Abstract
Our objective was to describe the CD4-mediated human immunodeficiency virus (HIV)-specific cell-mediated immunity (CMI) and its virologic and immunologic correlates in children with chronic HIV infection on highly active antiretroviral therapy (HAART). Twelve HIV-infected children on stable antiretroviral therapy with a median level of CD4+ lymphocytes (CD4%) of 25.5% and a median viral load (VL) of 786 HIV RNA copies/ml were enrolled in this study. Nine of these children were also cytomegalovirus (CMV) seropositive. Blood mononuclear cells, stimulated with HIV and CMV antigens, were used to measure lymphocyte proliferation and to enumerate gamma interferon (IFN-γ)-producing CD4+ cells. HIV CMI and CMV CMI were detected in similar proportions of patients and correlated with each other, although the HIV responses were less robust. HIV lymphocyte proliferation significantly increased with lower HIV VL and showed a trend to increase with higher CD4% and longer time on HAART. The in vitro IFN-γ response to HIV or CMV was not affected by CD4%, VL, or HAART. Pediatric patients with established HIV infection on HAART frequently exhibit HIV CMI despite undetectable HIV replication. We concluded that the association between HIV CMI and CMV CMI indicates that the same factors govern responsiveness to either antigen.
Human immunodeficiency virus (HIV) preferentially destroys CD4+ T lymphocytes and interferes with the functioning of the immune system, weakening defenses against infectious agents. Highly active antiretroviral therapy (HAART) restores CD4+ cell numbers and greatly decreases the incidence of opportunistic infections, indicating that significant immune recovery occurs in treated patients (17, 23, 28). Furthermore, individuals who previously met AIDS diagnostic criteria recover cytomegalovirus (CMV)-, Mycobacterium avium-, Mycobacterium intracellulare-, and Candida-specific cell-mediated immunity (CMI) while on HAART (8, 15, 18).
AIDS-associated impairment of the immune system includes the inability of the host to mount a protective response against HIV (21, 27). Although HIV-specific CD8-mediated cytotoxicity as detected in HIV-infected patients plays a protective role against the progression of infection (6, 13, 25), CD4-mediated immunity has been difficult to identify. Long-term nonprogressors and patients treated soon after primary infection display HIV-specific CD4-dependent lymphocyte proliferation (1, 20), but most chronically infected adults, including HAART recipients, do not (24, 29).
The objective of this study was to compare the HIV- and CMV-specific CD4-mediated responses of HIV-infected children on HAART by using two recently developed and highly sensitive immunological assays, the enzyme-linked immunospot (ELISPOT) assay (32) and intracellular cytokine flow enumeration (ICCK) (34), coupled with an inactivated whole-HIV antigen preparation (3, 26).
(Part of this study was presented at the 9th Conference on Retroviruses and Opportunistic Infections, 2002.)
MATERIALS AND METHODS
Patients.
The study enrolled HIV-infected children on HAART but excluded patients with opportunistic infections or immunomodulator therapy. Blood samples were obtained from each patient on at least two occasions. The CD4+-T-lymphocyte level and HIV viral load (VL) were measured pre-HAART and at each study visit. This study was approved by the Colorado Multiple Institute Review Board. The patients enrolled in this study and their parents understood and agreed to participation.
Specimen processing.
Peripheral blood mononuclear cells (PBMC) from heparinized blood were isolated on Ficoll-Histopaque gradients (Sigma), washed, counted, and studied within 24 h of collection or cryopreserved. For cryopreservation, cells were resuspended at 107 cells/ml in cold fetal calf serum containing 10% dimethyl sulfoxide, gradually brought to a temperature of −70°C or lower over 24 h with a Mr. Frosty device (Curtis Matheson Scientific), and transferred to a liquid nitrogen tank. On the day of the assay, the cells were thawed quickly to 4°C, and then RPMI 1640 (Cellgro) containing 10% human AB serum (Nabi) was slowly added. The cells were washed and counted in 0.5% trypan blue. Viability was invariably ≥85%.
LPA.
The lymphocyte proliferation assay (LPA) was performed as previously described (36). PBMC were resuspended in RPMI 1640 (Gibco) with glutamine-containing 10% human AB serum (Nabi) and 1% antibiotics (Gibco) (stimulation medium). PBMC were added at 105 cells/well to triplicate wells containing CMV antigen and mock-infected control each at a 1:200 dilution or HIV-inactivated virion and control (obtained from Jeffrey D. Lifson) each at 3 μg/ml. After 6 days of incubation at 37°C in a CO2 incubator, the wells were pulsed with 50 μCi of [3H]thymidine (Amersham) per well for 6 h. The cells were harvested and counted in a Packard scintillation counter with Biosafe II scintillation liquid (Research Product International Corp.). The stimulation index was calculated by dividing the average counts per minute in the stimulated wells by the average counts per minute in the unstimulated wells. A positive LPA result was defined as a stimulation index of ≥3.
Flow cytometric determination of intracellular cytokine secretion.
The ICCK assay was adapted from that described by Waldrop et al. (34). Two million frozen PBMC were thawed and resuspended in stimulation medium and transferred to 4-ml polypropylene tubes (Falcon). A 3-μg/ml concentration of the murine monoclonal antibodies anti-CD49d and -CD28 (Becton Dickinson) was added to the cells, followed by the addition of specific antigens. The antigens and concentrations used were as follows: CMV and control antigens, 1:10 dilution; HIV and control antigens, 3 μg/ml; Staphylococcus aureus enterotoxin B (Sigma), 10 μg/ml. After 6 h of incubation at 37°C in a CO2 incubator, brefeldin A (Sigma) was added to a final concentration of 10 μg/ml. Cells were further incubated for 16 h at 37°C in a CO2 incubator and then fixed and stained for CD69, gamma interferon (IFN-γ), CD4, and isotype controls (36). At least 10,000 cells were analyzed in each assay by use of a Coulter XL flow cytometer. Assays were considered positive when the number of CD4+ CD69+ IFN-γ+ cells was ≥0.2% of the total number of CD4+ cells.
ELISPOT assay.
The ELISPOT assay was adapted from that described by Smith et al. (32). Frozen cells were thawed and resuspended at 107 cells/ml in ELISPOT assay medium (RPMI 1640, 10% human serum, 10 mM HEPES [Cellgro], 2 mM l-glutamine, 100 U of penicillin-streptomycin/ml). Final antigen dilutions and concentrations were as follows: for HIV and mock HIV control antigens (3, 26), 3 μg/ml; for CMV antigen and control, 1:10 dilution; for phytohemagglutinin (Sigma), 20 μg/ml. A 50-μl volume containing 5 × 105 cells was added to 50 μl of twice-concentrated antigen. This mixture was then added to 96 microtiter wells precoated with anti-human recombinant IFN-γ monoclonal antibody (Endogen). The plates were incubated at 37°C in a CO2 incubator for approximately 20 h. The wells were washed six times with wash buffer (phosphate-buffered saline, 0.005% Tween 20), and 50 μl of biotin-labeled monoclonal anti-human recombinant IFN-γ antibody (Endogen) diluted to 1:1,000 in dilution buffer (phosphate-buffered saline, 5% fetal bovine serum, 0.005% Tween 20) was added to each well. After an overnight incubation at 4°C, the wells were washed six times in wash buffer. Streptavidin-alkaline phosphatase (Pierce) was diluted to 1:1,000 in dilution buffer, and 100 μl was added to each well. After a 2-h incubation at room temperature, 100 μl of chromogen substrate (1-Step NBT/BCIP [nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate]; Pierce) was added to each well. The plates were incubated at room temperature for 5 min, and the reaction was terminated by thoroughly washing the plates with running tap water. Numbers of spot-forming cells (SFC) were calculated by using a ×10 magnification for visual counts. Positive results were defined as ≥20 SFC/106 PBMC, which corresponds to the mean number of SFC measured in PBMC from seronegative individuals + 2 standard deviations. CD8 depletion did not decrease the number of SFC, indicating that the bulk of the response was mediated by CD4 cells.
Statistical analysis.
The data were analyzed using Statview version 5.0.1 software (SAS). Tests were chosen based on the distribution of values and numbers of observations. A P value of ≤0.05 was considered significant.
RESULTS
Patient characteristics.
This study enrolled 12 HIV-infected children and adolescents between 4 and 24 years of age, 9 of whom were CMV seropositive (Table 1). Nine patients had been perinatally infected, two had been infected by blood transfusions, and one had been infected by a sexual route. The subjects had received HAART for an average of 3.6 years. The median CD4+ lymphocyte level (CD4%) and HIV VL prior to HAART were 18% ± 11% and 4.3 ± 0.8 log10 RNA copies/ml, respectively. During the study, patients were seen on two to six occasions, separated by 1 month to 4 years. The CD4% and HIV VL of all visits were 26% ± 10% and 2.7 ± 1.4 log10 RNA copies/ml, respectively (mean ± standard deviation). Throughout the study, the HIV VL values were consistently ≤2.7 log10 RNA copies/ml in five patients, always >2.7 log10 RNA copies/ml in five patients, and alternated between visits in the remaining two patients. CD4% values were consistently ≥20% in eight patients, always <20% in two patients, and alternated between visits in the remaining two patients.
TABLE 1.
Clinical and laboratory characteristics of HIV-infected patients enrolled in the study of HIV-specific immunity
| Patient characteristica | Value |
|---|---|
| No. CMV seropositive/no. HIV seropositive | 9/12 |
| Age (yr) (mean ± SD) | 15 ± 6 |
| Sex (M:F) | 6:6 |
| Ethnicity (AA:C:H) | 1:7:4 |
| HIV acquisition (perinatal:sexual:transfusion) | 9:1:2 |
| Last pre-HAART data | |
| CD4% (mean ± SD) | 18 ± 11 |
| Log10 HIV RNA copies/ml (mean ± SD) | 4.3 ± 0.8 |
| Study visit data | |
| Median visits/patient (range) | 4 (2-6) |
| CD4% (mean ± SD)b | 26 ± 10 |
| Log10 HIV RNA copies/ml (mean ± SD)b | 2.7 ± 1.4 |
| Yr on HAART (mean ± SD)b | 3.6 ± 1.6 |
Abbreviations: AA, African-American; C, Caucasian; H, Hispanic; F, female; M, male.
Data represent mean results for all study visits for each patient.
HIV- and CMV-specific immunity in pediatric HAART recipients.
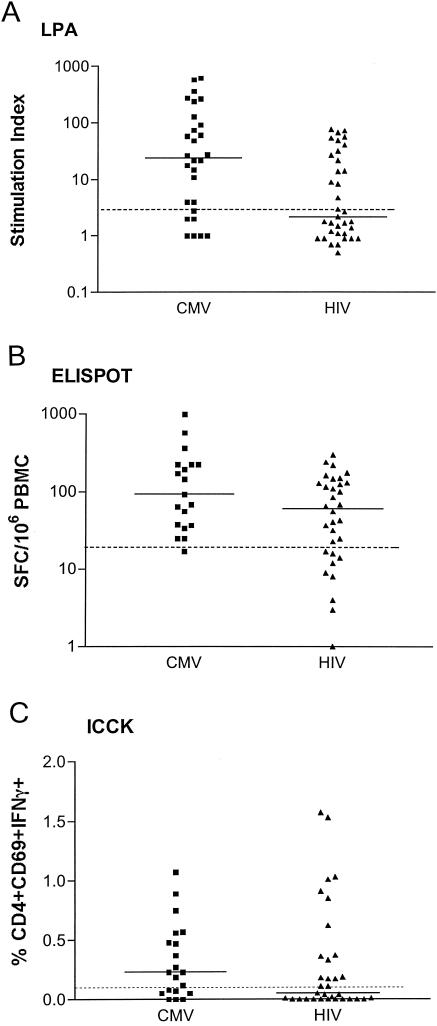
To determine if HIV immunity was differentially depressed by HIV infection and HAART, we compared HIV-specific cell-mediated immunity (CMI) to CMV-specific CMI by three different assays: LPA, ELISPOT assay, and ICCK (Fig. 1). Similar proportions of patients demonstrated HIV- and CMV-specific LPA responses at least once (66% versus 89%, P = 0.24). Seven of 12 patients had consistent HIV LPA responses throughout the study, whereas 5 demonstrated alternate positive and negative HIV LPA responses. In comparison, six of nine CMV-seropositive patients had consistent CMV LPA responses, indicating that intrapatient LPA variabilities were similar for HIV and CMV (P = 0.9, Fisher's exact test). Qualitative analysis of all LPA responses showed a significantly lower proportion of responses to HIV than to CMV (46% versus 75%, P = 0.02).
FIG. 1.
HIV- and CMV-specific CMI in HIV- infected children on HAART. Data represent results of assays performed with PBMC from 12 HIV-seropositive pediatric patients, 9 of whom were also CMV seropositive. Results for each patient contributed 2 to 6 data points. Dotted lines indicate thresholds for positive results. Solid lines indicate medians. (A) HIV LPA responses were significantly lower than those for CMV with respect to proportion of positive results (P = 0.02) and amplitude of response (P = 0.006). (B) The difference in the proportions of positive ELISPOT assay results for HIV and CMV did not reach statistical significance (P = 0.06), but the magnitude of the response was significantly lower for HIV than for CMV (P = 0.007).(C) ICCK responses were similar for HIV- and CMV-seropositive patients with respect to proportion of positive results (P = 0.16) and magnitude of the response (P = 0.3).
ELISPOT analysis showed that all patients responded to HIV and CMV on at least one occasion. Six of 11 patients who were tested by the HIV ELISPOT assay on at least two occasions had consistent positive results, but the results for 5 patients alternated between visits. By comparison, eight of nine CMV-seropositive patients had positive ELISPOT assay results at all visits, and one did not (P = 0.18 for HIV versus CMV intrapatient variability). Responses to HIV stimulation tended to be less frequent (74% versus 95%, P = 0.06) and significantly less robust (median SFC of 60 versus 90, P = 0.007) than the responses to CMV stimulation.
ICCK responses after HIV and CMV stimulation were similar with respect to the proportion of patients who responded on at least one occasion (66% versus 78%, P = 0.24), intrapatient variability (45% versus 29%, P = 0.7), proportion of responses across all study visits (46% versus 65%, P = 0.16), and number of IFN-γ-producing activated CD4% (median of 0.1% versus 0.2%, P = 0.3).
Two or more assays of HIV-stimulated responses were concordant on 44 to 63% of visits, which was similar to the concordance of assays of CMV-stimulated responses, which varied between 44 and 73%.
Immunologic and virologic correlates of HIV immunity in HIV-infected children on HAART.
To identify factors that affect the HIV- or CMV-specific responses, associations between HIV VL, CD4%, and length of time on HAART with LPA, ELISPOT, and ICCK results were investigated by Spearman rank correlation tests. HIV LPA results significantly increased with lower HIV VL (P = 0.02, ρ = −0.379). There were trends towards increases with higher CD4% and longer time on HAART, but they did not reach statistical significance (P of 0.06 and 0.09, respectively). CMV LPA showed a trend towards increase with longer time on HAART (P = 0.09). None of the other measurements of HIV or CMV CMI correlated with HIV VL, CD4%, or duration of HAART (P of 0.3 to 0.9). The intrapatient variability of LPA, ELISPOT, or ICCK results for CMV or HIV did not correlate with intervisit changes in CD4% or HIV VL above or below 20% and 2.7 log10 copies/ml, respectively. The likelihood of detecting an HIV CMI response was higher in patients with CMV CMI responses (P = 0.05), suggesting that a common set of factors affects the presence of HIV- and CMV-specific immunities.
There were no apparent differences in results with respect to age or mode of HIV acquisition. However, the number of patients was too small to draw significant conclusions.
DISCUSSION
These children with chronic HIV infection on HAART displayed HIV-specific CD4-mediated CMI at one or more study visits. This finding differs from those of previous reports, which showed much less frequent HIV-specific CD4-mediated immunity in adults with established HIV infection (2, 5) or in infants who started therapy in the first 3 months of life (19). The most likely explanation is that unique characteristics of the immune system of children, which are lacking in adults or young infants, facilitate the reconstitution of HIV CMI in children. There is evidence that the thymic function of HIV-infected children improves on HAART (16, 12, 10). The thymus may provide new T cells, which, if they are of the right specificity, may join the HIV memory CD4+ pool after antigenic stimulation. HAART, by inhibiting viral replication, would allow these cells to survive and compensate for HIV-specific CD4+ cells that are lost during the phase of unchecked viral replication. In adults, the thymus has a reduced potential of generating new T cells and may limit reconstitution of HIV immunity. Other factors related to antigen presentation and memory-T-cell generation might be limiting the HIV-specific immunologic memory in infants (19). Our observation that HIV LPA results increase with lower HIV VL, higher CD4%, and longer duration of HAART further supports the notion that CD4-specific HIV immunity in chronically infected children is a component of immune reconstitution.
Previous studies showed a direct relationship between HIV CMI and HIV VL (4, 14) that could not be verified in this study. In other reports, the relationship between the magnitude of HIV replication and intensity of the anti-HIV immune response tended to be more pronounced for cytotoxic T lymphocytes, which sometimes completely disappeared from the blood of patients with good control of viral replication (33). This finding has been interpreted as an indication that continuous antigenic stimulation is necessary for maintenance of HIV CD8+ and possibly CD4+ immunologic memory. We did not evaluate HIV CD8+ responses in this study, but our results indicate that HIV-specific CD4+ memory may not require continuous antigenic stimulation. In this study, five children with undetectable HIV VL during many years of HAART had in vitro HIV CMI responses. The use of very sensitive immunologic assays in conjunction with a whole-virus antigen might explain our increased ability to detect HIV CMI. Previous studies have mostly used recombinant HIV p24 as an in vitro stimulant of CD4+ responses. HIV p24 contains only a limited number of T-cell epitopes compared with that of the whole virus. In fact, in our laboratory, in vitro responses to p24 were less frequent and less robust than the responses to whole virus (data not shown).
Among the different measures of HIV and CMV CMI, the ELISPOT was the most sensitive assay. The ELISPOT and ICCK assays were performed with frozen PBMC, which might have decreased the magnitude of the measured responses, whereas the LPA was performed with fresh cells. Nevertheless, the ELISPOT assay had the highest proportion of positive results and there were no instances in this study of a negative ELISPOT result accompanied by a positive LPA or ICCK result. The ELISPOT assay measures IFN-γ secretion, an early event after the T-cell cognate encounter with antigen. In contrast, LPA involves several amplification steps subsequent to antigen-stimulated cytokine secretion, ultimately leading to proliferation. Impairment of the amplification steps would account for decreased sensitivity of the LPA in HIV-infected patients (7, 11, 22, 30, 31, 35). ICCK is limited by the number of events analyzed in an assay, which rarely surpasses the 10,000 figure, and by the low specificity of the fluorescent signal at frequencies of <0.1%. Therefore, while the HIV ELISPOT assay cannot detect <20 HIV-specific CD4+ cells/106 PMBC, HIV ICCK cannot detect <500 HIV-specific CD4+ cells/106 PBMC. The increased sensitivity of the ELISPOT assay was associated with a lower intervisit variability than those of the LPA and ICCK. Overall, there was a 44 to 63% concordance among the HIV assays used in this study, reflecting the different characteristics.
CMV CMI shared the assay characteristics described above for HIV CMI. Other observations common to HIV CMI and CMV CMI included similar proportions of patients and visits in which CMI was detected and a significant association between HIV CMI and CMV CMI results. The multiple similarities between CMV CMI and HIV CMI in children on HAART suggest a common mechanism controlling CMI against both pathogens. However, the magnitude of CMV CMI responses was higher than that of HIV CMI responses. This could be related solely to differences in the antigen preparations. Alternatively, it might indicate a lower frequency of HIV-specific responders than of CMV-specific responders, which would be in accordance with recent studies suggesting that HIV immunopathogenesis includes preferential destruction of HIV-specific CD4+ cells (9).
Our observation that continuous antigenic stimulation may not be necessary for maintenance of HIV CMI in children on HAART has important clinical ramifications and needs to be confirmed. The role of therapeutic vaccination and of structured treatment interruptions as an immune boost in the management of pediatric HIV infection would have to be evaluated in the context of an already existent HIV-specific immune response. The relatively weak HIV CMI (as compared with CMV CMI) indicates that there is room for improving HIV immunity. However, since a relationship between the magnitude of the immune response and protection against disease has not been established, it remains to be determined whether children who reconstitute HIV immunity on HAART will benefit from additional boosts.
Acknowledgments
We thank Julie Patterson for technical support, Sonya Swiney for help in the preparation of the manuscript, and Myron J. Levin for critical review of the manuscript.
Support of this study came from NICHD contract NO1-HD-3-3162.
REFERENCES
- 1.Al-Harthi, L., J. Siegel, J. Spritzler, J. Pottage, M. Agnoli, and A. Landay. 2000. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS 14:761-770. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J. B., K. G. Parato, A. Kumar, S. Kravcik, A. D. Badley, C. Fex, D. Ashby, E. Sun, and D. W. Cameron. 2001. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J. Infect. Dis. 183:546-554. [DOI] [PubMed] [Google Scholar]
- 3.Bess, J. W., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., D. S. Schiller, G. M. Ortiz, A. Hurley, D. F. Nixon, M. M. Markowitz, and J. P. Moore. 2000. The relationship between T cell proliferative responses and plasma viremia during treatment of human immunodeficiency virus type 1 infection with combination antiretroviral therapy. J. Infect. Dis. 181:1249-1263. [DOI] [PubMed] [Google Scholar]
- 5.Blankson, J. N., J. E. Gallant, and R. F. Siliciano. 2001. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657-661. [DOI] [PubMed] [Google Scholar]
- 6.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 7.Chougnet, C., S. Jankelevich, K. Fowke, D. Liewehr, S. M. Steinberg, B. U. Mueller, P. A. Pizzo, R. Yarchoan, and G. M. Shearer. 2001. Long-term protease inhibitor-containing therapy results in limited improvement in T cell function but not restoration of interleukin-12 production in pediatric patients with AIDS. J. Infect. Dis. 184:201-205. [DOI] [PubMed] [Google Scholar]
- 8.Connick, E., M. M. Lederman, B. L. Kotzin, J. Spritzler, D. R. Kuritzkes, M. St Clair, A. D. Sevin, L. Fox, M. H. Chiozzi, J. M. Leonard, F. Rousseau, J. D'Arc Roe, A. Martinez, H. Kessler, and A. Landay. 2000. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J. Infect. Dis. 181:358-363. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 10.Douek, D. C., R. A. Koup, R. D. McFarland, J. L. Sullivan, and K. Luzuriaga. 2000. Effect of HIV on thymic function before and after antiretroviral therapy in children. J. Infect. Dis. 181:1479-1482. [DOI] [PubMed] [Google Scholar]
- 11.Dybul, M., G. Mercier, M. Belson, C. W. Hallahan, S. Liu, C. Perry, B. Herpin, L. Ehler, R. T. Davey, J. A. Metcalf, J. M. Mican, R. A. Seder, and A. S. Fauci. 2000. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-γ in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J. Immunol. 165:1685-1691. [DOI] [PubMed] [Google Scholar]
- 12.Gibb, D. M., A. Newberry, N. Klein, A. de Rossi, I. Grosch-Woerner, A. Babiker, et al. 2000. Immune repopulation after HAART in previously untreated HIV-1-infected children. Lancet 255:1331-1332. [DOI] [PubMed] [Google Scholar]
- 13.Greenough, T. C., D. B. Brettler, F. Kirchhoff, L. Alexander, R. C. Desrosiers, S. J. O'Brien, M. Somasundaran, K. Luzuriaga, and J. L. Sullivan. 1999. Long-term nonprogressive infection with human immunodeficiency virus type 1 in a hemophilia cohort. J. Infect. Dis. 180:1790-1802. [DOI] [PubMed] [Google Scholar]
- 14.Haslett, P. A., D. F. Nixon, Z. Shen, M. Larsson, W. I. Cox, R. Manandhar, S. M. Donahoe, and G. Kaplan. 2000. Strong human immunodeficiency virus (HIV)-specific CD4+ T cell responses in a cohort of chronically infected patients are associated with interruptions in anti-HIV chemotherapy. J. Infect. Dis. 181:1264-1272. [DOI] [PubMed] [Google Scholar]
- 15.Havlir, D. V., R. D. Schrier, F. J. Torriani, K. Chervenak, J. Y. Hwang, and W. H. Boom. 2000. Effect of potent antiretroviral therapy on immune responses to Mycobacterium avium in human immunodeficiency virus-infected subjects. J. Infect. Dis. 182:1658-1663. [DOI] [PubMed] [Google Scholar]
- 16.Johnston, A. M., M. E. Valentine, J. Ottinger, R. Baydo, V. Gryszowka, C. Vavro, K. Weinhold, M. St Clair, and R. E. McKinney. 2001. Immune reconstitution in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy: a cohort study. Pediatr. Infect. Dis. J. 20:941-946. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher, A. D., A. Carr, J. Zaunders, and D. A. Cooper. 1996. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J. Infect. Dis. 173:321-329. [DOI] [PubMed] [Google Scholar]
- 18.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 19.Luzuriaga, K., M. McManus, M. Catalina, S. Mayack, M. Sharkey, M. Stevenson, and J. L. Sullivan. 2000. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J. Virol. 74:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra, U., M. M. Berrey, Y. Huang, J. Markee, D. J. Brown, S. Ap, L. Musey, T. Schacker, L. Corey, and M. J. McElrath. 2000. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:121-131. [DOI] [PubMed] [Google Scholar]
- 21.McMichael, A. J., and S. L. Rowland-Jones. 2000. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski, M. A., J. X. Gu, C. Kovacs, J. Freedman, M. A. Luscher, and K. S. MacDonald. 2001. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal interferon-γ and interleukin-10 responses. J. Infect. Dis. 184:1268-1278. [DOI] [PubMed] [Google Scholar]
- 23.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 24.Pontesilli, O., S. Kerkhof-Garde, D. W. Notermans, N. A. Foudraine, M. T. Roos, M. R. Klein, S. A. Danner, J. M. Lange, and F. Miedema. 1999. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J. Infect. Dis. 180:76-86. [DOI] [PubMed] [Google Scholar]
- 25.Pontesilli, O., M. R. Klein, S. R. Kerkhof-Garde, N. G. Pakker, F. de Wolf, H. Schuitemaker, and F. Miedema. 1998. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 178:1008-1018. [DOI] [PubMed] [Google Scholar]
- 26.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnittman, S. M., H. C. Lane, J. Greenhouse, J. S. Justement, M. Baseler, and A. S. Fauci. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. USA 87:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schooley, R. T. 1999. Longer-term immunologic effects and side effects of successful antiretroviral therapy. Clin. Infect. Dis. 29:12-28. [DOI] [PubMed] [Google Scholar]
- 29.Sester, M., U. Sester, H. Kohler, T. Schneider, L. Deml, R. Wagner, N. Mueller-Lantzsch, H. W. Pees, and A. Meyerhans. 2000. Rapid whole blood analysis of virus-specific CD4 and CD8 T cell responses in persistent HIV infection. AIDS 14:2653-2660. [DOI] [PubMed] [Google Scholar]
- 30.Sieg, S. F., D. A. Bazdar, C. V. Harding, and M. M. Lederman. 2001. Differential expression of interleukin-2 and gamma interferon in human immunodeficiency virus disease. J. Virol. 75:9983-9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieg, S. F., C. V. Harding, and M. M. Lederman. 2001. HIV-1 infection impairs cell cycle progression of CD4(+) T cells without affecting early activation responses. J. Clin. Investig. 108:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, J. G., X. Liu, R. M. Kaufhold, J. Clair, and M. J. Caulfield. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin. Diag. Lab. Immunol. 8:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel, H. M., E. DeFalcon, G. S. Ogg, M. Larsson, T. J. Beadle, P. Tao, A. J. McMichael, N. Bhardwaj, C. O'Callaghan, W. I. Cox, K. Krasinski, H. Pollack, W. Borkowsky, and D. F. Nixon. 1999. Changes in frequency of HIV -1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J. Infect. Dis. 180:359-368. [DOI] [PubMed] [Google Scholar]
- 34.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg, A., D. A. Wohl, R. J. Barrett, and C. van der Hoost. 2001. Inconsistent reconstitution of cytomegalovirus-specific cell-mediated immunity in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J. Infect. Dis. 184:707-712. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg, A., D. A. Wohl, D. G. Brown, G. B. Pott, L. Zhang, M. G. Ray, and C. van der Hoost. 2002. Effect of cryopreservation on measurement of cytomegalovirus-specific cellular immune response in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 25:109-114. [DOI] [PubMed] [Google Scholar]