Abstract
Cryptococcus neoformans is surrounded by an antiphagocytic capsule whose primary constituent is glucuronoxylomannan (GXM). An epitope shared by GXM serotypes A, B, C, and D is immunodominant when mice are immunized with serotype A GXM. In contrast, an epitope shared only by serotypes A and D is immunodominant when mice are immunized with serotype D. Hybridomas secreting antibodies reactive with subdominant epitopes were identified through a positive-negative screening procedure in which antibody-secreting colonies were characterized by reactivity with both the immunizing polysaccharide and GXMs from each of the four major serotypes. In this manner, a monoclonal antibody (MAb) that was reactive with an epitope shared only by serotypes A and B was identified and designated F10F5. Such an epitope has not been described previously. Immunization of mice with de-O-acetylated serotype A GXM generated a hybridoma that secreted an antibody, designated F12D2, that was reactive with all four serotypes. Unlike previously described monoclonal and polyclonal panspecific antibodies, the reactivity of MAb F12D2 was not altered by de-O-acetylation of GXM. These results indicate that there are at least two panspecific GXM epitopes; one epitope is dependent on O acetylation for antibody reactivity, and the other is independent of O acetylation. This study identifies strategies for production of MAbs that are reactive with subdominant or cryptic GXM epitopes and provides new information regarding the antigenic makeup and the humoral immune response to GXM, an essential virulence factor that is a target for active and passive immunization.
Cryptococcus neoformans is a pathogenic yeast that is surrounded by an antiphagocytic polysaccharide capsule. The primary constituent of the capsule is glucuronoxylomannan (GXM), a polysaccharide that is a linear (1→3)-α-d-mannopyranan with single β-d-xylopyranosyl and β-d-glucopyranosyl-uronic acid substituents (4, 10). The mannose backbone is also variably O acetylated at C-6 (44). The O-acetyl substituent is a major epitope in the recognition of GXM by polyclonal antibodies (8, 24) and monoclonal antibodies (MAbs) (1, 13). GXM occurs in five major serotypes, A, B, C, D, and A/D (20, 46). The degrees of xylose substitution and O acetylation are the major determinants of structure for the GXM of each serotype.
GXM antibodies have numerous biological activities. GXM antibodies (i) are opsonic for phagocytosis by macrophages (23, 38), (ii) activate the classical complement pathway leading to early deposition of C3 fragments on the yeast (22), (iii) suppress overall accumulation of C3 via the alternative pathway, (iv) facilitate clearance of GXM from serum in vivo, leading to increased accumulation of GXM in tissues that are rich in cells of the mononuclear phagocyte system (16, 18, 28), (v) are protective in murine models of cryptococcosis (11, 33), and (vi) facilitate various aspects of cellular immunity to C. neoformans (see reference 45 for a review of the humoral immunity-cellular immunity axis in cryptococcosis). Comparative studies using MAbs with different epitope specificities found that the ability of GXM MAbs to mediate many of the above biological activities is critically dependent on the epitope specificity of the antibody. For example, MAbs that are reactive with an epitope that is shared by serotypes A, B, C, and D activate the classical pathway (22), suppress overall C3 accumulation via the alternative pathway (22), exhibit a high level of Fc-dependent opsonization (34), and mediate opsonization in an Fc-independent manner (34). In contrast, MAbs that are reactive with an epitope found only on serotypes A and D fail to activate the classical pathway, have no effect on C3 accumulation via the alternative pathway, exhibit a low level of Fc-dependent opsonization, and fail to mediate Fc-independent opsonization.
Studies of the role of GXM antibody epitope specificity in host resistance to cryptococcosis are hampered by the limited spectrum of GXM MAbs that recognize different GXM epitopes. For example, the overwhelming number of polyclonal antibodies that are produced by immunization with serotype A GXM (31) and the MAbs derived from mice immunized with serotype A GXM are reactive with an apparent immunodominant epitope that is shared by serotypes A, B, C, and D (5). For the purposes of this study, immunodominance is taken to mean an epitope that generates the strongest antibody response in the case of polyclonal antibodies or the highest frequencies of antibody-secreting hybridomas in the case of MAbs. The concept of immunodominant and immunorecessive epitopes on protein antigens has received considerable attention in the literature, but few if any studies have addressed the question of immunorecessive epitopes on polysaccharide antigens. The present study (i) reports the use of complementary immunization and screening strategies that allow for production of MAbs that are reactive with epitopes that induce antibody-secreting cells in lesser numbers (immunorecessive epitopes) than are induced by more-immunodominant epitopes and (ii) describes the properties of two antibodies that are reactive with immunorecessive epitopes on serotype A GXM. The results reveal further information regarding the immune response to GXM and MAb-GXM interactions that will aid studies aimed at active or passive immunization as a means to prevent or treat cryptococcosis.
MATERIALS AND METHODS
C. neoformans and GXM.
C. neoformans strains were provided by R. Cherniak (Georgia State University, Atlanta). The chemotypes and structural components of polysaccharides produced by these strains, as defined by Cherniak et al. (10), are summarized in Table 1. Since many strains of C. neoformans produce GXMs having a mixture of structure reporter groups (10), representative strains of each serotype were selected for the present study largely on the basis of having 100% of the structure reporter group that is characteristic of each serotype. GXM was isolated from supernatant fluids of each strain. Yeast cells were grown for 4 days at 30°C on synthetic medium (9) and killed by overnight treatment with formaldehyde, and the GXM was isolated and purified by differential precipitation with ethanol and hexadecyltrimethylammonium bromide as described previously (7, 14). Studies that examined the capsular “quellung” reaction produced by binding of MAbs to whole cells used yeast cells that were grown on synthetic medium supplemented with 24 mM sodium bicarbonate and 25 mM HEPES and were incubated at 37°C with 5% CO2. Growth in the presence of bicarbonate and CO2 induces the production of large capsules (17, 30). For some experiments, yeast cells or GXMs were chemically de-O-acetylated by alkaline hydrolysis. The pH of a suspension of cells or a solution of GXM was adjusted to 11.2 with ammonium hydroxide and incubated overnight at room temperature. De-O-acetylated whole cells were washed three times with phosphate-buffered saline (PBS) before use. De-O-acetylated GXM was recovered by precipitation with ethanol (24). Efficacy of the de-O-acetylation procedure was confirmed by use of the Hestrin assay.
TABLE 1.
Serotype, chemotype, and GXM structure of C. neoformans strainsa
| Strain | Serotype | Chemotype | % of repeating units with structure reporter group:
|
|||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | |||
| MU-1 | A | 4 | 100 | |||||
| CN6 | A | 5 | 67 | 17 | 15 | |||
| 409 | B | 7 | 100 | |||||
| 24066 | C | 8 | 100 | |||||
| KT24066 | C | 8 | 100 | |||||
| 9375B | D | 1 | 100 | |||||
| 34875 | D | 1 | 27 | 25 | 48 | |||
Serotype, chemotype, and structure reporter group data are from reference 10 and R. Cherniak (personal communication).
Production of MAbs.
Mice were immunized and hybridomas were prepared largely as described previously (13). Briefly, BALB/c mice (Animal Production Program, Frederick Cancer Research and Development Center, Frederick, Md.) were immunized by intravenous injection of 0.2 ml of a 0.1% suspension of GXM-coated sheep erythrocytes (SRBC) (21). The use of laboratory animals for this purpose was approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee and was compliant with relevant federal guidelines. The immunization was repeated from three to five times at intervals of 1 to 3 weeks or more until levels of anti-GXM immunoglobulin G (IgG) antibodies reached a titer of approximately 1/5,000.
Spleen cells were obtained from immune mice 3 days after the last immunization and were fused with cells of the X63-Ag8.653 cell line by use of polyethylene glycol (26). Peritoneal cells were collected from Swiss Webster mice for use as feeder cells and were used at 4 × 104 cells per well of 48-well cell culture plates (product no. 3548; Costar, Corning, N.Y.). Cells from the polyethylene glycol fusion were distributed among four 48-well plates. All other aspects of the hybridoma production were as described previously (13).
Hybridoma cultures were screened for GXM antibody production by enzyme-linked immunosorbent assays (ELISAs) using the same GXM as was used for immunization as well as GXMs from all four serotypes. The ELISA was performed as described previously (13), with the exception that 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, Md.) was the substrate for the horseradish peroxidase (HRPO)-coupled secondary antibody. Culture supernatant fluids were screened for antibodies of the IgG class only. A signal that was approximately two times the background level was considered positive for antibody production. Colonies that produced GXM antibodies of interest were cloned three times by limiting dilution.
Large-scale production of MAbs was done by in vitro culture in a Tecnomouse system (Integra Biosciences, Ijamsville, Md.). MAbs were isolated by protein A affinity chromatography. Concentrations of MAbs were determined by UV spectroscopy, using an optical density of 1.43 at 280 nm for 1 mg of IgG/ml (39). The IgG subclass of the MAbs was determined by an antigen capture ELISA in which antibodies specific for murine IgG subclasses (Southern Biotechnology, Inc., Birmingham, Ala.) were used in the solid phase and HRPO-labeled antibodies specific for murine IgG (Southern Biotechnology) were used as indicators.
MAbs 3C2 and 471 are antibodies that were generated in previous studies (2, 41). The properties of these antibodies are summarized in Table 2.
TABLE 2.
Characteristics of GXM MAbs
| MAb | GXM used for immunization | Isotype | Serotype reactivitya | Source or reference(s) |
|---|---|---|---|---|
| 3C2 | Serotype C | IgG1 | A, B, C, and D | 2, 41 |
| 471 | Serotype A | IgG1 | A, B, C, and D | 2, 41 |
| F10F5 | Serotype B | IgG1 | A and B | This study |
| F12D2 | De-O-Ab | IgG3 | A, B, C, and D | This study |
Determined by capsular reaction with whole cells and ELISA using GXM of known serotypes.
De-O-A, de-O acetylated GXM of serotype A.
Assays for antibody activity.
The ELISA used to quantitatively compare the reactivities of MAbs with GXMs of different serotypes was a variation of an assay described by Leinonen and Frasch for detection of antibody to meningococcal polysaccharide (27). Briefly, microtiter plates were coated for 5 h with poly-l-lysine at a concentration of 5 μg/ml of PBS, washed with PBS, and incubated overnight with GXM (4 μg/ml of PBS). The plates were washed with PBS-Tween (PBS [pH 7.4] containing 0.05% Tween 20) and incubated for 90 min at 37°C with a blocking solution (PBS-0.5% Tween containing 5% skim milk). After the blocking step, the plates were washed with blocking solution, 100 μl of serial twofold dilutions of GXM MAbs in blocking solution was added, and the plates were incubated for 90 min at room temperature. After incubation with GXM MAbs, the plates were washed with blocking solution and incubated for 90 min at room temperature with 100 μl of HRPO-labeled secondary antibody specific for mouse IgG heavy chains (Southern Biotechnology) at a 1:5,000 dilution in blocking solution. After incubation with secondary antibody, the plates were washed with PBS-Tween and incubated for 30 min at room temperature with 100 μl of TMB substrate (KPL, Gaithersburg, Md.). Stop solution (1 M H3PO4) was added to each well, and the absorbance was read at 450 nm. Results are reported as the optical density at 450 nm versus the antibody dilution.
Double immunodiffusion in agar was done as described previously (13). Capsular quellung reactions were done and assessed by differential interference contrast (DIC) microscopy as described earlier (30). Previous studies of capsular quellung-type reactions produced by incubation of encapsulated cryptococci with GXM MAbs showed that MAbs can (i) fail to react, in which case the capsule cannot be visualized by DIC microscopy; (ii) produce an annular or “rim” pattern that is characterized by a sharp increase in the optical gradient at the capsular edge followed directly by a decrease in the optical gradient; or (iii) produce a pattern termed “puffy” in which there is an increase in the optical gradient at the capsular surface and the absence of the immediate decrease that is characteristic of the rim pattern (30).
RESULTS
Mice were immunized with serotype A, B, C, or D GXM that had been coupled to SRBC. The density of cells used for initial plating of hybrid cells was adjusted so that <50% of the wells typically contained antibody-producing cultures. As a consequence, most culture wells likely contained the progeny of a single hybridoma. The results from nine experiments are summarized in Table 3. Analysis of the serotype specificity of antibody-secreting hybridomas showed that the apparent immunodominant epitope depended in a large part on the serotype of the immunizing GXM. For example, 88% of positive cultures from mice immunized with serotype A GXM produced antibodies that were reactive with an epitope shared by serotypes A, B, C, and D. In contrast, 67% of antibody-secreting cultures prepared from mice immunized with serotype D GXM were reactive with an epitope shared only by serotypes A and D.
TABLE 3.
Frequency of colonies producing antibodies reactive with GXM of serotypes A, B, C, or D
| Fusion no.a | GXM used for immunization
|
Positive wells (%)b | No. of wells showing reactivity with GXM of serotypes:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Serotype | A, B, C, D | A, B, D | A, B, C | A, B | A, D | A | ||
| 6 | 9375B | D | 2 | 2 | 0 | 0 | 0 | 1 | 0 |
| 7a | 9375B | D | 15 | 9 | 0 | 0 | 0 | 19 | 0 |
| 7b | 9375B | D | 46 | 7 | 21 | 0 | 0 | 60 | 0 |
| 9 | MU-1 | A | 21 | 31 | 4 | 0 | 2 | 4 | 0 |
| 10 | 409 | B | 4 | 4 | 0 | 0 | 4 | 0 | 0 |
| 11 | MU-1 | A | 97 | 181 | 0 | 3 | 2 | 0 | 0 |
| 12 | De-O-MU-1c | A | 6 | 8 | 0 | 3 | 0 | 0 | 0 |
| 16 | CN6 | A | 43 | 61 | 5 | 0 | 12 | 3 | 1 |
| 17 | KT24066 | C | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Fusions were done on different days over the course of 2 years. Fusions 7a and 7b were done on the same day with spleens from two mice immunized in an identical manner.
Percentage of culture wells producing GXM antibodies as determined by ELISA screening of supernatant fluids using the immunizing GXM in the solid phase.
De-O-MU-1, de-O-acetylated GXM from strain MU-1.
Characterization of a MAb that is reactive with GXMs of serotypes A and B.
Ikeda et al. used polyclonal antibodies raised in rabbits against heat-killed cryptococci and reciprocal cross-absorption procedures to characterize the distribution of putative epitopes among and between serotypes A, B, C, and D (20). An epitope that is shared only by serotypes A and B was not found in this earlier study; consequently, hybridomas secreting antibodies that were reactive only with serotypes A and B (fusion numbers 9, 10, 11, and 16) were of interest because they recognized a heretofore undescribed epitope. One of the colonies from fusion 10 that produced antibodies reactive with serotypes A and B was cloned by limiting dilution, and a MAb, designated MAb F10F5, was characterized in detail.
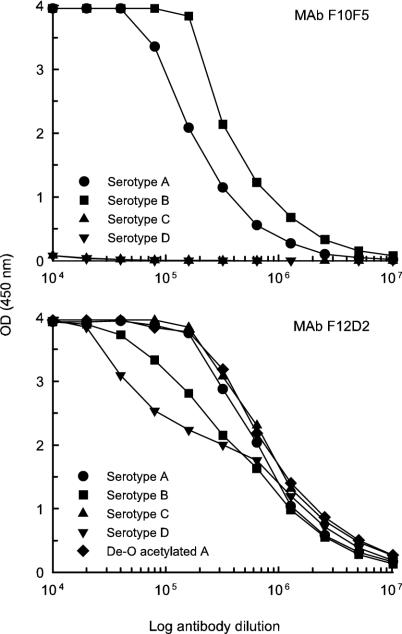
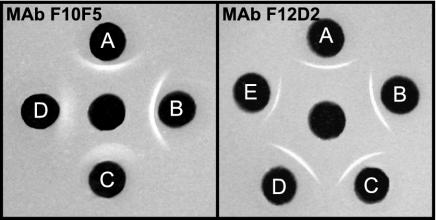
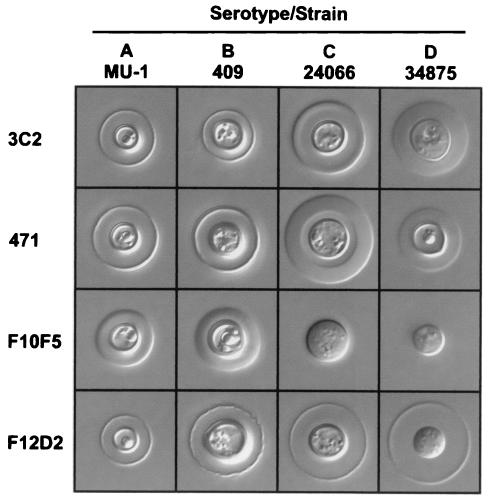
Analysis of the reactivity of MAb F10F5 by ELISA using GXMs of serotype A, B, C, or D in the solid phase showed specificity only for serotypes A and B (Fig. 1). The assay of serotype specificity by double immunodiffusion showed crisp and distinct precipitin bands with GXMs of serotypes A and B (Fig. 2). Precipitin bands were also noted with GXMs of serotypes C and D; however, the bands were diffuse, weak in intensity, and located near the wells containing antigen. Examination of the capsular reaction produced by MAb F10F5 and cells of serotype A, B, C, or D showed prominent puffy patterns with cells of serotypes A and B and no apparent capsule reaction with cells of serotypes C and D (Fig. 3).
FIG. 1.
Reactivity of MAbs F10F5 and F12D2 with serotype A (MU-1), B (409), C (24066), and D (9375B) GXM or de-O-acetylated serotype A (MU-1) GXM by ELISA. Serial dilutions were made from a stock 2-mg/ml antibody solution.
FIG. 2.
Reactivity of MAbs F10F5 (left panel) and F12D2 (right panel) with GXM by immunodiffusion. The assay was done with each MAb in the center well (2 mg/ml) and GXM in the outer wells (2 mg/ml). (A) serotype A (MU-1); (B) serotype B (409); (C) serotype C (24066); (D) serotype D (9375B); (E) de-O-acetylated serotype A (MU-1).
FIG. 3.
Capsule reactions of MAbs 3C2, 471, F10F5, and F12D2 with yeast cells of serotypes A (MU-1), B (409), C (24066), and D (34875).
Production and characterization of a MAb that is reactive with de-O-acetylated GXM.
Previous studies of monoclonal and polyclonal antibodies that are reactive with the panspecific epitope shared by serotypes A, B, C, and D found that the antibodies are highly O-acetyl dependent in the recognition of GXM (1, 8, 13, 24). As a consequence, production of a high frequency (73%) of hybridomas from mice immunized with de-O-acetylated serotype A GXM that produced antibodies reactive with all four serotypes was not predictable on the basis of previous reports. One of the colonies from fusion 12 that exhibited panspecific reactivity was cloned by limiting dilution, and MAb F12D2 was characterized in detail.
Analysis of the reactivity of MAb F12D2 by ELISA using GXMs of serotype A, B, C, or D in the solid phase showed strong binding to GXMs of all four serotypes (Fig. 1). Reactivity with GXMs of all four serotypes was also found by double immunodiffusion (Fig. 2). Also shown in Fig. 1 and 2 are the binding of MAb F12D2 to de-O-acetylated serotype A GXM in ELISA and double immunodiffusion assays, respectively. The patterns of reactivity are indistinguishable from the fully O-acetylated GXM. Examination of capsular reactions of MAb F12D2 by DIC microscopy showed prominent rim patterns with cells of serotypes A, B, and C. Strain 9375B, which was used for production of the serotype D GXM used for experiments whose results are shown in Fig. 1 and 2, failed to respond to capsule induction conditions and produced a smaller capsule that did not allow for an unequivocal capsule reaction. As a consequence, serotype D strain 34875 was used for assessment of the capsule reaction for serotype D cells. MAb F12D2 typically produced a rim pattern; however, a minority of cells in a field produced a puffy pattern (not shown). Also shown in Fig. 3 are the capsule reactions for MAbs 3C2 and 471. Like MAb F12D2, MAbs 3C2 and 471 are reactive with all four serotypes; however, MAbs 3C2 and 471 were generated from mice immunized with native O-acetyl-positive GXM.
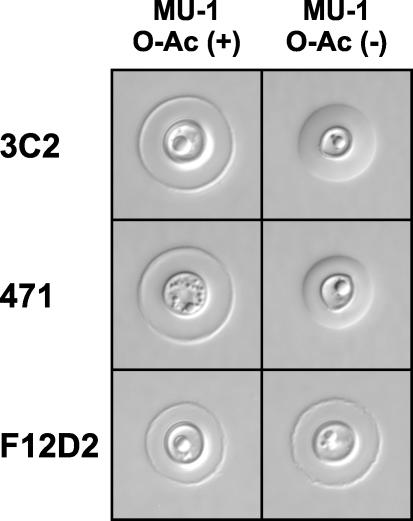
Since de-O-acetylation influences the reactivity of panspecific MAbs such as 3C2 and 471 with soluble GXM (2, 13), we compared the capsular reactions produced by MAbs 3C2, 471, and F12D2 with cells of serotype A and cells that had been de-O-acetylated by alkaline hydrolysis. The results (Fig. 4) showed that all three MAbs produced rim patterns with untreated serotype A cells. However, MAbs 3C2 and 471 produced a puffy pattern with de-O-acetylated cells whereas the pattern of MAb F12D2 remained that of a rim. These results provide further evidence for the dependence of MAbs 3C2 and 471 on O acetylation for full reactivity with serotype A GXM and demonstrate that binding of MAb F12D2 is independent of O acetylation.
FIG. 4.
Capsule reactions of MAbs 3C2, 471, and F12D2 with serotype A cells of C. neoformans (MU-1) and cells that have been chemically de-O-acetylated.
DISCUSSION
One of the earliest uses of the term immunodominant was attributed to Heidelberger in reference to that portion of a polysaccharide epitope that has the highest affinity for antibody (29); however, most studies of immunodominance have examined factors that influence stimulation of the T-lymphocyte system by protein antigens (reviewed in reference 40). A dominant T-cell determinant induces a strong T-cell response when the native antigen is used in an appropriate adjuvant (40). A subdominant determinant induces a weak immune response in comparison to dominant determinants. A cryptic determinant induces little if any immune response, unless the antigen has been altered to facilitate display of the determinant. The extent to which previous studies of dominance and crypticity apply to polysaccharide antigens such as GXM is not known. For example, major histocompatibility complex-deficient mice produce antibody in response to immunization with T-independent antigens (19). In contrast, dominance and crypticity in the context of protein molecules is dependent on antigen processing, major histocompatibility complex binding, and recognition by T-cell receptors. GXM has been described as a T-independent antigen (42). However, in the present study, mice were immunized with GXM-SRBC conjugates that likely invoke a considerable T-dependent response. As a consequence, components of dominance and crypticity of T-cell antigenic determinants may be at play in the immune response to GXM-SRBC. Regardless of the immunological basis for dominance and crypticity of antigenic determinants of GXM, the general definitions of dominant, subdominant, and cryptic determinants are useful for a discussion of the immune response to GXM. For the purposes of this report, the term dominant epitope describes an epitope that generates a high frequency of antibody-secreting hybridomas, subdominant epitopes generate antibody-secreting hybridomas in a low frequency, and cryptic determinants generate antibody-secreting hybridomas only if the immunizing antigen has been structurally altered to expose an epitope.
An epitope that is shared by serotypes A, B, C, and D is immunodominant when mice are immunized with an SRBC conjugate of serotype A GXM. In three independent immunizations and fusions, 88% of the hybridomas secreted an antibody that was reactive with a panspecific antigen. However, by use of a rigorous selection approach in which supernatant fluids were screened for reactivity with GXMs of all four serotypes, it was possible to identify additional hybridomas that secreted antibodies specific for apparent subdominant epitopes. Of 309 antibody-secreting colonies, 3% were reactive with serotypes A, B, and D, 1% were reactive with serotypes A, B, and C, 5% were reactive with serotypes A and B, and 2% were reactive with serotypes A and D. MAbs that are reactive with an epitope shared by serotypes A, B, and D and serotypes A and D have been described previously (37). MAbs reactive with serotypes A, B, and C and serotypes A and B have not been described previously.
The immunodominant epitope in immunization with SRBC serotype D GXM is an epitope that is shared by GXMs from serotypes A and D. Of 119 antibody-secreting colonies, 67% were reactive only with serotypes A and D, 18% were reactive with serotypes A, B and D, and 15% were reactive with all four serotypes. Immunization with serotype B GXM produced still a different pattern of apparent immunodominant epitopes, producing an equal number of colonies secreting antibodies reactive with the panspecific determinant and a determinant shared by serotypes A and B.
The synthesis of antibodies reactive with an epitope shared only by serotype A and B GXMs was unexpected, because an antibody with this pattern of reactivity was not reported in earlier studies of polyclonal antibodies by Ikeda et al. (20). All of the remaining patterns of serotype reactivity found in our screening of positive colonies had previously been identified by the cross-absorption studies of Ikeda et al. Our identification of a MAb that recognizes an epitope that was not predicted by the classical study of Ikeda et al. may have been due to our immunization of mice rather than rabbits, as used in Ikeda's study. Mice may produce an immune response that recognizes one or more GXM epitopes that are not recognized by rabbits. One colony secreting antibody reactive to A/B was cloned by limiting dilution from the fusion derived from serotype B-immunized mice. The antibody produced by this clone, termed MAb F10F5, demonstrated reactivity only with serotypes A and B by both ELISA and capsule reactions; however, weak reactivity with serotypes C and D was noted when antigen-antibody binding was examined by double immunodiffusion. The reason for this discrepancy, particularly in view of the very limited sensitivity of double immunodiffusion, is not known. However, we have previously reported a lack of agreement between results obtained by analysis of GXM MAbs by ELISA and immunoprecipitation (12).
Belay and Cherniak described factor 1 antibodies (reactive with an epitope that is shared by all serotypes) as being O-acetyl dependent (1). However, our studies of MAb F12D2 show that induction of a MAb that is reactive with all serotypes does not inherently require the presence of an O-acetyl group on the immunizing GXM. As a consequence, we conclude from these results that there are two distinct panspecific epitopes: one epitope is O-acetyl dependent, and the other is O-acetyl independent. Further evidence for the existence of two distinct panspecific epitopes is provided by examination of capsular reactions by DIC microscopy. The panspecific MAbs 3C2 and 471 produce prominent rim-type patterns with cells of serotype A. In contrast, these same antibodies produce puffy patterns with chemically de-O-acetylated yeast cells, demonstrating for the first time that the ability to produce a given capsule reaction for this class of antibodies requires an O-acetyl group. In contrast, MAb F12D2 produced a rim pattern with untreated and de-O-acetylated cells.
MAb F12D2 has recently been used to evaluate the antigenic characteristics of a mutant strain of C. neoformans serotype D that is deficient in xylosylation of GXM (25). The results showed that MAb F12D2 failed to react with the xylose-deficient GXM. These results indicate that MAb F12D2 is dependent on the xylosylation of GXM for its reactivity. The extent to which results from study of the contribution of xylosylation to the antigenicity of serotype D GXM can be extended to the GXMs of the remaining serotypes is not known.
The epitope recognized by MAb F12D2 most likely falls into the subdominant or cryptic category. The MAb was generated by using GXM that had been chemically de-O-acetylated to remove the dominant panspecific O-acetyl determinant. In the absence of the dominant epitope, colonies secreting antibodies reactive with a second panspecific epitope became apparent. We cannot exclude the possibility that antibodies reactive with the O-acetyl-independent, panspecific epitope are produced in response to immunization with native serotype A GXM. Our initial screen for antibody production was not constructed to make such a distinction. However, most panspecific antibodies examined to date, whether polyclonal or monoclonal, have been O-acetyl dependent (1, 8, 13, 24). Notably, an IgM MAb designated 21D2 was produced from mice immunized with a GXM-protein conjugate and shows limited binding to de-O-acetylated GXM from serotype A strain 371 and no reactivity with de-O-acetylated GXM from serotype A strain 24064 (6).
The importance of O acetylation to the immunogenicity and immunoreactivity of capsular polysaccharides and protein conjugates of polysaccharides varies considerably from one polysaccharide to another. De-O-acetylated capsular polysaccharides of Neisseria meningitidis serogroup A polysaccharide (3), the Vi polysaccharide of Salmonella enterica serovar Typhi (43), and the Escherichia coli K1 capsular polysaccharide (35) are dramatically less immunogenic than the O-acetyl-positive parent polysaccharides. In contrast, meningococcal serogroup C polysaccharide (36), Staphylococcus aureus type 5 and type 8 capsular polysaccharides (15), and pneumococcal type 9V (32) do not require O acetylation for induction of antibodies that are reactive with the O acetylated parent polysaccharides.
In summary, in the present paper we report the production of MAbs that are reactive with a previously undescribed GXM epitope that is shared by GXMs from serotypes A and B. In addition, we have found that immunization of mice with de-O-acetylated serotype A GXM led to the production of MAbs that are reactive with a previously undescribed O-acetyl-independent, panspecific epitope that is found on all four of the major GXM serotypes. Importantly, MAb reactive with the O-acetyl-independent epitope produced a rim-type capsular reaction with yeast cells of all four serotypes. In contrast, the panspecific MAbs 3C2 and 471 produced a rim pattern on cells of serotypes A and B and a puffy pattern on cells of serotypes C and D. Given the close association between production of a rim pattern and protective efficacy of MAbs in a murine model of cryptococcosis (30), these results raise the possibility that the O-acetyl-independent epitope defined by MAb F12D2 would be an ideal target for active or passive immunization that would be effective against all serotypes of C. neoformans.
Acknowledgments
This work was supported in part by Public Health Service Grant AI14209 from the National Institute for Allergy and Infectious Diseases.
REFERENCES
- 1.Belay, T., and R. Cherniak. 1995. Determination of antigen binding specificities of Cryptococcus neoformans factor sera by enzyme-linked immunosorbent assay. Infect. Immun. 63:1810-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belay, T., R. Cherniak, T. R. Kozel, and A. Casadevall. 1997. Reactivity patterns and epitope specificities of anti-Cryptococcus neoformans monoclonal antibodies by enzyme-linked immunosorbent assay and dot enzyme assay. Infect. Immun. 65:718-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, D. S., F. Lynn, C.-H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharjee, A. K., J. E. Bennett, and C. P. J. Glaudemans. 1984. Capsular polysaccharides of Cryptococcus neoformans. Rev. Infect. Dis. 6:619-624. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., M. DeShaw, M. Fan, F. Dromer, T. R. Kozel, and L. Pirofski. 1994. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., J. Mukherjee, S. J. N. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 165:1086-1093. [DOI] [PubMed] [Google Scholar]
- 7.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherniak, R., E. Reiss, M. E. Slodki, R. D. Plattner, and S. O. Blumer. 1980. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol. Immunol. 17:1025-1032. [DOI] [PubMed] [Google Scholar]
- 9.Cherniak, R., E. Reiss, and S. H. Turner. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103:239-250. [Google Scholar]
- 10.Cherniak, R., H. Valafar, L. C. Morris, and F. Valafar. 1998. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin. Diagn. Lab. Immunol. 5:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duro, R. M., D. Netski, P. Thorkildson, and T. R. Kozel. 2003. Contribution of epitope specificity to the binding of monoclonal antibodies to the capsule of Cryptococcus neoformans and the soluble form of its major polysaccharide, glucuronoxylomannan. Clin. Diagn. Lab. Immunol. 10:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert, T. F., and T. R. Kozel. 1987. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect. Immun. 55:1895-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falugi, F., R. Petracca, M. Mariani, E. Luzzi, S. Mancianti, V. Carinci, M. L. Melli, O. Finco, A. Wack, A. Di Tommaso, M. T. De Magistris, P. Costantino, G. Del Giudice, S. Abrignani, R. Rappuoli, and G. Grandi. 2001. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: a model for new conjugate vaccines. Eur. J. Immunol. 31:3816-3824. [DOI] [PubMed] [Google Scholar]
- 15.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 63:3448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger, D. L., J. R. Perfect, and D. T. Durack. 1985. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Investig. 76:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grinsell, M., L. C. Weinhold, J. E. Cutler, Y. Han, and T. R. Kozel. 2001. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J. Infect. Dis. 184:479-487. [DOI] [PubMed] [Google Scholar]
- 19.Grusby, M. J., H. Auchincloss, Jr., R. Lee, R. S. Johnson, J. P. Spencer, M. Zijlstra, R. Jaenisch, V. E. Papaioannou, and L. H. Glimcher. 1993. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA 90:3913-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda, R., T. Shinoda, Y. Fukazawa, and L. Kaufman. 1982. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J. Clin. Microbiol. 16:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozel, T. R., and J. Cazin, Jr. 1972. Immune response to Cryptococcus neoformans soluble polysaccharide. I. Serological assay for antigen and antibody. Infect. Immun. 5:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozel, T. R., B. C. H. deJong, M. M. Grinsell, R. S. MacGill, and K. K. Wall. 1998. Characterization of anti-capsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect. Immun. 66:1538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozel, T. R., and J. L. Follette. 1981. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect. Immun. 31:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel, T. R., and E. C. Gotschlich. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129:1675-1680. [PubMed] [Google Scholar]
- 25.Kozel, T. R., S. M. Levitz, F. Dromer, M. A. Gates, P. Thorkildson, and G. Janbon. 2003. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect. Immun. 71:2868-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane, R. D., R. S. Crissman, and M. F. Lachman. 1984. Comparison of polyethylene glycols as fusogens for producing lymphocyte-myeloma hybrids. J. Immunol. Methods 72:71-76. [DOI] [PubMed] [Google Scholar]
- 27.Leinonen, M., and C. E. Frasch. 1982. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect. Immun. 38:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. S. Zuckier. 1998. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 177:1647-1659. [DOI] [PubMed] [Google Scholar]
- 29.Lüderitz, O., A. M. Staub, and O. Westphal. 1966. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol. Rev. 30:192-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacGill, T. C., R. S. MacGill, A. Casadevall, and T. R. Kozel. 2000. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J. Immunol. 164:4835-4842. [DOI] [PubMed] [Google Scholar]
- 31.MacGill, T. C., R. S. MacGill, and T. R. Kozel. 2001. Capsular reaction of Cryptococcus neoformans with polyspecific and oligospecific polyclonal anticapsular antibodies. Infect. Immun. 69:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeely, T. B., J. M. Staub, C. M. Rusk, M. J. Blum, and J. J. Donnelly. 1998. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 66:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netski, D., and T. R. Kozel. 2002. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect. Immun. 70:2812-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orskov, F., I. Orskov, A. Sutton, R. Schneerson, W. Lin, W. Egan, G. E. Hoff, and J. B. Robbins. 1979. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J. Exp. Med. 149:669-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savoy, A. C., D. M. Lupan, P. B. Manalo, J. S. Roberts, A. M. Schlageter, L. C. Weinhold, and T. R. Kozel. 1997. Acute lethal toxicity following passive immunization for treatment of murine cryptococcosis. Infect. Immun. 65:1800-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlageter, A. M., and T. R. Kozel. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 58:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal, D. M. 1995. Antibody detection and preparation, p. 2.0.1-2.13.16. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley and Sons, Inc., New York, N.Y.
- 40.Sercarz, E. E., P. V. Lehmann, A. Ametani, G. Benichou, A. Miller, and K. Moudgil. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11:729-766. [DOI] [PubMed] [Google Scholar]
- 41.Spiropulu, C., R. A. Eppard, E. Otteson, and T. R. Kozel. 1989. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect. Immun. 57:3240-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundstrom, J. B., and R. Cherniak. 1992. The glucuronoxylomannan of Cryptococcus neoformans serotype A is a type 2 T-independent antigen. Infect. Immun. 60:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szu, S. C., X. R. Li, A. L. Stone, and J. B. Robbins. 1991. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59:4555-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner, S. H., and R. Cherniak. 1991. Multiplicity in the structure of the glucuronoxylomannan of Cryptococcus neoformans, p. 123-142. In J. P. Latgé and D. Boucias (ed.), Fungal cell wall and immune response. Springer-Verlag, Berlin, Germany.
- 45.Vecchiarelli, A., and A. Casadevall. 1998. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res. Immunol. 149:321-333. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, D. E., J. E. Bennett, and J. W. Bailey. 1968. Serologic grouping of Cryptococcus neoformans. Proc. Soc. Exp. Biol. Med. 127:820-823. [DOI] [PubMed] [Google Scholar]