the study transudation rates for total serum IgA were highest in filter wick-collected samples, with no statistically significant differences between the rates for specimens from the proximal or the distal small intestine, neither when rates for individual immunization groups nor when the averages over all groups were compared (one-way ANOVA, P ≥ 0.096 and P = 0.44, respectively). Averaged over all groups and both collection sites, plasma leakage accounted for 1.75% ± 0.14% (mean ± SEM; n = 47) of filter wick-extracted IgA. Compared to that value, the relative amounts of transudated IgA in feces and intestinal lavage fluid were significantly lower (one-way ANOVA, P ≤ 0.005) and not significantly different from each other, neither when the amounts for individual immunization groups nor when the averages over all groups were compared (one-way ANOVA, P ≥ 0.26 and P = 0.58, respectively). Since the transudate content of feces reflects the naturally occurring baseline level of plasma leakage, the filter wick absorption procedure clearly generates artifactually higher IgA measurements for mucosal secretions. The reason for this may be attributed either to physical damage of the epithelium caused by application of the filter wicks or to capillary suction of the filter fabric.
In light of this observation, it is important to elucidate the conditions under which artifactual serum IgA leakage caused by filter wicks is tolerable or not. As the filter wick absorption procedure is predominantly used to analyze antibody immune responses after mucosal infection or immunization, we also determined the transudation rates of specific serum IgA after experimental mucosal immunization (Table 2). Again, serum IgA transudation was highest in filter wick-collected samples, but in contrast to total IgA, transudation of specific IgA turned out to be antigen dose dependent.
TABLE 2.
Antigen-specific IgA titers and percent transudated specific IgA in collected samplesa
| Sample type | Antigen dose (mg) | Log (titer + 1) (mean ± SEM) | % Transudated specific IgA (mean ± SEM) |
|---|---|---|---|
| Serum | 0.2 | 4.43 ± 0.34 | NAb |
| 2 | 4.79 ± 0.13 | NA | |
| 20 | 6.14 ± 0.05 | NA | |
| Allc | 5.12 ± 0.21 | NA | |
| Feces | 0.2 | 5.28 ± 0.22 | 0.08 ± 0.04 |
| 2 | 5.58 ± 0.19 | 0.38 ± 0.13 | |
| 20 | 6.89 ± 0.16 | 0.12 ± 0.04 | |
| All | 5.92 ± 0.20 | 0.19 ± 0.06 | |
| Small intestinal lavage fluidd | 0.2 | 3.28 ± 0.25 | 0.61 ± 0.35 |
| 2 | 3.73 ± 0.14 | 0.04 ± 0.02 | |
| 20 | 4.18 ± 0.17 | 2.33 ± 1.36 | |
| All | 3.73 ± 0.14 | 1.00 ± 0.50 | |
| Filter wick-collected secretions from proximal small intestine | 0.2 | 4.69 ± 0.20 | 1.44 ± 0.46 |
| 2 | 5.54 ± 0.20 | 0.25 ± 0.08 | |
| 20 | 5.54 ± 0.09 | 11.52 ± 3.14 | |
| All | 5.25 ± 0.14 | 4.40 ± 1.58 | |
| Filter wick-collected secretions from distal small intestine | 0.2 | 4.94 ± 0.25 | 0.61 ± 0.09 |
| 2 | 5.29 ± 0.15 | 0.49 ± 0.12 | |
| 20 | 5.54 ± 0.09 | 11.72 ± 3.91 | |
| All | 5.25 ± 0.11 | 4.27 ± 1.77 |
Some of the titer data have been reported earlier in another context (2).
NA, not applicable.
Mean for all groups.
Titers depend on the amount of lavage fluid used. Percent transudate values are absolute measures; i.e., they are independent of the lavage fluid volume.
For ≤2 mg of ovalbumin (i.e., for the groups receiving 0.2 and 2 mg combined), the amount of specific serum IgA in filter wick-collected samples did not differ significantly from that in feces or intestinal lavage fluid, no matter whether filter wick-collected samples from the proximal and the distal small intestine were analyzed separately or the average for both sites was used for comparison (one-way ANOVA, P ≥ 0.071). The contributions of serum IgA to the mucosal secretion titers for these antigen doses were 0.23% ± 0.08% (mean ± SEM; n = 12) in feces, 0.33% ± 0.19% (mean ± SEM; n = 12) in small intestinal lavage fluid, and 0.70% ± 0.15% (mean ± SEM for both sampling sites; n = 24) in filter wick-collected samples, with the highest rate in an individual animal being 3.5%.
However, when 20 mg of antigen, which corresponds to 1.25 mg of antigen per g of body weight of the mice at the day of priming (body weight, 16.02 ± 0.60 g [mean ± SEM; n = 6]), was used, a dramatic increase in the relative amount of specific serum IgA in filter wick-collected samples was observed. It reached up to 31% in individual animals and was significantly different from those in feces and intestinal lavage fluid (one-way ANOVA, P ≤ 0.007).
We attribute this to the high specific serum IgA titers generated by such an antigen dose and conclude that the filter wick absorption procedure is prone to erroneous results when high serum IgA titers and/or low secretory IgA titers are expected. J-chain-knockout (7, 11) or polymeric immunoglobulin receptor-knockout (8) mice, which are presumed to be deficient in secretory IgA production but which show up to 35-fold higher total serum IgA titers, are a prototype example for this constraint. For these animals, plasma-derived monomeric IgA should give rise to amounts of IgA in filter wick-collected samples nearly similar to those in wild-type animals. In view of this, it seems questionable whether alternative transport mechanisms are responsible for the high levels of monomeric IgA in filter wick-collected specimens from jch−/− mice, as proposed by Hendrickson et al. (7).
For applications other than that, however, the filter wick absorption procedure appears to be well suited for determination of the secretory IgA status at mucosal surfaces. Even for extreme hyperimmunizations, like those achieved with the 20-mg dose (which, in case of human applications, would translate into the enormous dose of about 15 g of antigen for a 2-year-old toddler on the basis of body weight), almost 90% of the specific IgA in a filter wick-collected sample is still locally produced; and it seems unlikely that an average deviation of 0.7% for moderate antigen doses would affect titer readouts for serially diluted samples to a significant extent. In support of this assumption, we observed that correlations between specific antibody titers in serum and filter wick-collected intestinal secretions vanish with increasing antigen doses (2). If the contribution of plasma transudate-derived IgA to the readouts for filter wick-collected samples had been of significance, these correlations should have increased along with the antigen dose instead of going down.
Thus, in wild-type mice more than 98% of total and specific mucosal IgA antibodies which have been sampled by either procedure are true secretory IgA antibodies; i.e., the IgA is dimeric or polymeric and carries the secretory component. This is in good agreement with data obtained by use of yet another sampling procedure, the so-called perfusion extraction method, by which serum IgA transudation rates of 1.5% have been measured for the murine small intestine (9). The transudation rates obtained for mice by either sampling technique also correspond well to those in the human small intestine, in which 98% of luminal IgA was found to be of the secretory type (10).
On the basis of our data, however, it is difficult to assess where the secretory IgA antibodies are in fact transported. In addition to the intestinal epithelium, the hepatobiliary system constitutes another translocation site for those immunoglobulins. Even filter wick-collected specimens may contain bile-derived secretory IgA that diffused from the lumen of the gut toward the epithelial layer. This concern is of particular relevance in the murine system, since rodents were shown to have a considerably higher biliary secretory IgA transport rate than humans (1).
Transudation of serum IgG into feces, intestinal lavage fluid, and filter wick-collected samples.
Since IgG is believed to be not actively transported across the intestinal epithelium in adult rodents, we expected transudation rates of about 100% for this immunoglobulin and the ratio of the MSA concentration to the total IgG concentration to be identical to that for serum in all mucosal sample types. This, however, was not the case.
When the data for all groups were combined, the MSA concentration to total IgG concentration ratios were significantly lower for feces and intestinal lavage fluid (two-tailed, paired t test, P = 0.044 and P < 0.0001, respectively) and significantly higher for filter wick-collected samples than for serum (two-tailed, paired t test, P ≤ 0.015). Ratios were also significantly lower for gut lavage fluid than for feces (two-tailed, paired t test, P = 0.005) but not different between filter wick-collected specimens from the two sites (two-tailed, paired t test, P = 0.43). When the data were analyzed by group, the same tendency was observed, but there were fewer significant differences.
According to these data, the amount of IgG collected from small intestinal surfaces with filter wicks was less than the amount expected from the MSA contents of these samples, resulting in IgG transudation rates above 100% (Table 1). IgG must therefore be either actively reabsorbed at the intestinal epithelium or more rapidly degraded than MSA. Which of these possibilities finally holds true cannot be assessed on the basis of the present data. However, our measurements clearly indicate that IgG is not actively transported across the small intestinal epithelium into the lumen of the gut. This is not in conflict with the observation that about 60% of the IgG in intestinal lavage fluid is locally produced, since luminal IgG can be bile derived in mice (1). While en route to the large intestine, part of this bile-derived IgG may again either be broken down more rapidly than MSA or be reabsorbed, which would explain the nominally higher transudation rate which we observed for feces.
Conclusions.
Taken together, we showed that the three samples commonly used for detection of intestinal IgA, i.e., fecal samples, intestinal lavage fluid specimens, and filter wick-collected specimens, are equally reliable for wild-type mice with respect to artifactually caused plasma leakage. Only in situations in which extremely high serum IgA concentrations are to be expected, like in J-chain- or polymeric immunoglobulin receptor-knockout mice, the filter wick procedure may provide erroneously high readouts due to contaminating serum IgA transudate. In addition to these technical aspects, we showed that in mice ≥98% of intestinal IgA is of the secretory type, whereas the majority of intestinal IgG appears to be plasma transudate or bile derived.
Acknowledgments
This work was supported by DFG research grants FR 958/2-2 and FR 958/2-3 (to A.F.) and SCHM770/10-1 and SCHM770/10-2 (to M.A.S.).
REFERENCES
- 1.Delacroix, D. L., G. N. Malburny, and J.-P. Vaerman. 1985. Hepatobiliary transport of plasma IgA in the mouse: contribution to clearance of intravascular IgA. Eur. J. Immunol. 15:893-899. [DOI] [PubMed] [Google Scholar]
- 2.Externest, D., B. Meckelein, M. A. Schmidt, and A. Frey. 2000. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect. Immun. 68:3830-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey, A., B. Meckelein, D. Externest, and M. A. Schmidt. 2000. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 233:47-56. [DOI] [PubMed] [Google Scholar]
- 4.Frey, A., J. Di Canzio, and D. Zurakowski. 1998. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221:35-41. [DOI] [PubMed] [Google Scholar]
- 5.Guy, B. 2002. Evaluation of events occurring at mucosal surfaces: techniques used to collect and analyze mucosal secretions and cells. Clin. Diagn. Lab. Immunol. 9:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J.-P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 62:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrickson, B. A., L. Rindisbacher, B. Corthesy, D. Kendall, D. A. Waltz, M. R. Neutra, and J. G. Seidman. 1996. Lack of association of secretory component with IgA in J chain-deficient mice. J. Immunol. 157:750-754. [PubMed] [Google Scholar]
- 8.Johansen, F.-E., M. Pekna, I. N. Norderhaug, B. Haneberg, M. A. Hietala, P. Krajci, C. Betsholtz, and P. Brandtzaeg. 1999. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson, E.-L., C. Rask, M. Frederiksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonard, P. P., J. C. Rambaud, C. Dive, J.-P. Vaerman, A. Galian, and D. L. Delacroix. 1984. Secretion of immunoglobulins and plasma proteins from the jejunal mucosa. J. Clin. Investig. 74:525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lycke, N., L. Erlandsson, L. Ekman, K. Schön, and T. Leanderson. 1999. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J. Immunol. 163:913-919. [PubMed] [Google Scholar]
- 12.Phalipon, A., A. Cardona, J.-P. Kraehenbuhl, L. Edelman, P. J. Sansonetti, and B. Corthésy. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17:107-115. [DOI] [PubMed] [Google Scholar]
- 13.Stubbe, H., J. Berdoz, J.-P. Kraehenbuhl, and B. Corthésy. 2000. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164:1952-1960. [DOI] [PubMed] [Google Scholar]


